Novel salts for the manufacture of pharmaceutical compositions
A technology for medicines and mixtures, applied in the field of new salts for preparing pharmaceutical compositions, can solve the problems of not providing dabigatran etexilate crystalline properties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
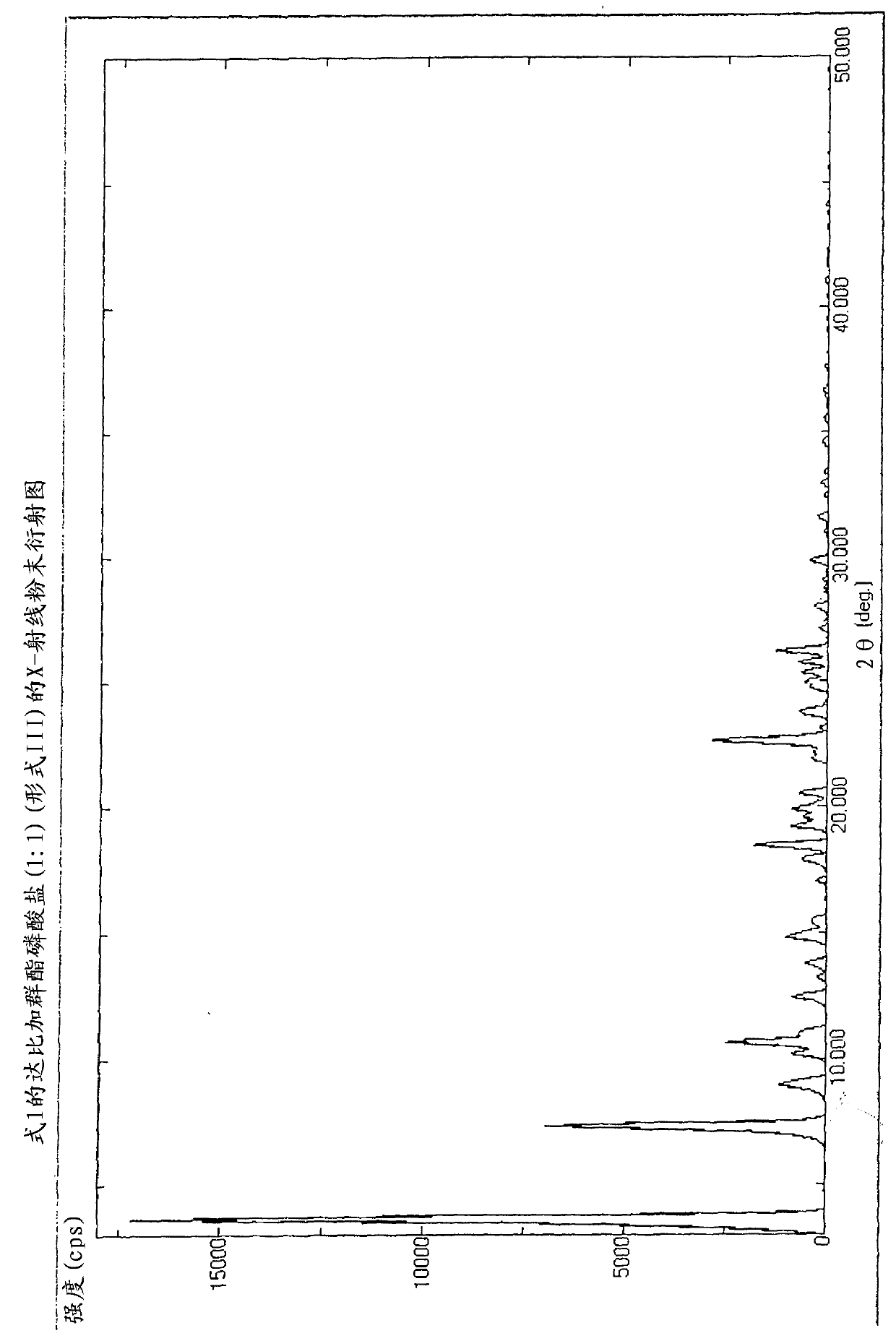
[0236] Dabigatran etexilate phosphate (1:1) of Formula 1 was prepared (Form III).
[0237] 486mg (0.77mmol) dabigatran etexilate, 1.0ml N,N-dimethyl-formamide and 89mg 85w / w% phosphoric acid (0.77mmol) were measured into 10cm 3 in a round bottom flask. While stirring, the suspension was warmed to 65°C. The mixture was completely dissolved at this temperature. Then, it was cooled to room temperature within 2 hours and kept at 5°C overnight. The crystalline suspension was filtered, washed with a little N,N-dimethyl-formamide and acetonitrile, and dried under vacuum at room temperature to constant weight.
[0238] Yield: 590 mg (81.3%).
[0239] Melting point: 154-156°C.
[0240] HNMR (DMSO, 500MHz): 8-10(b, 5H), 8.38(m, 1H), 7.92(m, 2H), 7.54(m, 1H), 7.48(d, J=1.1Hz, 1H), 7.40 (d, J=8.4Hz, 1H), 7.16 (dd, J1=1.5Hz, J2=8.4Hz, 1H), 7.12(m, 1H), 7.0(b, 1H), 6.89(m, 1H), 6.78 (m, 2H), 4.60(bs, 2H), 4.23(t, J=7.0Hz, 2H), 4.00(m, 2H), 3.98(q, J=7.1Hz, 2H), 2.68(t, J= 7.2Hz, 2H)...
Embodiment 2
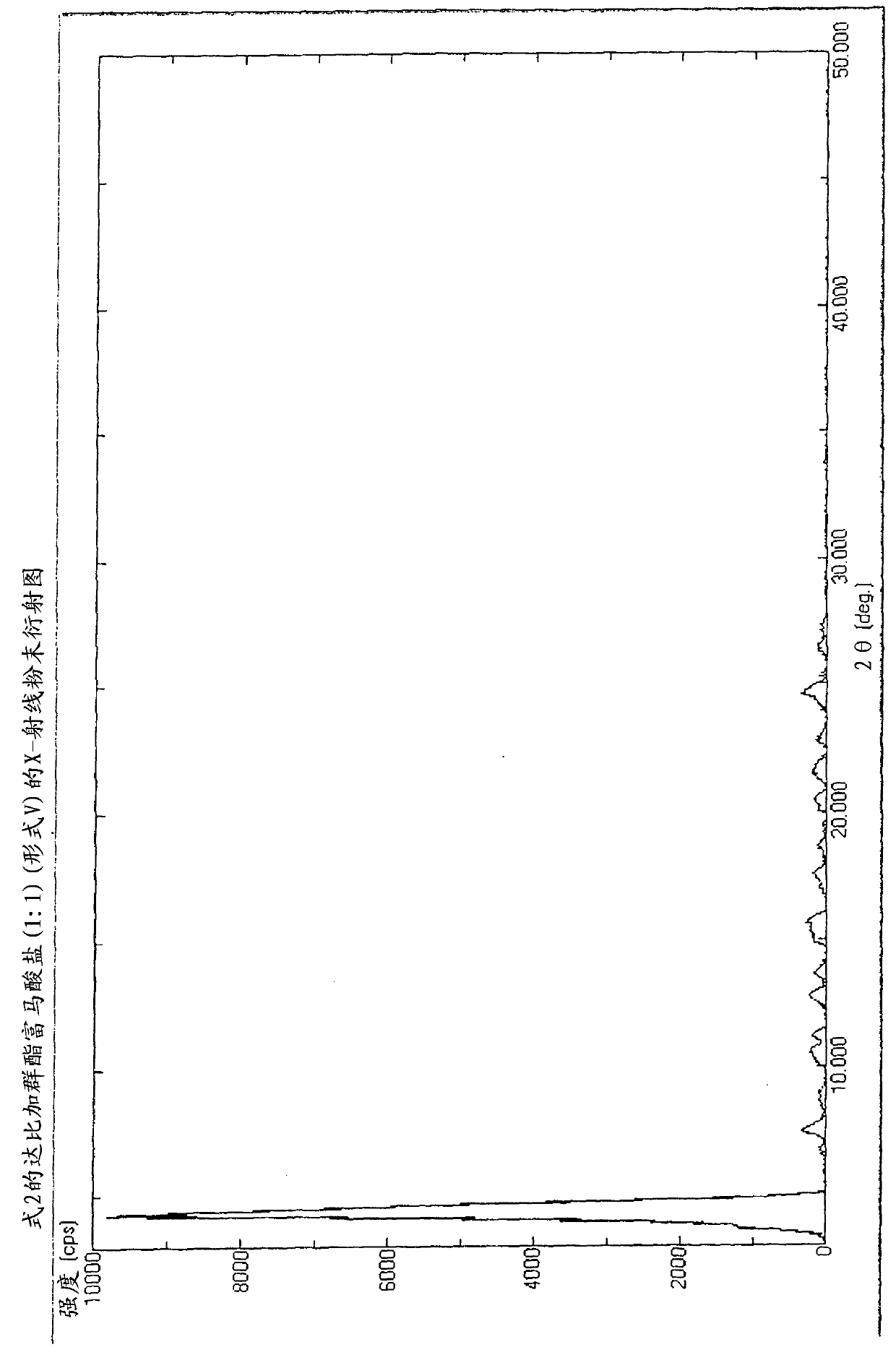
[0243] Dabigatran etexilate fumarate (1:1) of Formula 2 was prepared (Form V).
[0244] Add 636mg (1.0mmol) of dabigatran etexilate, 2.5ml of ethanol and 116mg (1.0mmol) of fumaric acid into the 10cm 3 in a round bottom flask. While stirring, the suspension was warmed to 60°C. The mixture was completely dissolved at this temperature. The mixture was cooled to room temperature over 2 hours and kept at 5 °C overnight. The crystalline suspension was filtered, washed with a small amount of ethanol, and dried under vacuum at room temperature to constant weight.
[0245] Yield: 673mg (90.5%)
[0246] Melting point: 113-115°C.
[0247]HNMR (DMSO, 500MHz): 9(b), 8.39(m, 1H), 7.80(~d, J=8.8Hz, 2H), 7.54(m, 1H), 7.48(d, J=1.1Hz, 1H) , 7.40(d, J=8.4Hz, 1H), 7.16(dd, J1=1.5Hz, J2=8.6Hz, 1H), 7.12(m, 1H), 6.94(bt, 1H), 6.89(m, 1H) , 6.77(~d, J=8.8Hz, 2H), 6.62(s, 2H), 4.60(d, J=5.1Hz, 2H), 4.23(t, J=7.2Hz, 2H), 3.98(m, 2H ), 3.98(q, J=7.0Hz, 2H), 3.77(s, 3H), 2.68(t, J=7.1Hz, 2H), ...
Embodiment 3
[0250] Preparation of dabigatran etexilate sulfate (1:1) of Formula 3 (Form I).
[0251] Add 630mg (1.0mmol) of dabigatran etexilate, 1.1ml of ethanol and 8.0ml of ethyl acetate into the 25cm 3 in a round bottom flask. 0.2 g of 50 w / w% sulfuric acid (1.0 mmol) was added to the suspension. The mixture was kept at 5°C overnight. The crystalline suspension was filtered, washed with a small amount of a mixture of ethyl acetate and ethanol (10:1 ), and dried under vacuum at room temperature to constant weight.
[0252] Yield: 543mg (74.6%)
[0253] Melting point: 171-173°C.
[0254] HNMR (DMSO, 500MHz): 11.84 (b, 1H), 10.59 (b, 1H), 9.99 (b, 1H), 8.39 (m, 1H), 7.65 (~d, J=9.0Hz, 1H), 7.59 ( b, 1H), 7.55(m, 1H), 7.47(m, 1H), 7.42(d, J=8.4Hz, 1H), 7.17(dd, J1=1.5Hz, J2=8.4Hz, 1H), 7.12( m, 1H), 6.91(m, 1H), 6.87(~d, J=9.2Hz, 2H), 4.69(d, J=3.3Hz, 2H), 4.25(m, 2H), 4.22(t, J= 7.1Hz, 2H), 3.98(q, J=7.1Hz, 2H), 3.78(s, 3H), 2.68(t, J=7.1Hz, 2H), 1.68(m, 2H), 1.38(m, 2H) , 1.30(m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com