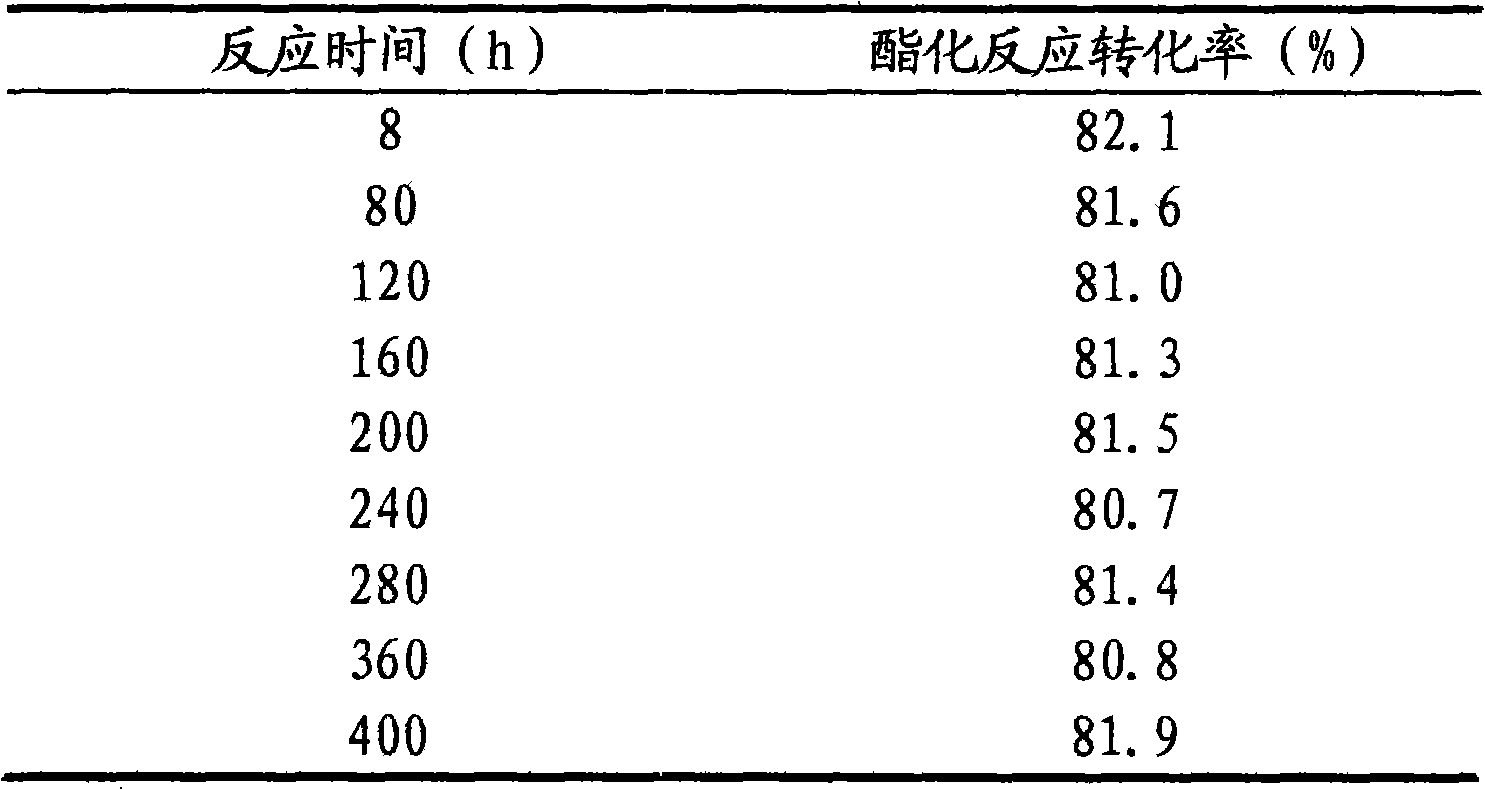
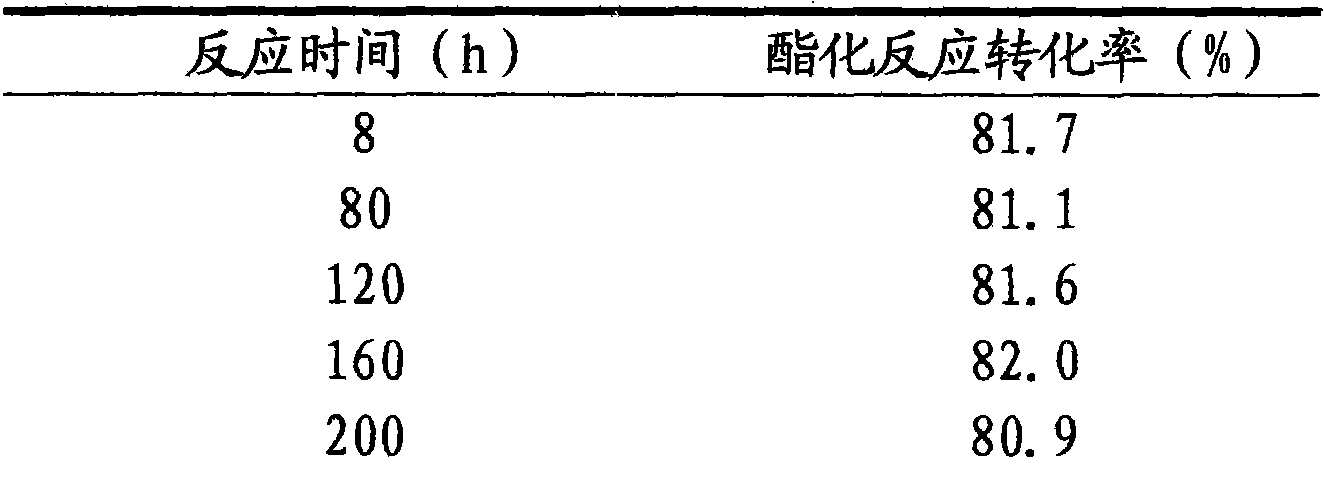
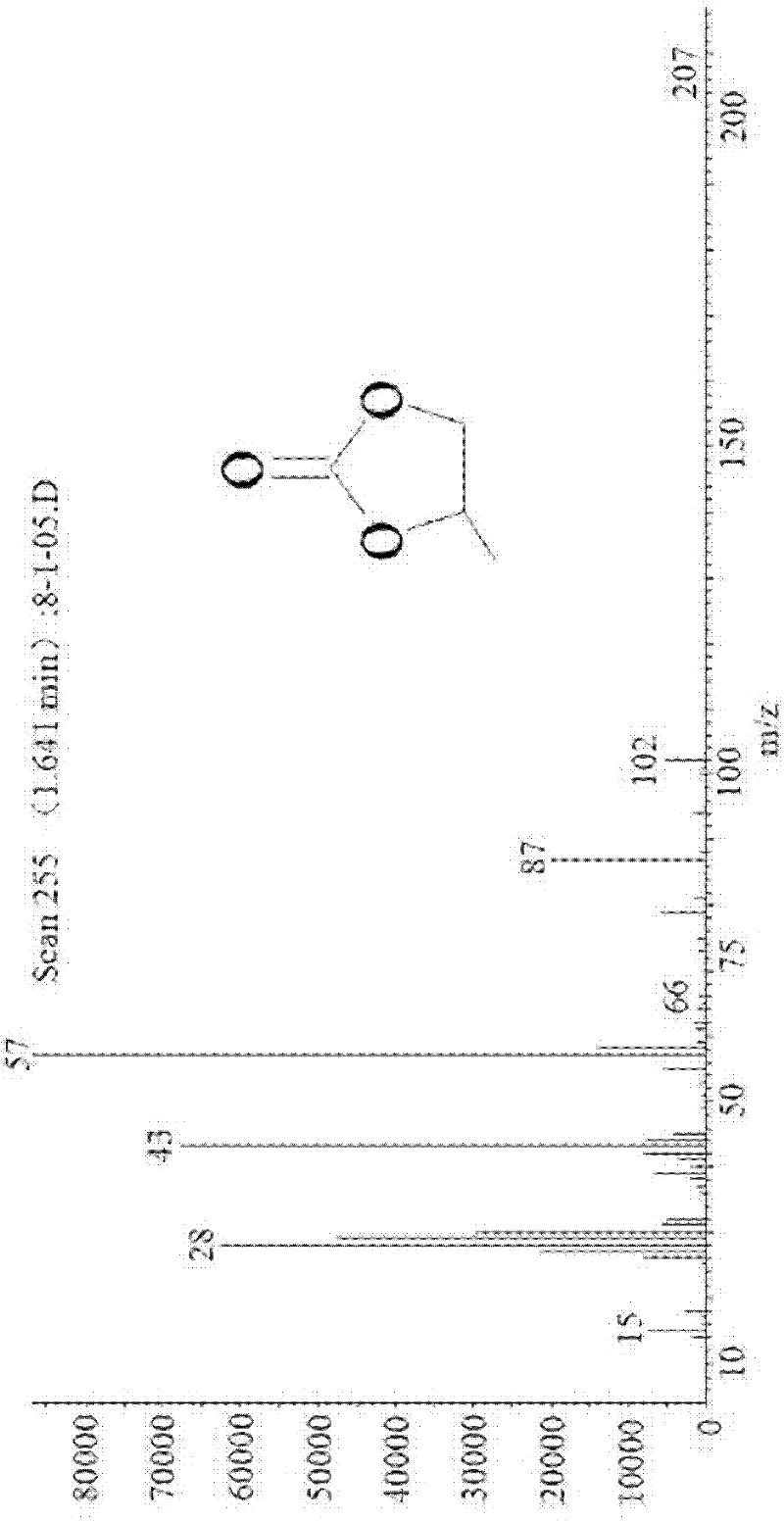
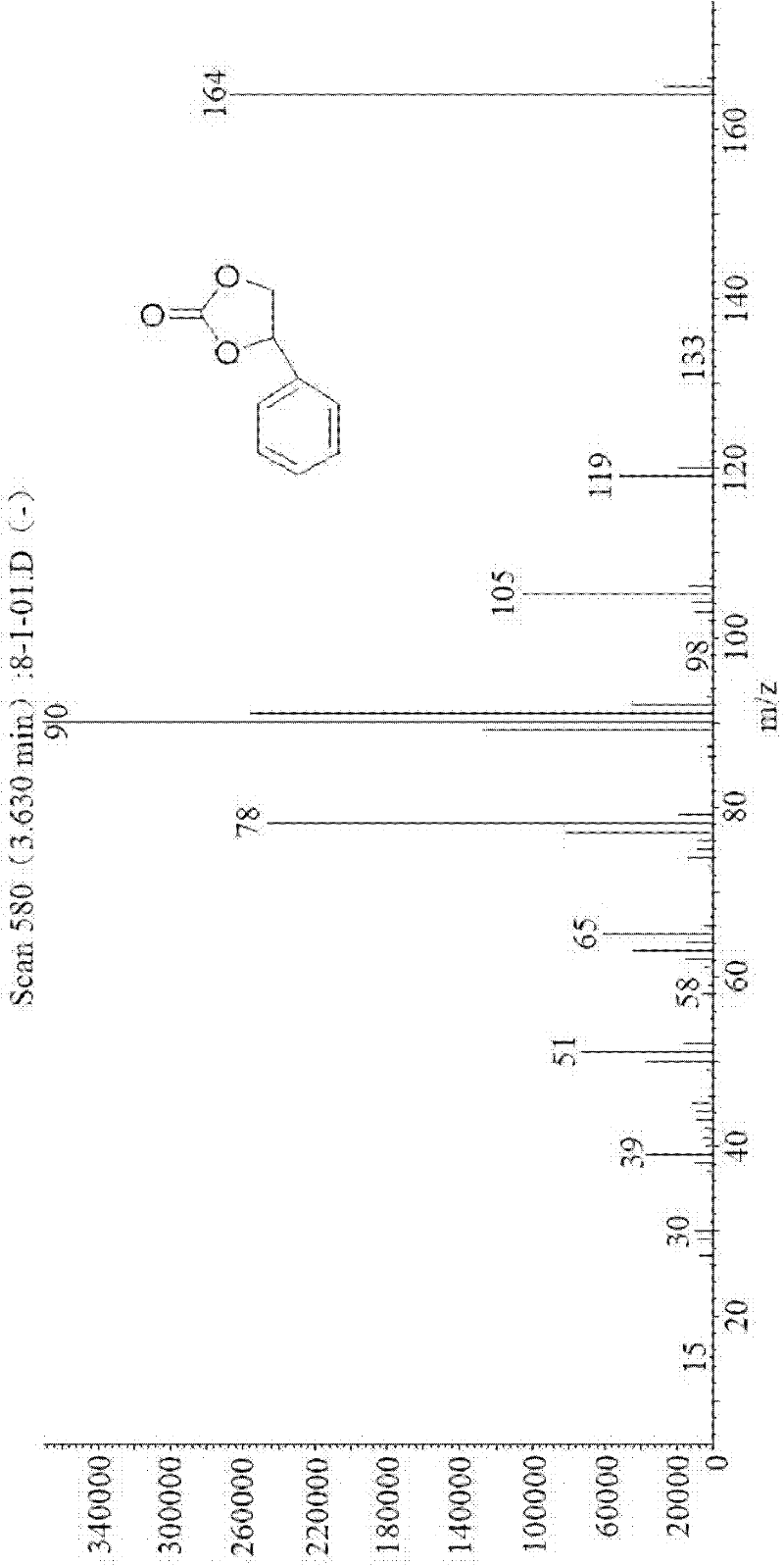
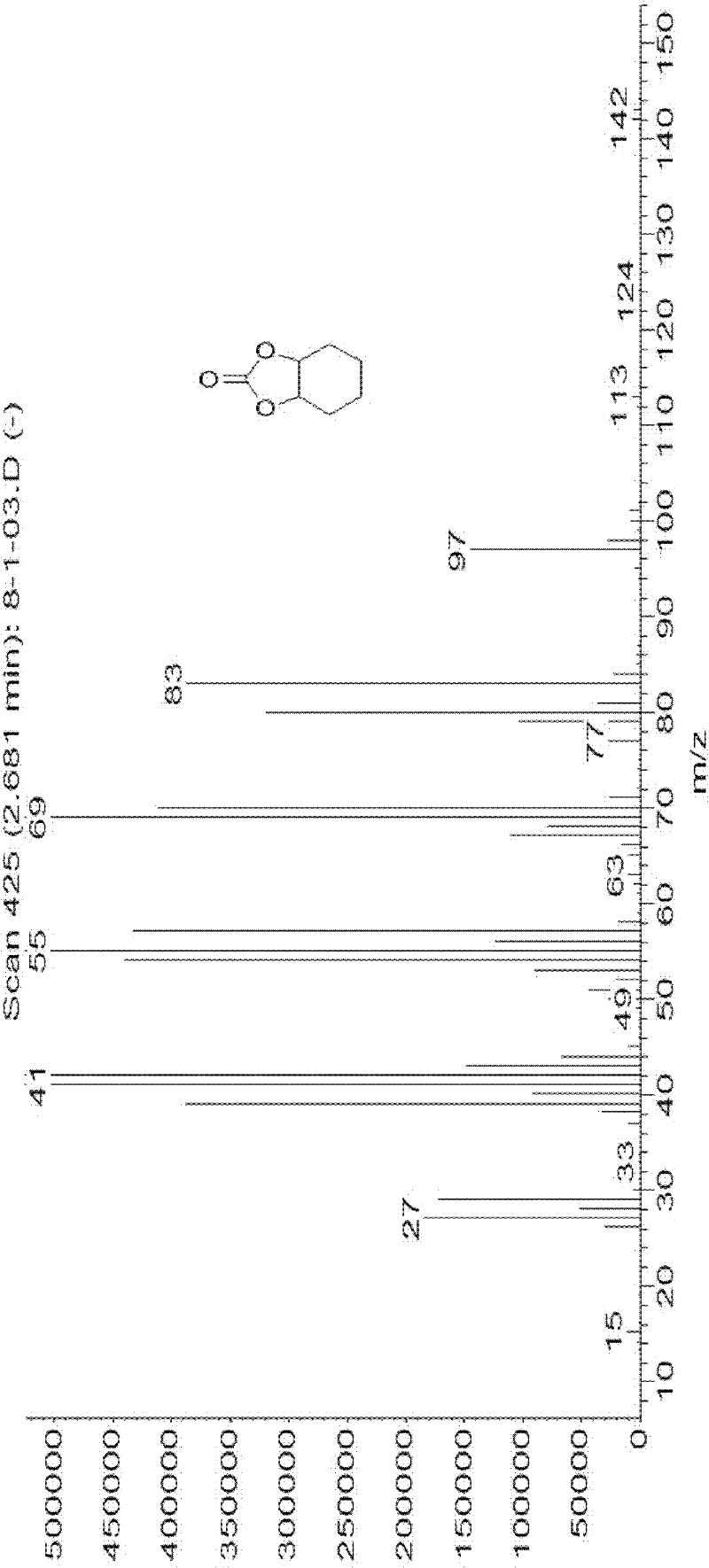
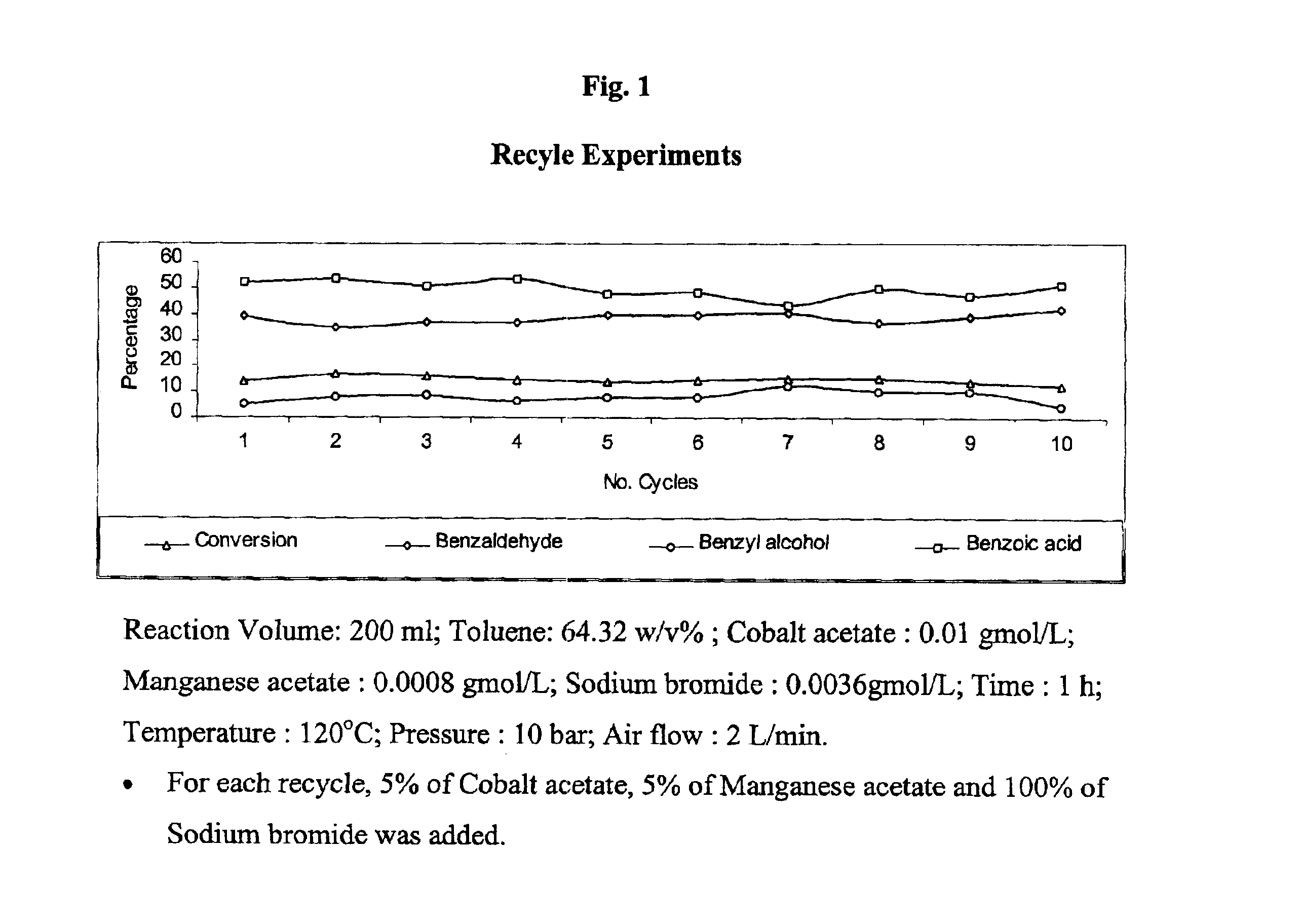
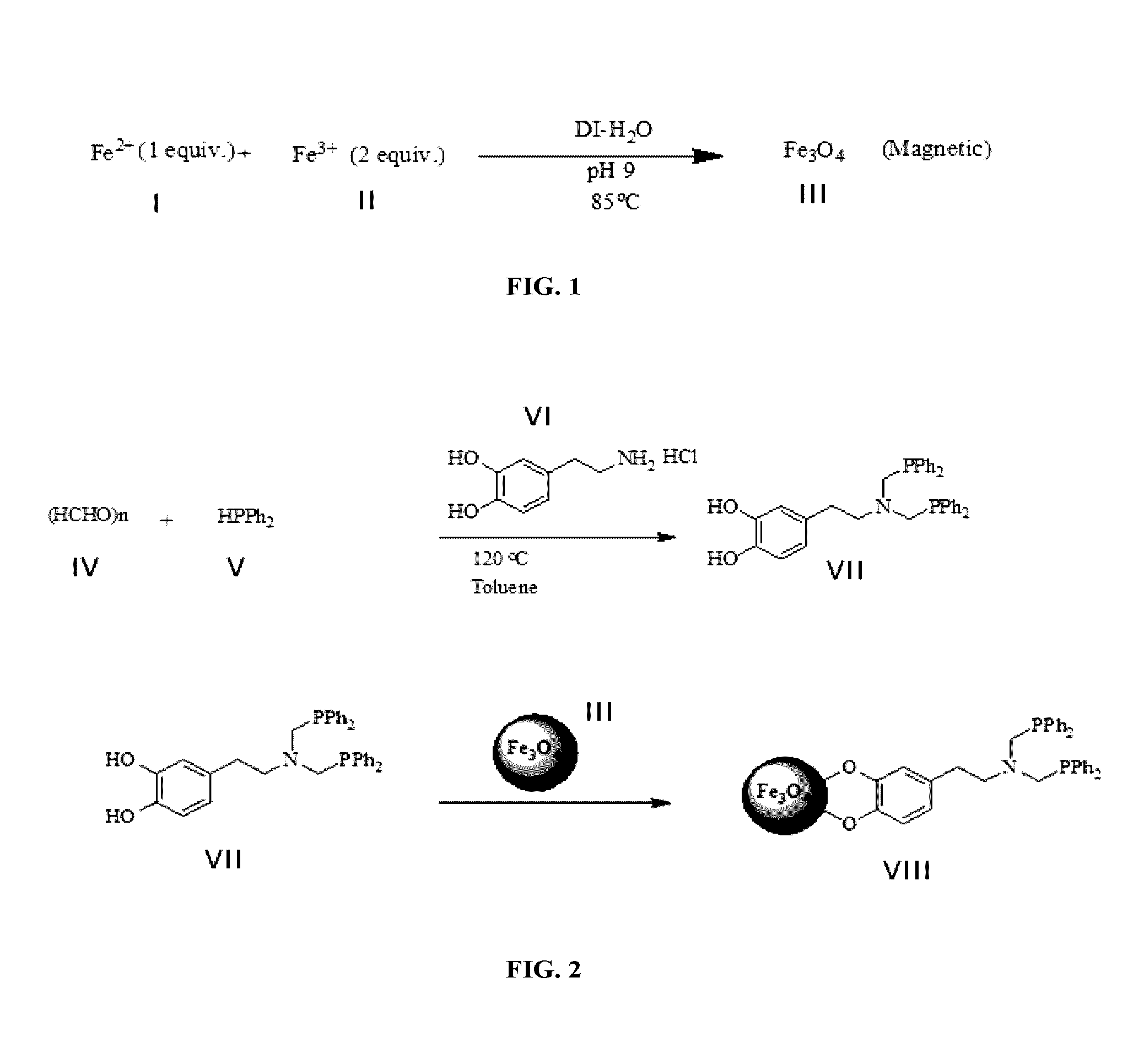
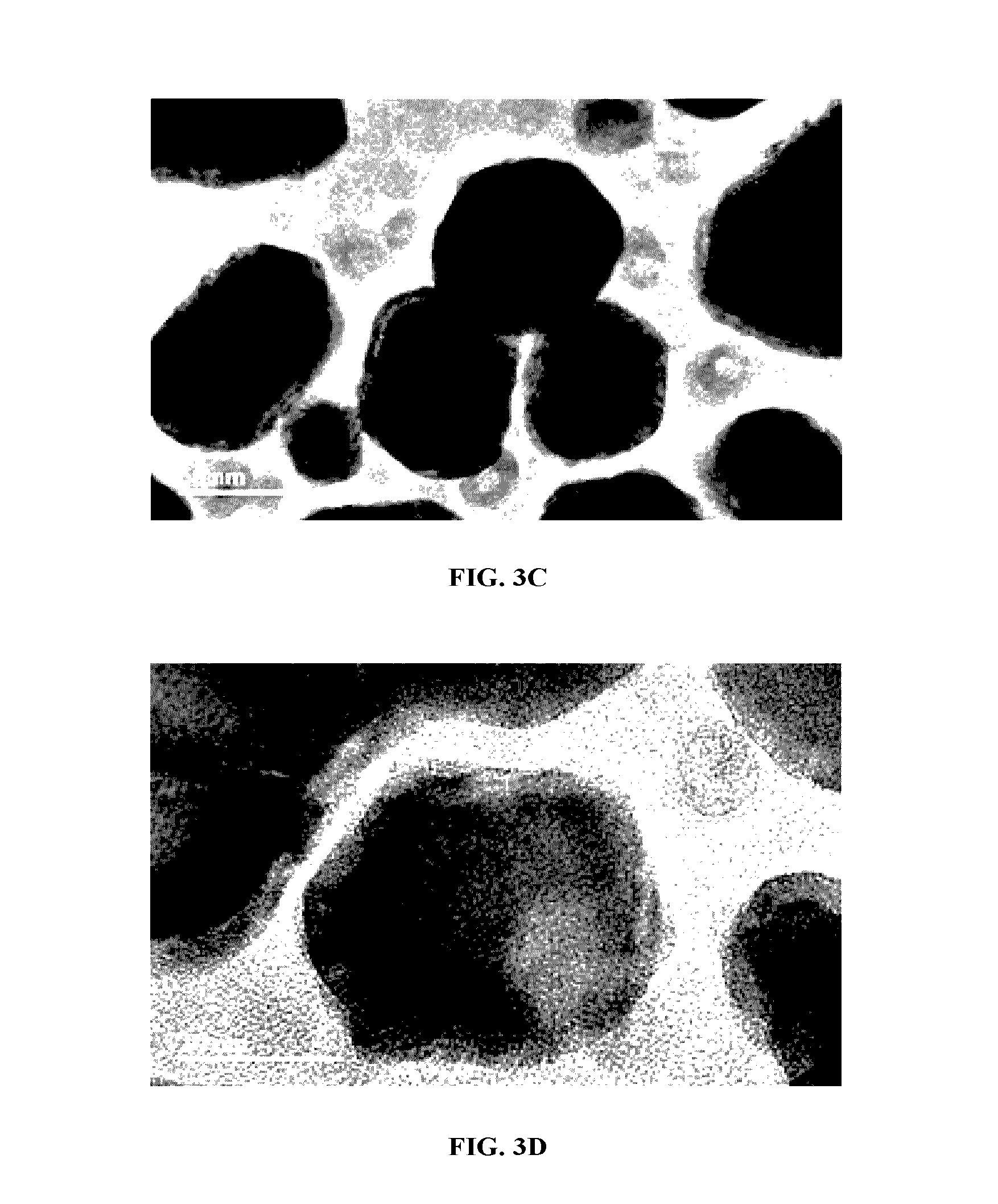
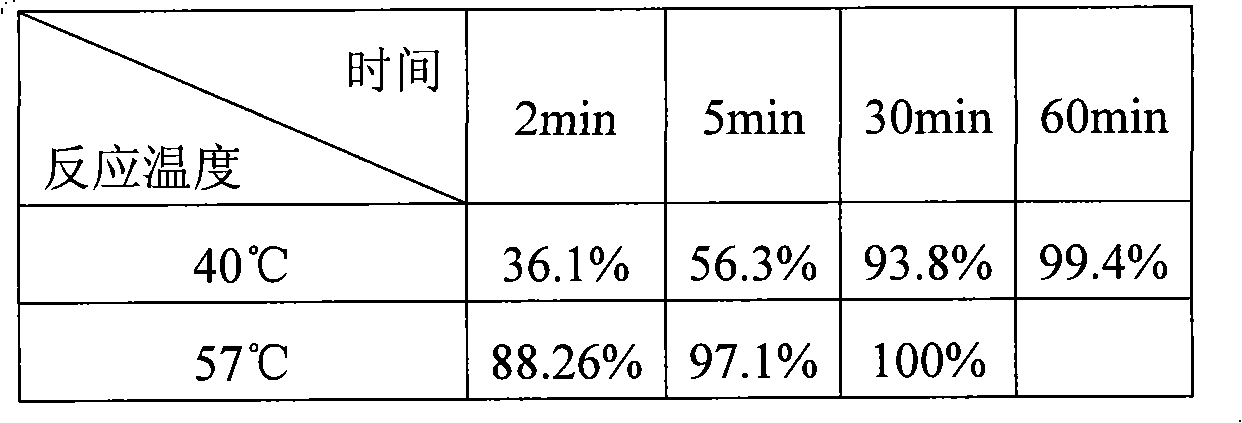
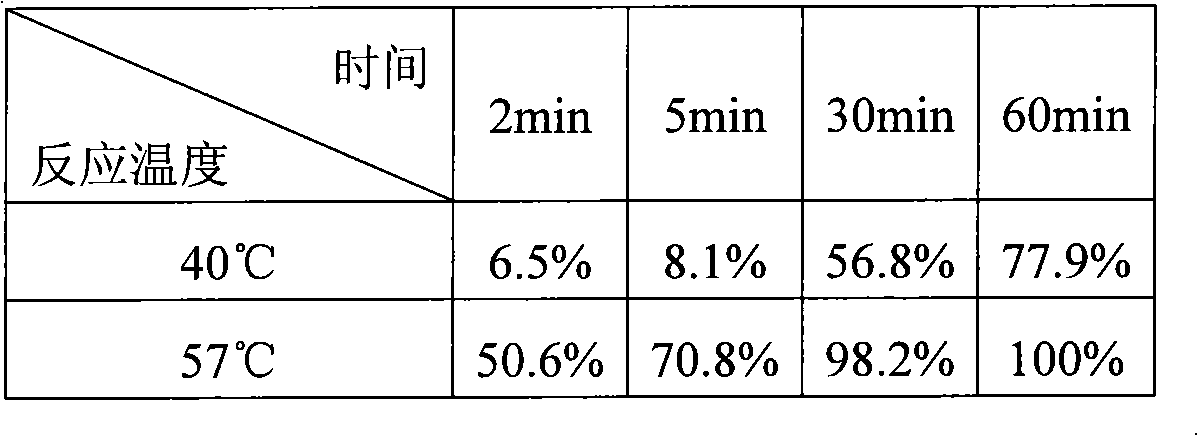
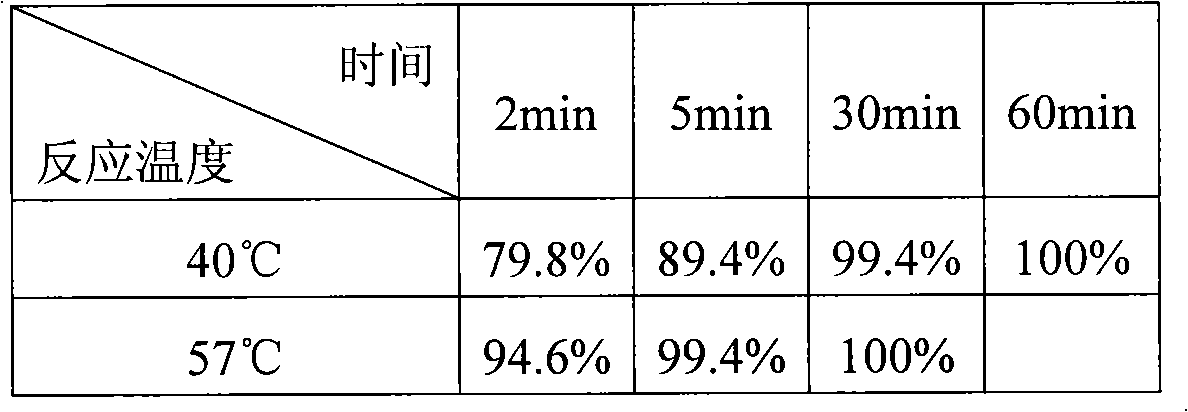
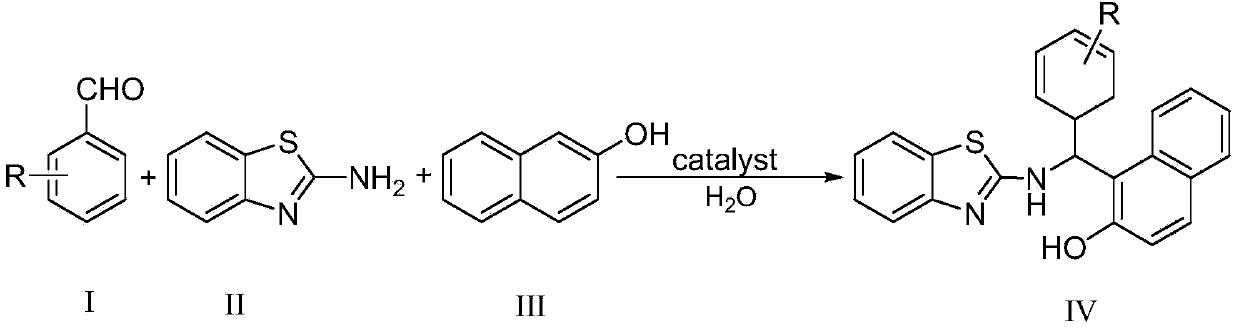
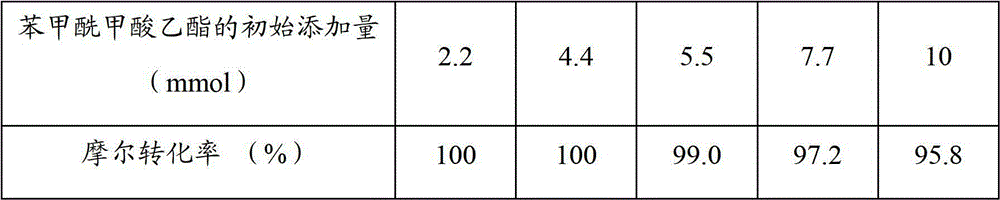
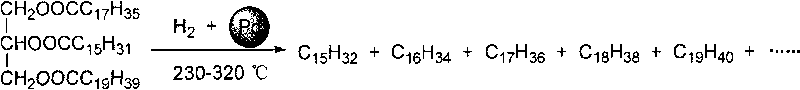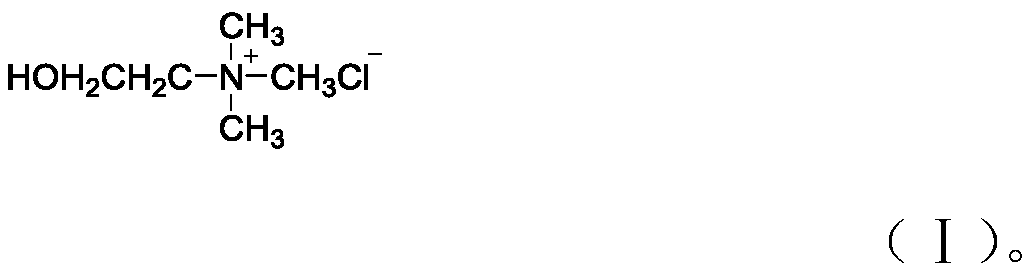Patents
Literature
128 results about "Recyclable catalyst" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Recoverable and Recyclable Catalysts is a valuable reference source for academic researchers and professionals from a range of pharmaceutical and chemical industries, particularly those working in catalysis, organic synthesis and sustainable chemistry.
Method for preparing diesel components by catalytic hydrodeoxygenation of vegetable oil
InactiveCN101709225AHigh catalytic activityImprove catalytic selectivityBiofuelsLiquid carbonaceous fuelsRecyclable catalystLiquid product
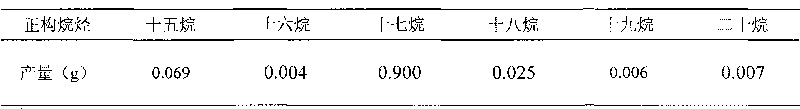
The invention provides a method for preparing diesel components by catalytic hydrodeoxygenation of vegetable oil, which prepares high heat-value alkane by catalytic hydrodeoxygenation of the vegetable oil. The method comprises the following reaction steps: adding the vegetable oil, barium sulphate-loaded palladium serving as a catalyst, as well as a solvent selected from n-hexane, n-heptane, n-octane and dodecane to a reactor; supplying hydrogen; allowing reaction pressure to be between 1 and 10 MPa; stirring and heating at 230 to 320 DEG C; performing reaction for 3 to 12 hours and then stopping the reaction; allowing the catalyst and liquid products to automatically separate after the obtained product is cooled to room temperature; and obtaining the diesel components containing high heat-value alkane without centrifugation, filtration and other complicated post treatment. The method has the advantages of simple preparation process, low reaction temperature, low solvent consumption, no cracking of carbon chain parts of the oil, high combustion heat value of target products and recyclable catalyst.
Owner:ZHEJIANG UNIV
Method of preparing adipinic acid using bionic catalytic oxggen to oxidize cyclohexane
InactiveCN1231449CReduce dosageHigh catalytic activityOrganic compound preparationCarboxylic compound preparationRecyclable catalystEnzyme structure
Owner:BEIJING UNIV OF TECH
Novel oxidation catalyst, the process for the preparation thereof and green process for selective aerobic oxidation
ActiveUS20150336090A1High yieldShort time spanAluminium compoundsMolecular sieve catalystsRecyclable catalystAlkaline earth metal
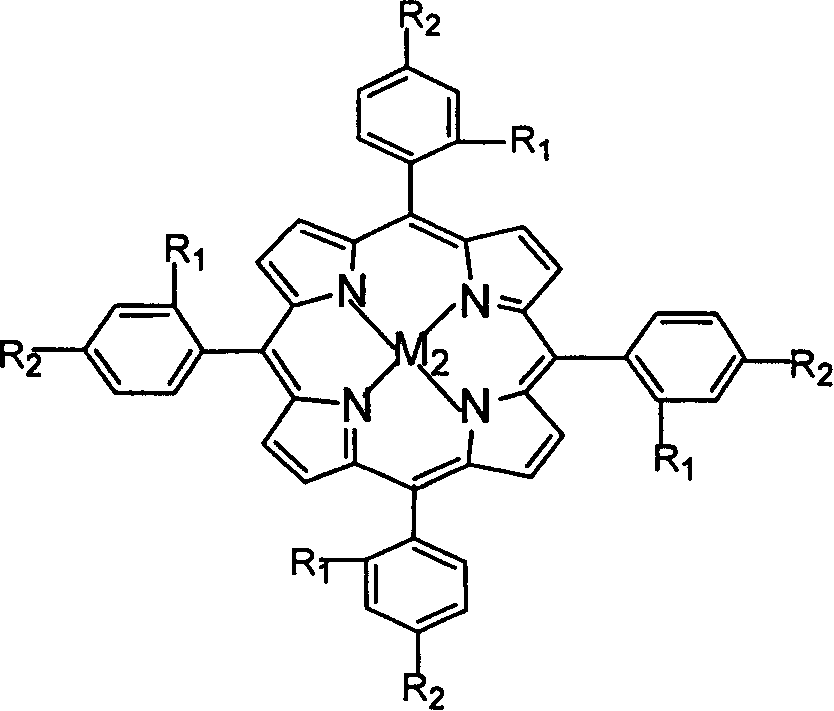
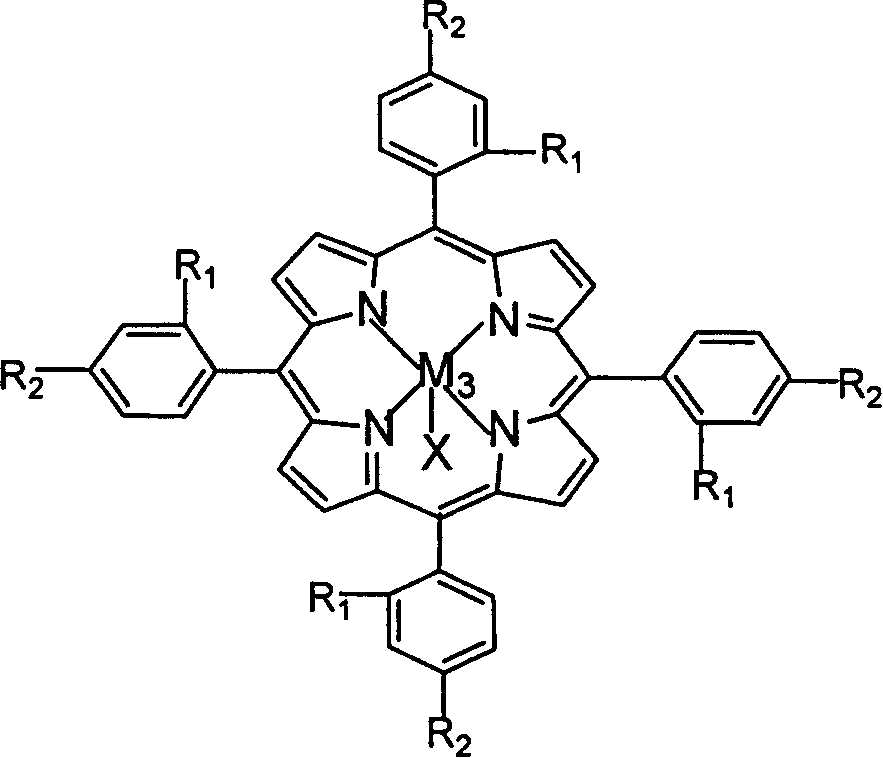
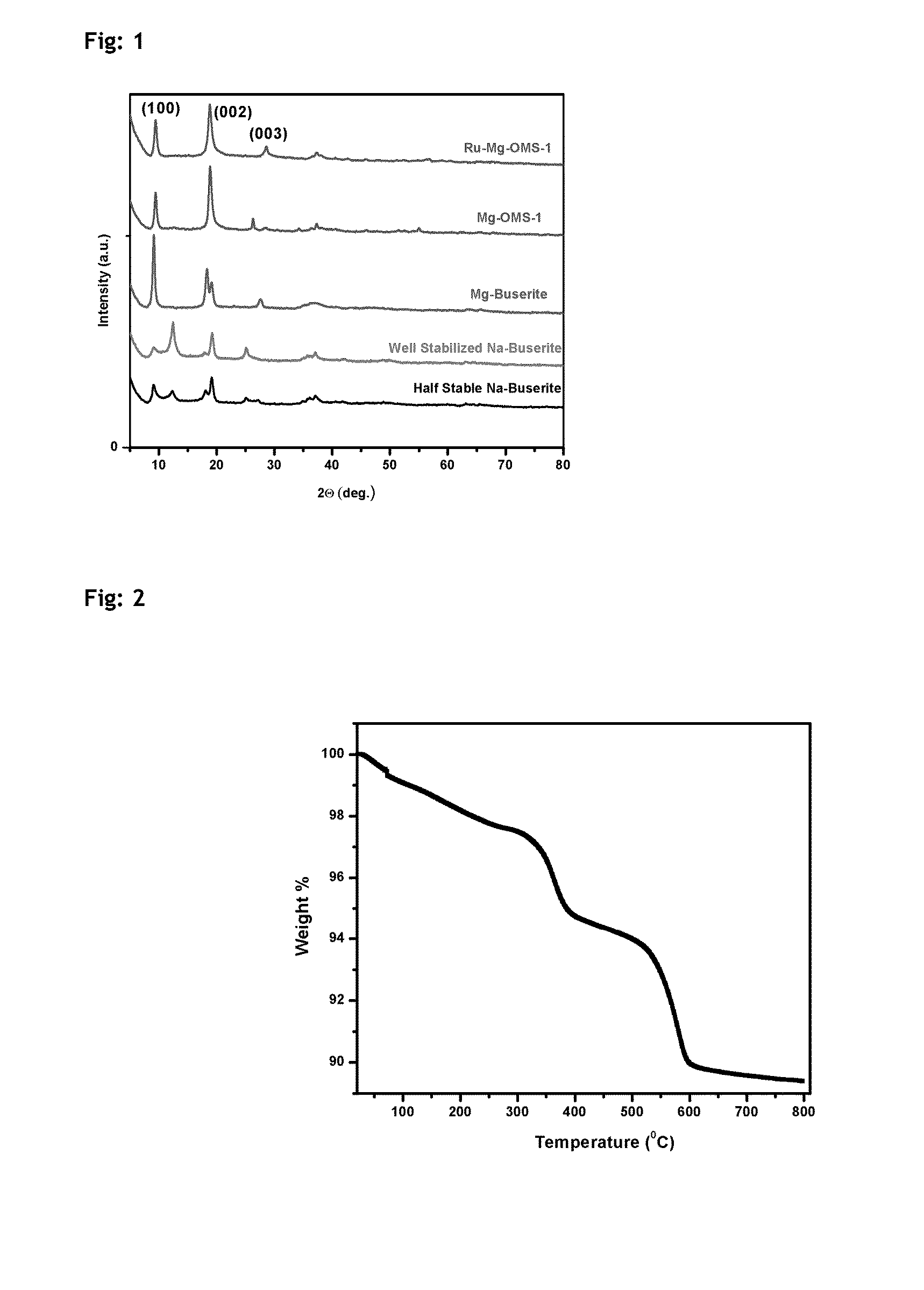
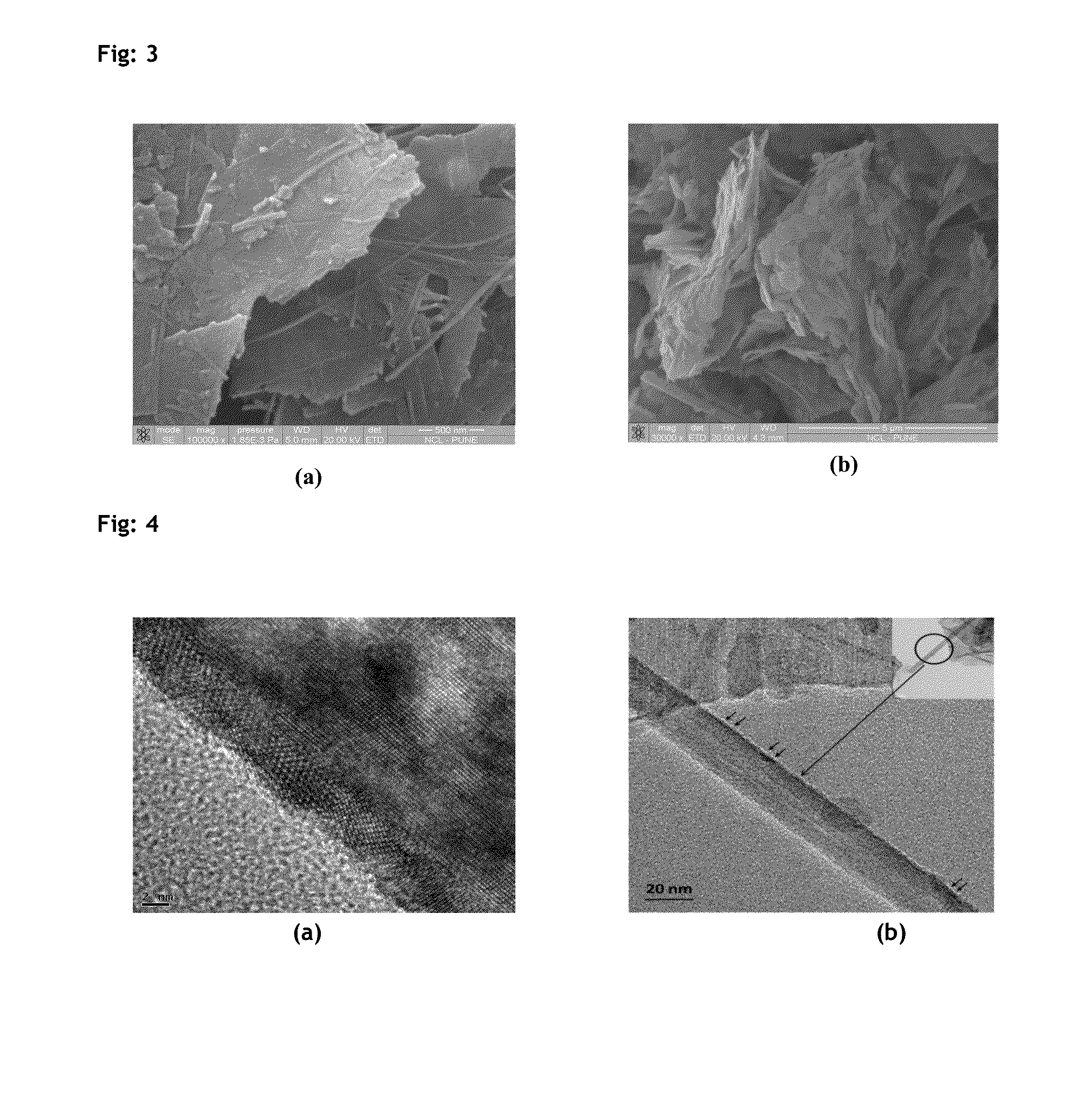
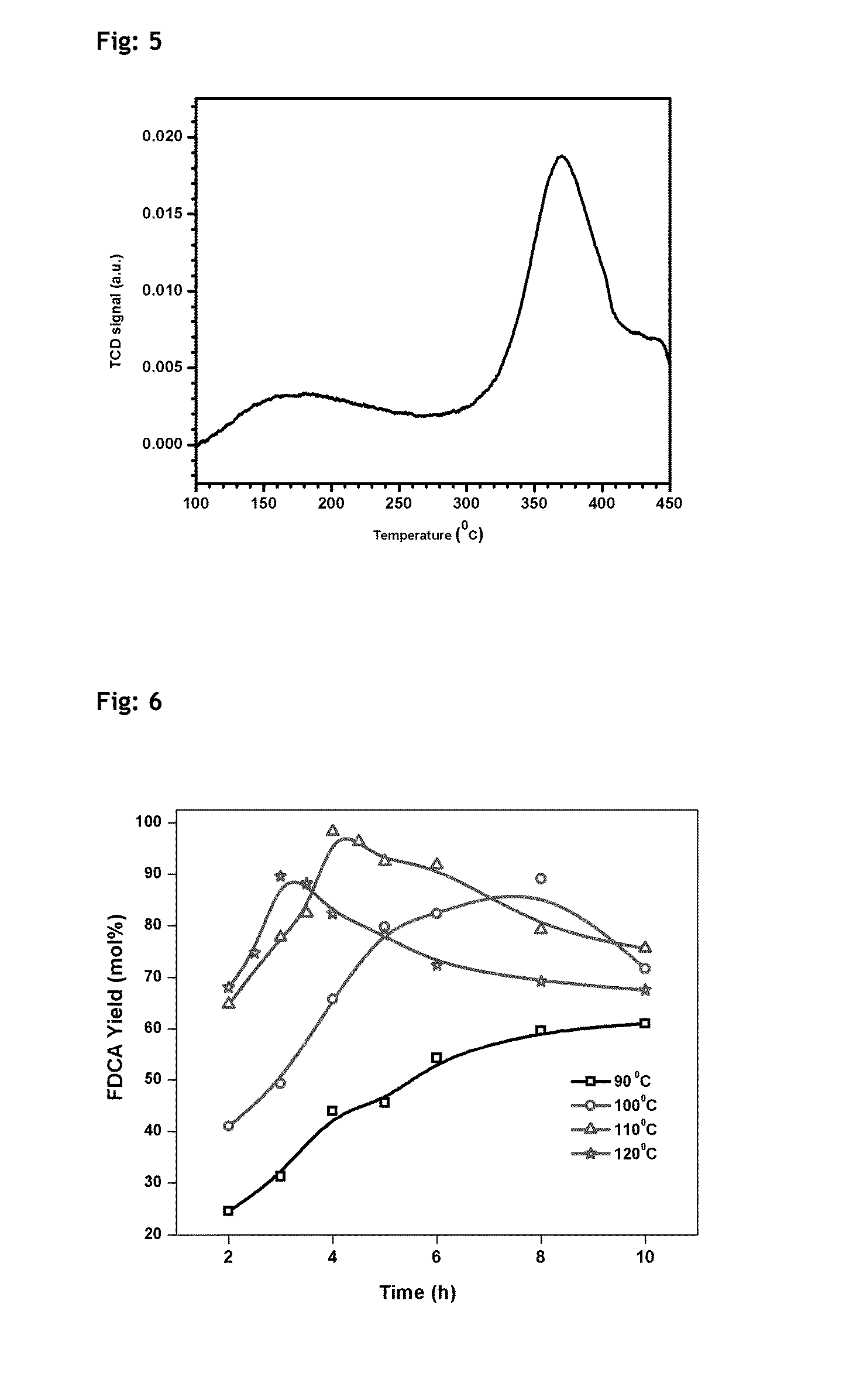
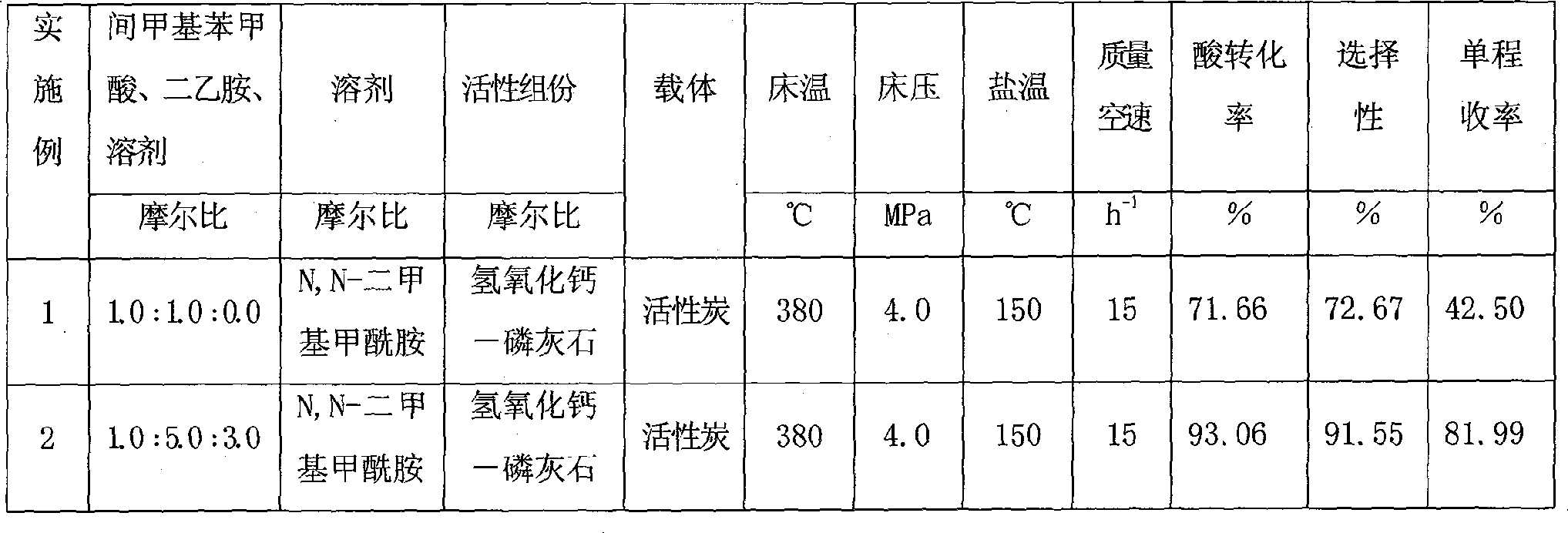
Described herein is a novel, cost effective, stable and recyclable catalyst composition i.e. A-B-OMS, wherein ‘A’ is selected from noble and transition metals; ‘B’ is selected from alkali or alkaline earth metals; and OMS is octahedral molecular sieve which includes synthetic todorokite (OMS-1) and cryptomelane (OMS-2); and characterization thereof. Further the invention provides base free green process for selective aerobic oxidation of 5-hydroxymethylfurfural (HMF) catalysed by said catalyst composition under optimized reaction conditions to obtain high yield of 2,5-furandicarboxylic acid (FDCA) in a shorter span of time. Invention also provides for selective oxidation of glucose to Gluconic Acid, furfural to furoic Acid and glycerol to Glyceric acid. Invention can also applicable for the selective oxidation of hexoses, pentoses, disaccharides to corresponding acids.
Owner:COUNCIL OF SCI & IND RES
Method for one-step synthesizing N,N-diethyl-m-methyl benzamide in fixed bed
InactiveCN101362707ALow costIncrease profitOrganic compound preparationCarboxylic acid amides preparationRecyclable catalystBenzoic acid
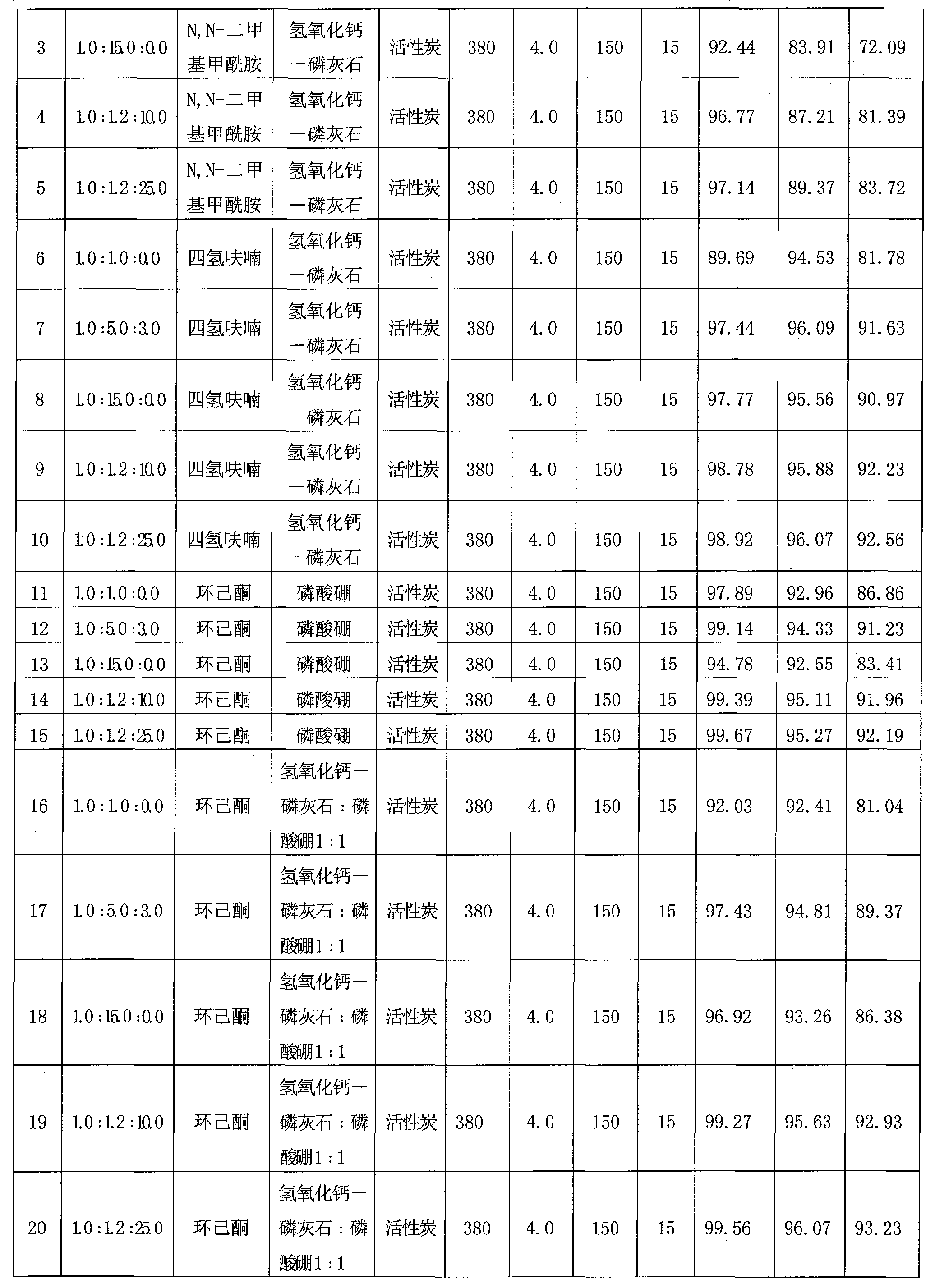
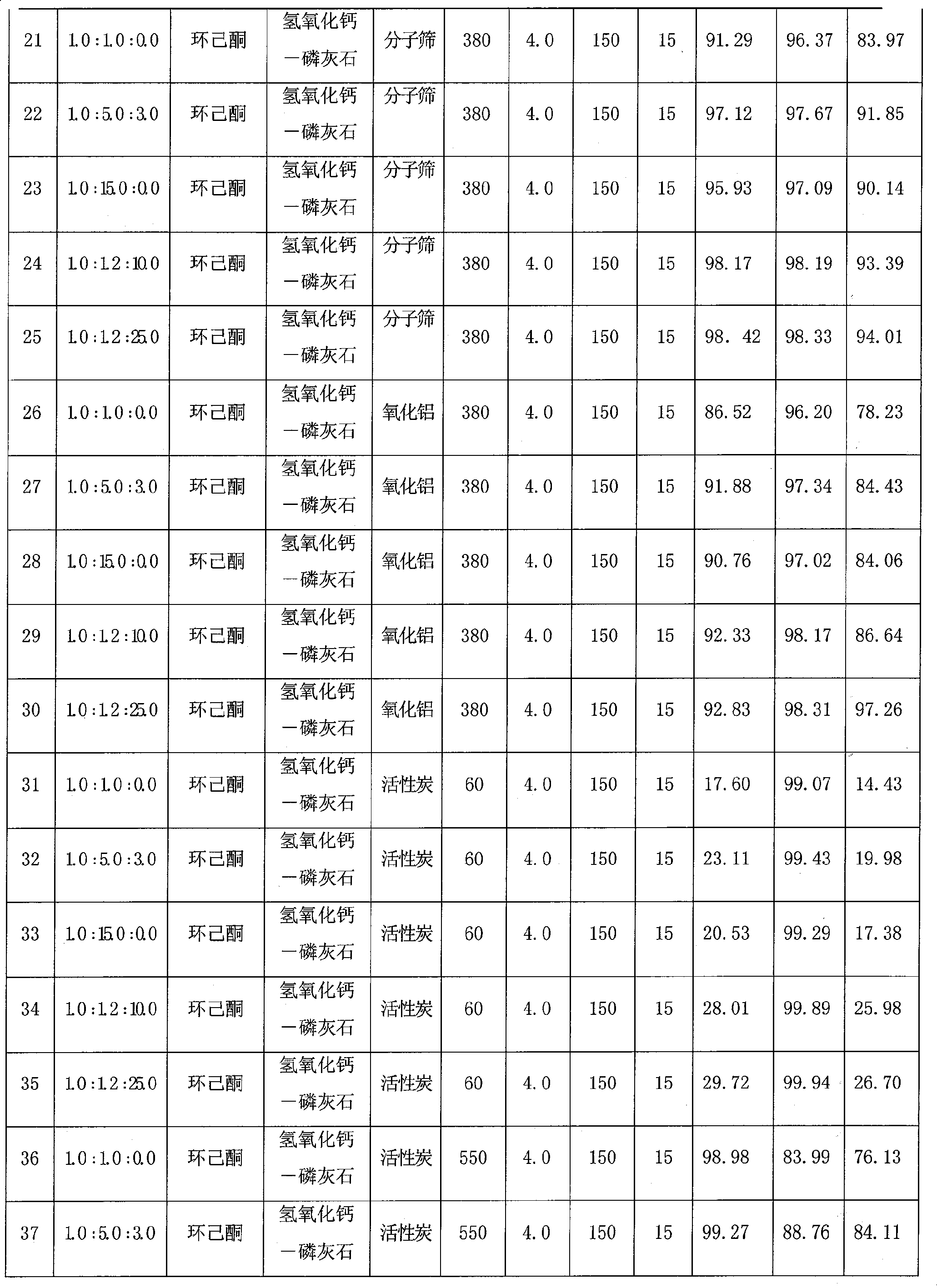
The invention discloses a one-step synthesis method of N, N-diethyl-3-methyl-benzamide with a fixed bed, which comprises the steps that: 3-methyl benzoic acid and diethylamine are stirred under a solvent system for reaction to generate a complex salt compound of the 3-methyl benzoic acid and diethylamine, and then materials are fed to the catalyzing fixed bed continuously under constant temperature and constant pressure, then dehydrated continuously to obtain a crude product; the solvent is recovered by distillation under ordinary pressure; finally the pure product of the N, N-diethyl-3-methyl-benzamide is obtained after rectification under vacuum. The molar ratio of the 3-methyl benzoic acid to the diethylamine to the solvent is 1.0: (1.0-15.0): (0-25.0), the mass velocity is 0.1 h<-1> to 20.0 h<-1>, the reaction temperature of the fixed bed is 60 DEG C to 550 DEG C and the reaction pressure of the fixed bed is 0.1 MPa to 5.0 MPa. The synthesis technology has the advantages of short technological process, high conversion ratio, good selectivity, high yield rate, small amount of waste gas, waste water and waste residues, reproducible and recyclable catalyst and low cost.
Owner:JIANGSU PANOXI CHEM
Preparation and application of highly crosslinked imidazole ionic liquid porous organic polymer
InactiveCN108948350AWide areaSimple preparation processOrganic-compounds/hydrides/coordination-complexes catalystsChemical recyclingRecyclable catalystEthylene oxide
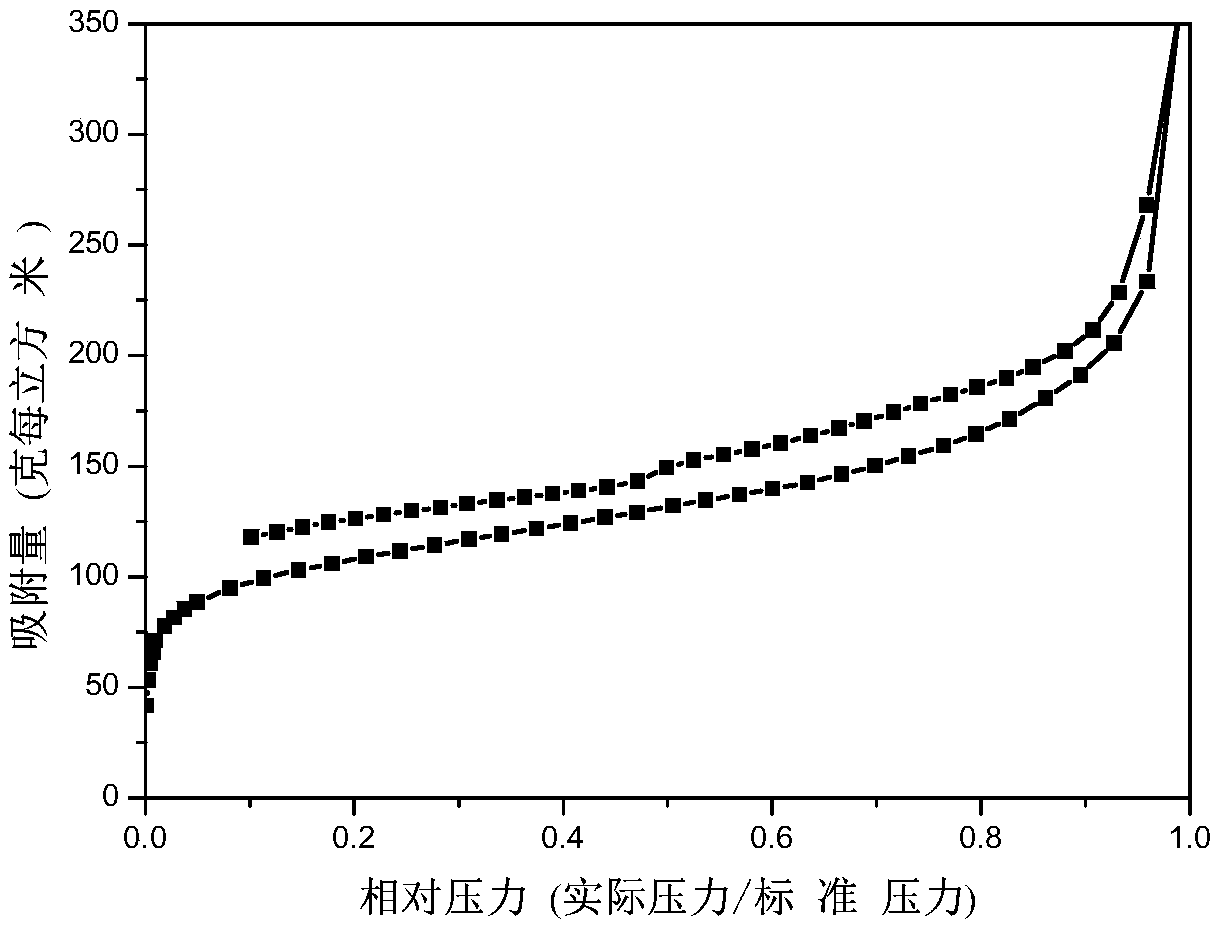
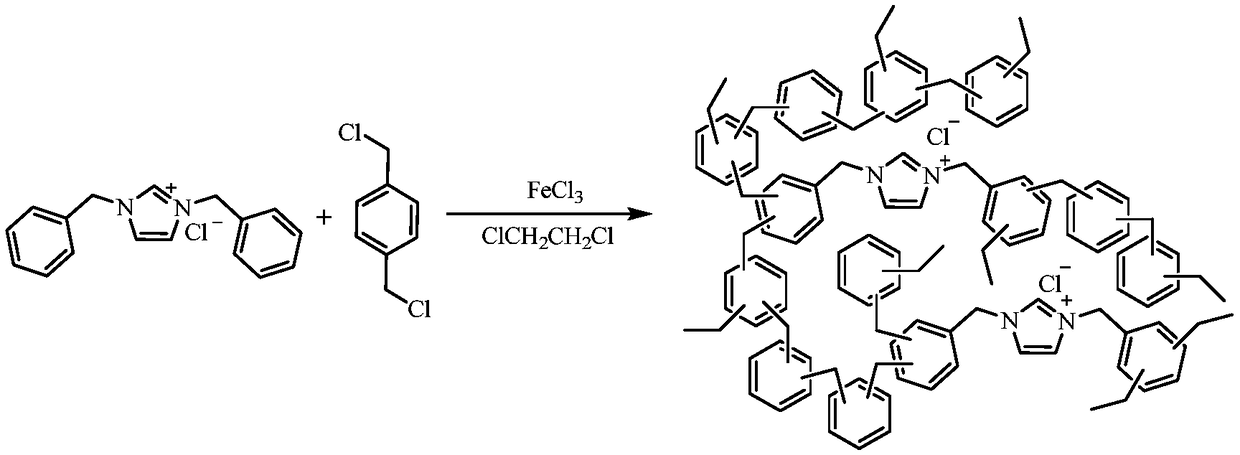
The invention discloses preparation and application of a highly crosslinked imidazole ionic liquid porous organic polymer. The polymer is characterized by being prepared by dissolving 1,3-dibenzylimidazolium chloride, 1-(dichloromethyl)-4-methylbenzene and iron trichloride in 1,2-dichloroethane, and carrying out a reaction. The prepared organic polymer has high catalytic activity in a reaction ofCO2 with ethylene oxide and derivatives thereof to synthesize a cyclic carbonate compound. Compared with the prior art, the highly crosslinked imidazole ionic liquid porous organic polymer has the advantages of good product selectivity, high catalyst activity, short reaction time, mild reaction conditions, easy separation and recovery of the catalyst, and is a recyclable catalyst for efficiently preparing cyclic carbonate compounds by using CO2. Therefore, the highly crosslinked imidazole ionic liquid porous organic polymer has great potential for development in the field of CO2 utilization catalysis.
Owner:EAST CHINA NORMAL UNIV
Method for lignin aryl-ether bond catalytic pyrolysis with ReO<x>/AC
ActiveCN107473944ABreak through dependenceAvoid it happening againOrganic compound preparationCatalystsRecyclable catalystCatalytic pyrolysis
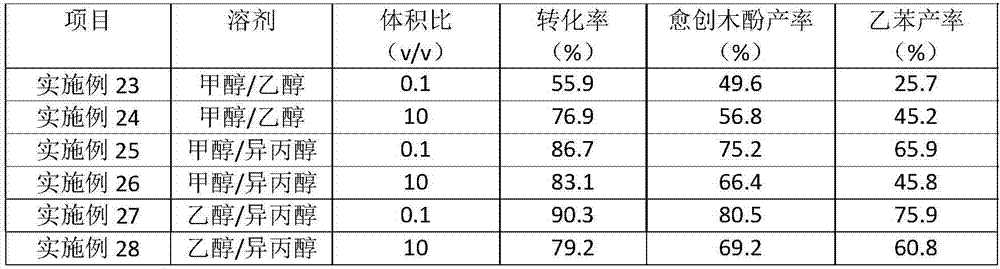
The invention provides a new method for converting a lignin model compound dimer and a lignin raw material to a corresponding aromatic compound through a hydrogen transfer reaction by taking ReO<x> / AC as a catalyst. According to the method, one or two micromolecular alcohol is taken as a reaction regent, ReO<x> / AC is taken as a catalyst, and the lignin model compound and the lignin raw material are catalyzed and converted to a micromolecular aromatic chemical in a mild condition. Compared with a traditional lignin depolymerization method, the method has distinct features: no addition of hydrogen and oxygen sources, mild reaction conditions, high selectivity of monophenol products, fast reaction speed, recyclable catalyst, and simple operation. The aromatic chemicals can be prepared from renewable lignin sources, a mild depolymerization strategy without consuming hydrogen sources is developed, and a new approach is developed for producing an aromatic compound in a non-petroleum route.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method for substituted molybdophosphate crystal catalyst
InactiveCN102218348ASolve the problems of high toxicity and serious pollutionEasy to recycleOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsRecyclable catalystChemical industry
The invention relates to a preparation method for a substituted molybdophosphate crystal catalyst. The design and synthesis of a high-activity, selective, environmentally-friendly and recyclable catalyst have an important significance in the research and chemical industry fields. The preparation method provided by the invention comprises two steps: step one, mixing sodium molybdate Na2MoO4.2H2O, transition metal sulfate, phosphoric acid H3PO4 and 1,3-bis(4-pyridyl)-propane or ethylene diamine at a molar ratio of 6: 2.9: 30: 2.6 in water with the molar fraction of 2000 and stirring for 30 minutes to obtain a mixed liquid; and step two, enclosing the mixed liquid obtained in the step one in a polytetrafluoroethylene-lined stainless steel reaction kettle at a filling degree of 60-70%, then placing the reaction kettle in a 165 DEG C oven to heat and crystallize for 7 days, and then naturally cooling to room temperature to obtain the dark red polyacid crystal catalyst. The preparation method can be used for preparing the substituted molybdenum phosphate crystal catalyst.
Owner:HARBIN NORMAL UNIVERSITY
Continuous production method of propyl acetate
InactiveCN102001936ANon-volatileImprove solubilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSolubilityRecyclable catalyst
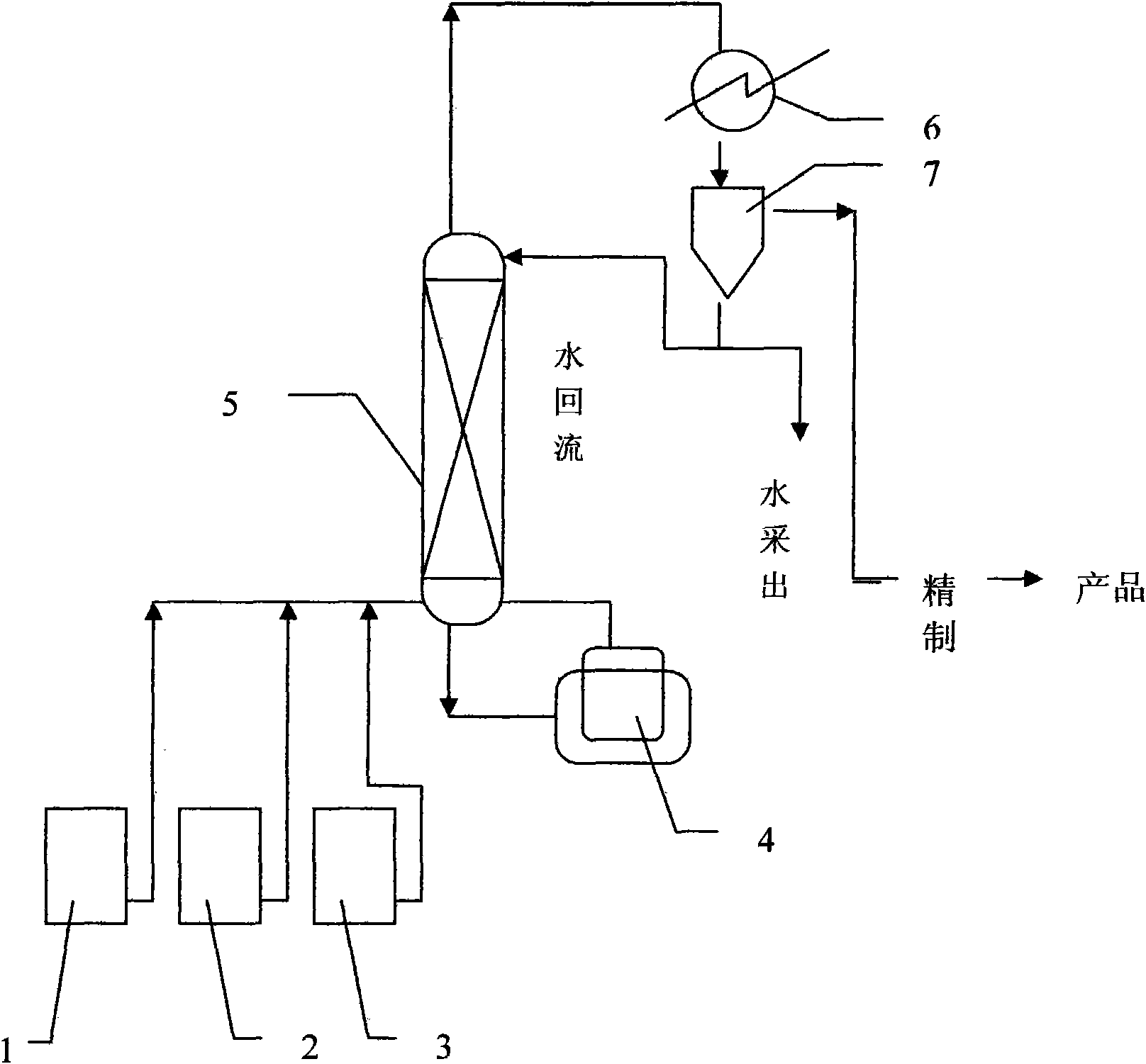
The invention relates to a continuous production method of propyl acetate, and is characterized by comprising the following steps: adding ionic liquid dissolving inorganic compound of the Lewis acid into the glacial acetic acid and the normal propyl alcohol as the catalyst; heating the components in an esterification kettle for esterification reaction; adding the mixed raw material of the glacial acetic acid and the normal propyl alcohol with the same proportion into the esterification kettle continuously after the reaction begins to reflux; separating the propyl acetate generated in the reaction in an esterification tower, and sending the unreacted raw materials back to the esterification kettle from the bottom of the kettle to continue the reaction; steaming the water and part of the normal propyl alcohol from the tower top; after the cooling, refluxing one part of the steam to the tower top, and sending the other part of the steam to a stratifying device at the tower top; refluxing part of the aqueous phase at the lower layer to the esterification tower through a reflux ratio distributor, and recovering the other part of the aqueous phase; and sending the ester phase at the upper layer to the production purification stage for the further separation to obtain the propyl acetate product. The method of the invention has simple process, high esterification conversion rate, good product selectivity, recyclable catalyst, no device corrosion, environmental protection, simple synthesis method of the selected ionic liquid catalyst, and better solubility of the acid inorganic compound.
Owner:BEIJING INSTITUTE OF PETROCHEMICAL TECHNOLOGY
Synthesis method for cyclic carbonate under catalysis of supported Bronsted acidic ionic liquid catalyst
InactiveCN102206199AHigh reactivityMild reaction conditionsOrganic chemistryChemical recyclingRecyclable catalystInternal pressure
The invention provides a synthesis method for a cyclic carbonate under catalysis of a supported acidic ionic liquid catalyst, relates to a synthesis method for the cyclic carbonate, and mainly solves the technical problems that the catalyst is difficult to recover and the energy consumption is high during the separation process in the existing cyclic carbonate preparation methods. The method provided by the invention comprises the following steps of: adding the supported acidic ionic liquid catalyst to an autoclave, adding an epoxy compound, closing the autoclave, introducing carbon dioxide to the autoclave until the internal pressure of the autoclave is 0.5-5 MPa, heating to the reaction temperature of 80-200 DEG C, reacting while maintaining the pressure and temperature for 1.0-10 hours, cooling the autoclave to room temperature, slowly discharging carbon dioxide, filtering and distilling under reduced pressure to obtain the cyclic carbonate. The catalytic synthesis method has the advantages of mild reaction conditions, high catalyst activity, easy separation of the catalyst from a product, recyclable catalyst, etc.
Owner:HEILONGJIANG UNIV
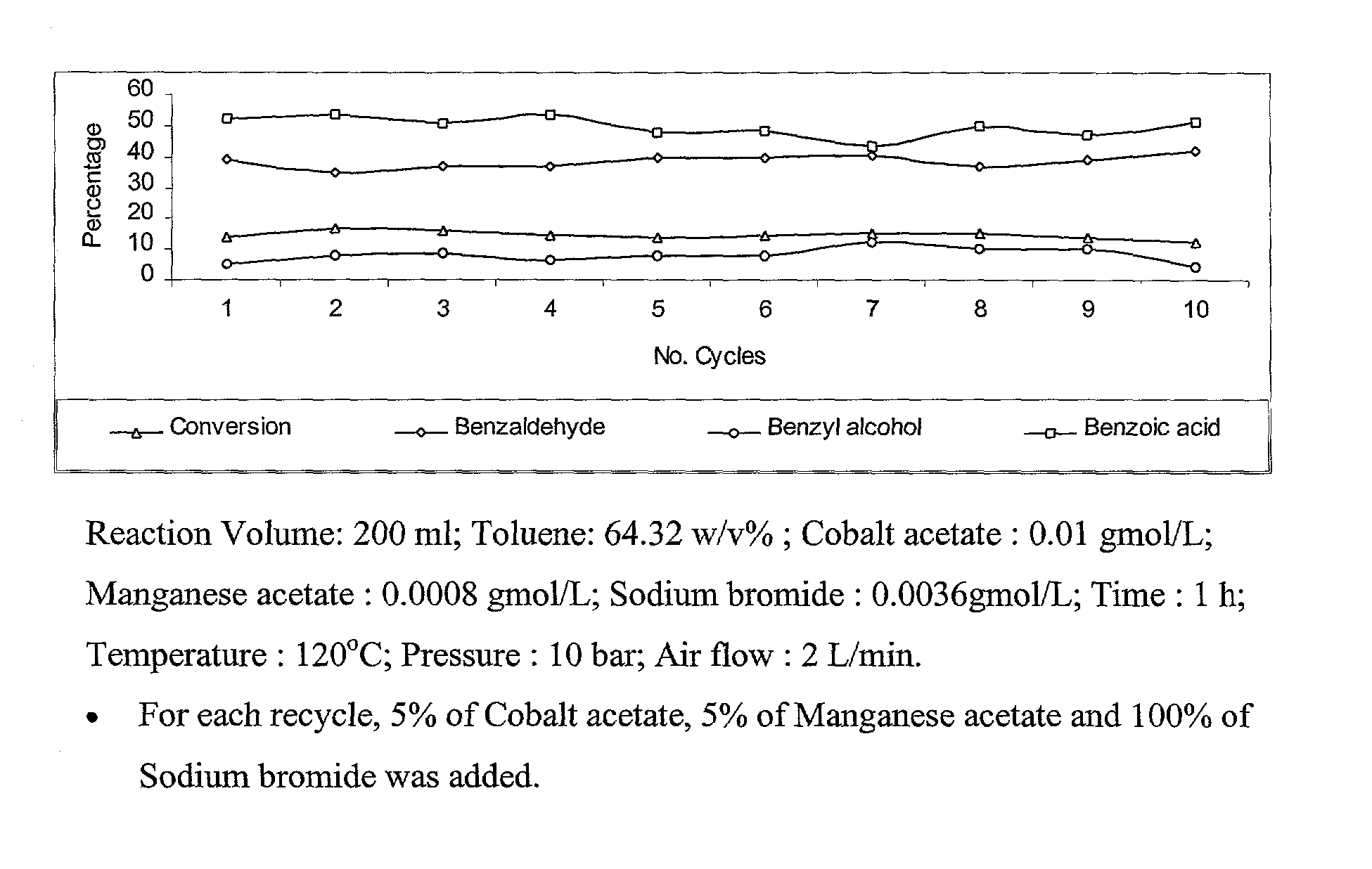
Selective liquid phase air oxidation of toluene catalysed by composite catalytic system
InactiveUS6743952B2Preparation by oxidation reactionsOrganic compound preparationRecyclable catalystZinc bromide
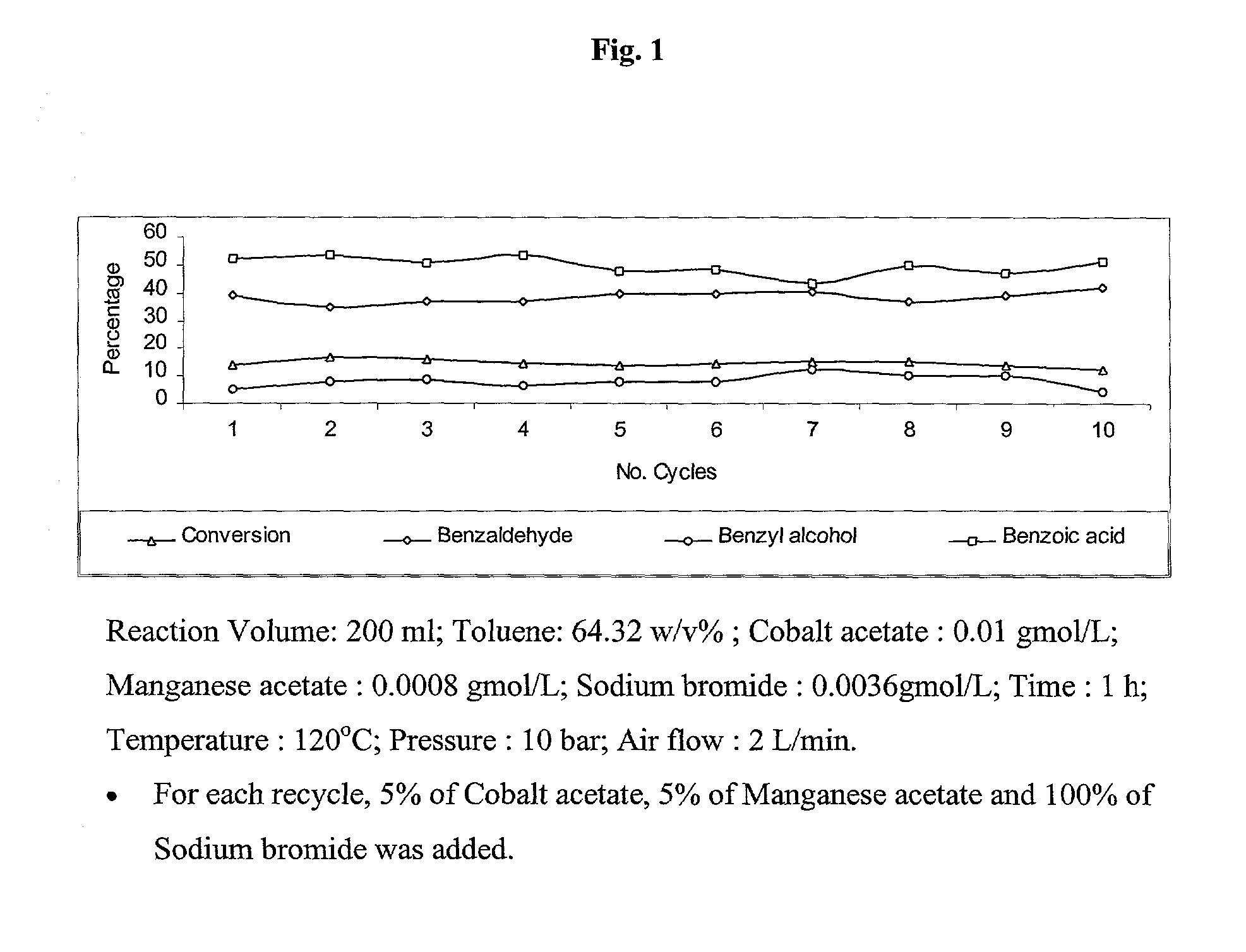
The present invention provides an improved process for the production of benzaldehyde with 40-50% selectivity by the catalytic liquid phase air oxidation of toluene using a composite catalytic system comprising salts of iron, cobalt, manganese, molybdenum or nickel as recyclable catalyst, salts of manganese or copper as recyclable co-catalyst and cobalt bromide, sodium bromide, sodium chloride and zinc bromide as promoter.
Owner:COUNCIL OF SCI & IND RES
Surface bonded Rh-bis(diarylphosphine) on magnetic nanoparticles as a recyclable catalyst for hydroformylation of olefins
InactiveUS9480978B1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsRecyclable catalystChemical transformation
A functionalized nanomaterial having an average particles size of less than 10 nm comprising an iron oxide nanoparticle core and a bis(diarylphosphinomethyl) dopamine based ligand layer anchored to the iron oxide nanoparticle core is disclosed. In addition, a catalyst composition for use in a variety of chemical transformations wherein the bisphosphine groups of the functionalized nanomaterial chelate a catalytic metal is disclosed. In addition, a method for producing the functionalized nanomaterial and a method for the hydroformylation of olefins to aldehydes employing the functionalized nanomaterial with high conversion percentage and high selectivity are disclosed.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS
Method for decomposing hydrogen phosphide cumene to prepare phynol and acetone
ActiveCN101343212AEasy to separateThe progress of the reaction is easy to controlPhysical/chemical process catalystsOrganic chemistryRecyclable catalystDecomposition
The invention provides a method for preparing phenol and acetone by composing hydrogen peroxide iso-propyl benzene, which is characterized in that the method adopts a smectite solid-acid catalyst modified by inorganic ammonium salt as a reaction catalyst to make the hydrogen peroxide iso-propyl benzene have the decomposition reaction, with a decomposition temperature of between 35 and 80 DEG C, and a decomposition pressure of 0.1 to 5atm. The method makes the reaction under a mild condition, and has advantages of easy control of extent of reaction, less by-products, non corrosion, easy separation of products, recyclable catalyst, and non phenolic wastewater.
Owner:PETROCHINA CO LTD
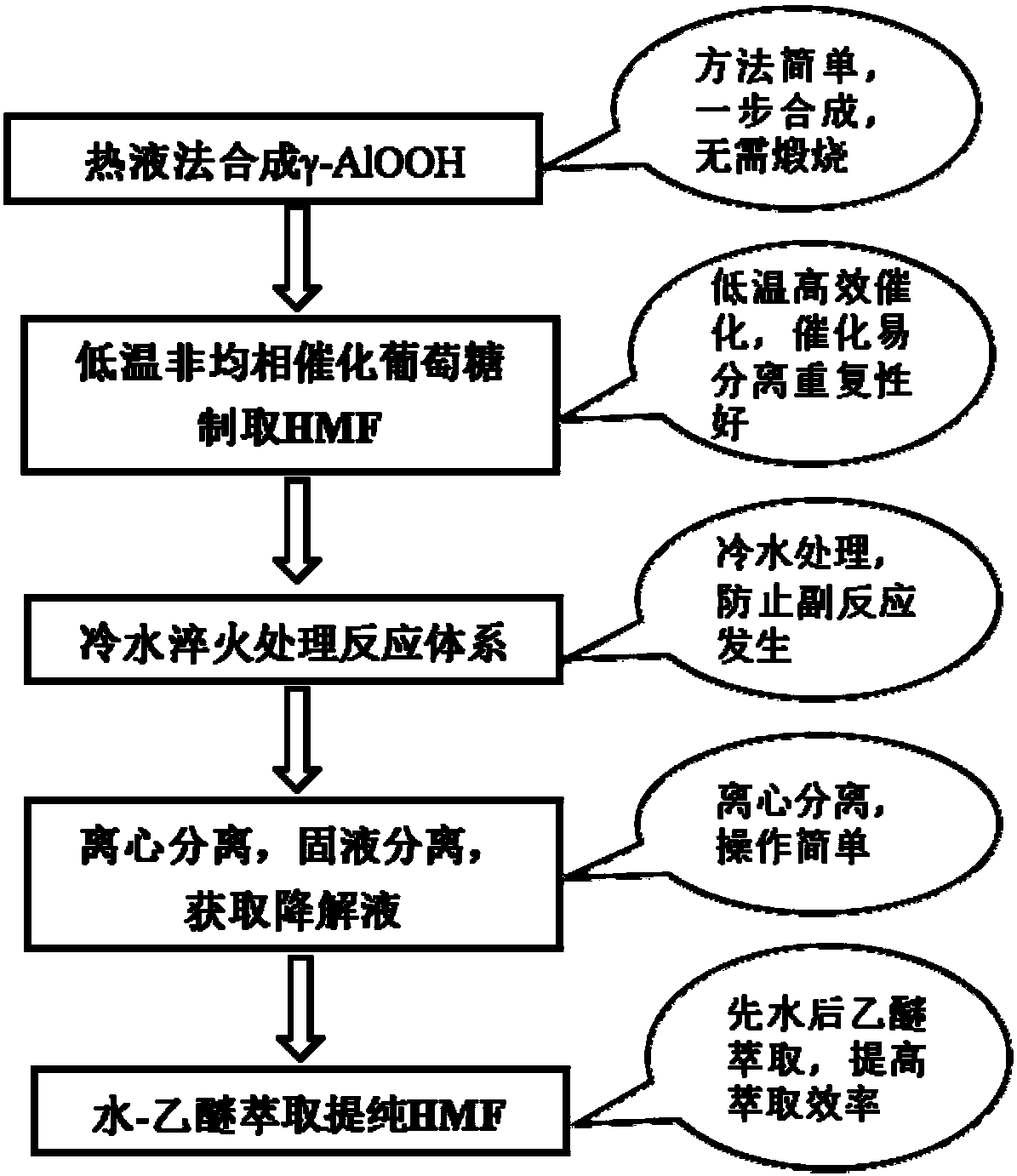
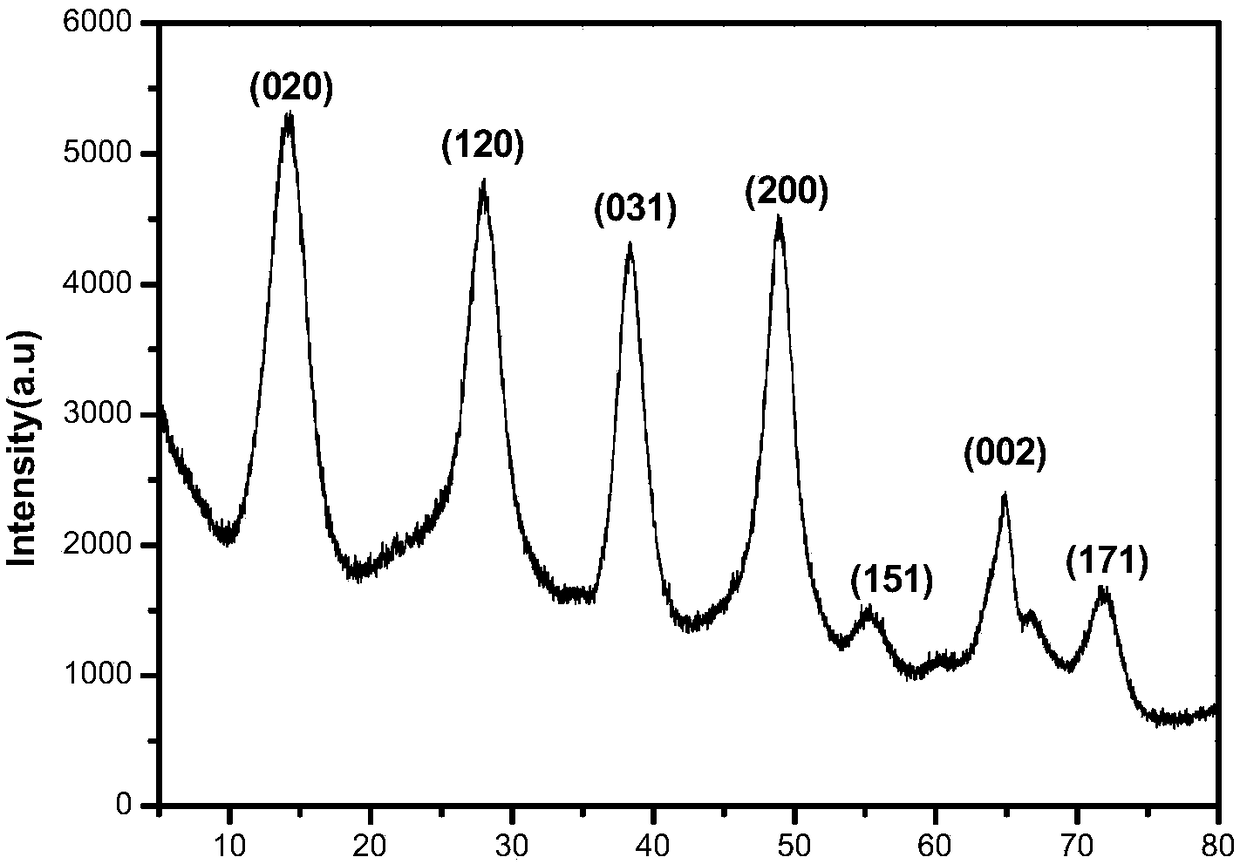
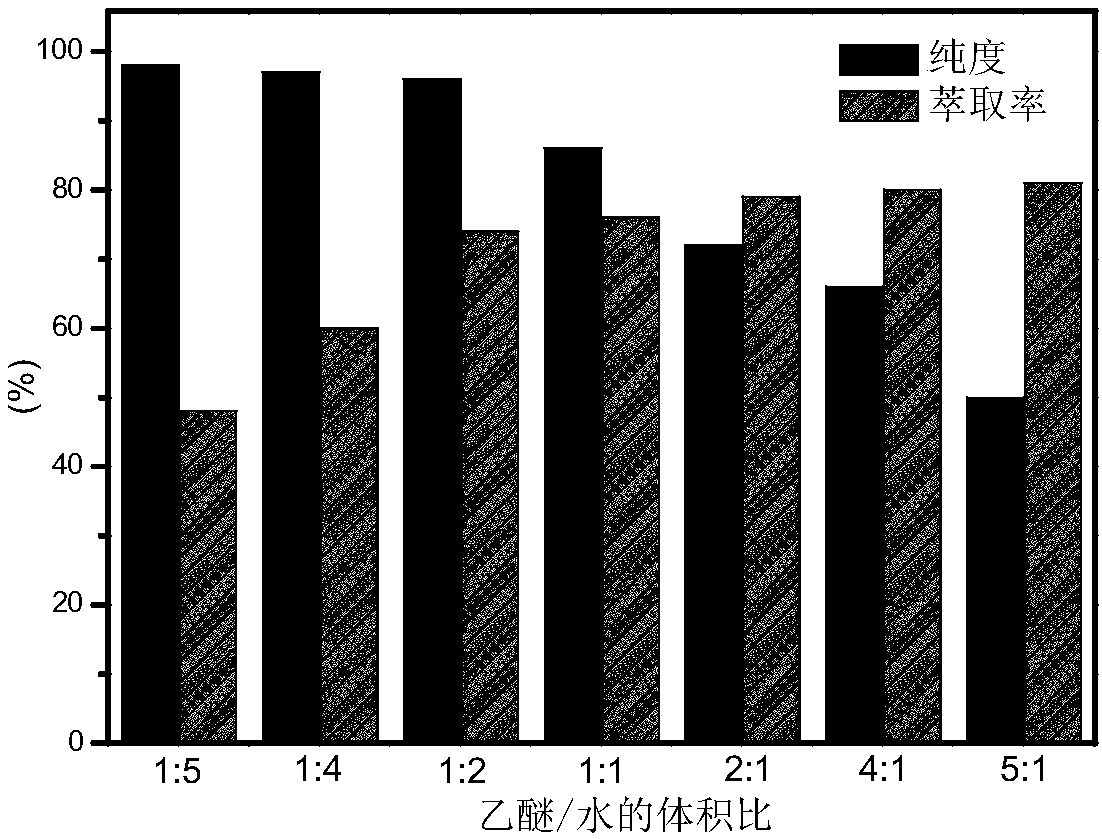
Method for preparing HMF (5-hydroxymethylfurfural) from glucose by lower-temperature catalysis with boehmite
ActiveCN108610311ARaw materials are cheap and easy to getEasy to prepareOrganic chemistryChemical recyclingRecyclable catalystSide effect
The invention discloses a method for preparing HMF (5-hydroxymethylfurfural) from glucose by lower-temperature catalysis with boehmite. The method specifically comprises the following steps: boehmitegamma-AlOOH and glucose are added to dimethyl sulfoxide, the substances are mixed and transferred to a position at 80-180 DEG C for a stirring reaction, deionized water is added to a reaction liquid after the reaction for quenching treatment, then centrifugation is performed, an upper liquid is collected, and a degradation liquid containing HMF is obtained. When HMF is prepared from glucose by catalysis with the method, the method has the characteristics of low temperature, high efficiency (high HMF yield and high selectivity), easily separable and recyclable catalyst, low energy consumption and the like, and has quite high application value; a large quantity of side effects can be avoided, product selectivity can be improved, and product separation cost can be reduced.
Owner:YANCHENG INST OF TECH
Preparation method of compound ecological fertilizer from plant stalks
InactiveCN105601374AImprove catalytic degradation efficiencyAvoid mildew and spoilageBio-organic fraction processingBioloigcal waste fertilisersRecyclable catalystPlant stalk
The invention relates to a preparation method of a compound ecological fertilizer from plant stalks. The compound fertilizer is prepared by degrading a main raw material of plant stalks under the action of small molecule organic acid in the presence of a solid super acid catalyst, and combining with distiller's grains, bone meal, bran leather, humic acid, and NPK fertilizer. The raw materials consist of, by weight, 60-80 parts of plant stalks, 5-10 parts of distiller's grains, 5-15 parts of bone meal, 5-15 parts of wheat bran, 5-10 parts of humic acid and 30-50 parts of NPK fertilizer. The method uses a catalytic degradation technology for preparation of the compound ecological fertilizer, reaches high catalytic degradation efficiency of stalks, completes the conversion from stalks to compound ecological fertilizer within 16h at the highest speed, and has the advantages of simple operation, low cost, recyclable catalyst, and no corrosion of equipment, and can quickly obtain the product. The prepared compound ecological fertilizer can effectively enhance fertilizer efficiency, stimulate crop growth, control pests and diseases, improve the quality of agricultural products, and the absorb soil pollutants, improve the water retention capacity of soil and fertilizer and improve soil structure, and is conducive to the sustainable development of agriculture.
Owner:CHENDU NEW KELI CHEM SCI CO LTD
Method for synthesizing 1,1,1,3,5,5,5-heptamethyltrisiloxane by continuous catalysis of solid phase catalyst
ActiveCN101921287AEasy to separateSimple processSilicon organic compoundsChemical recyclingRecyclable catalystReaction temperature
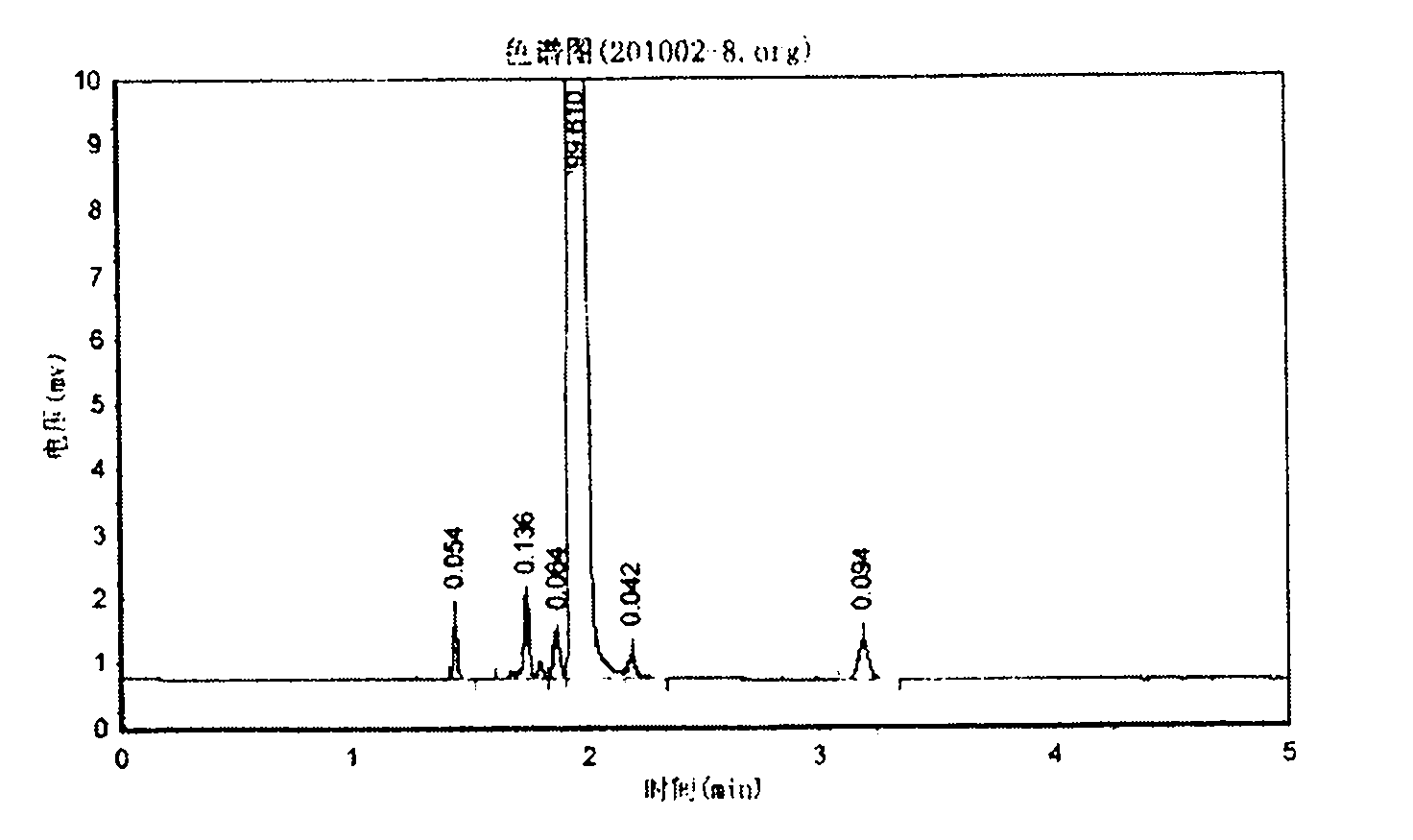
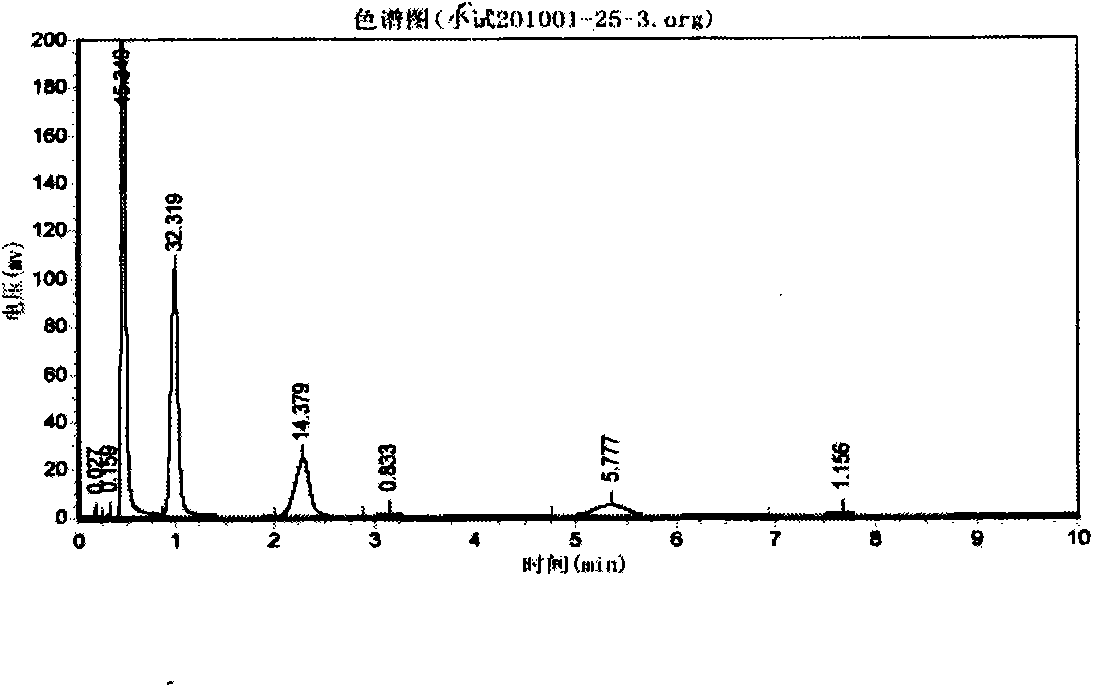
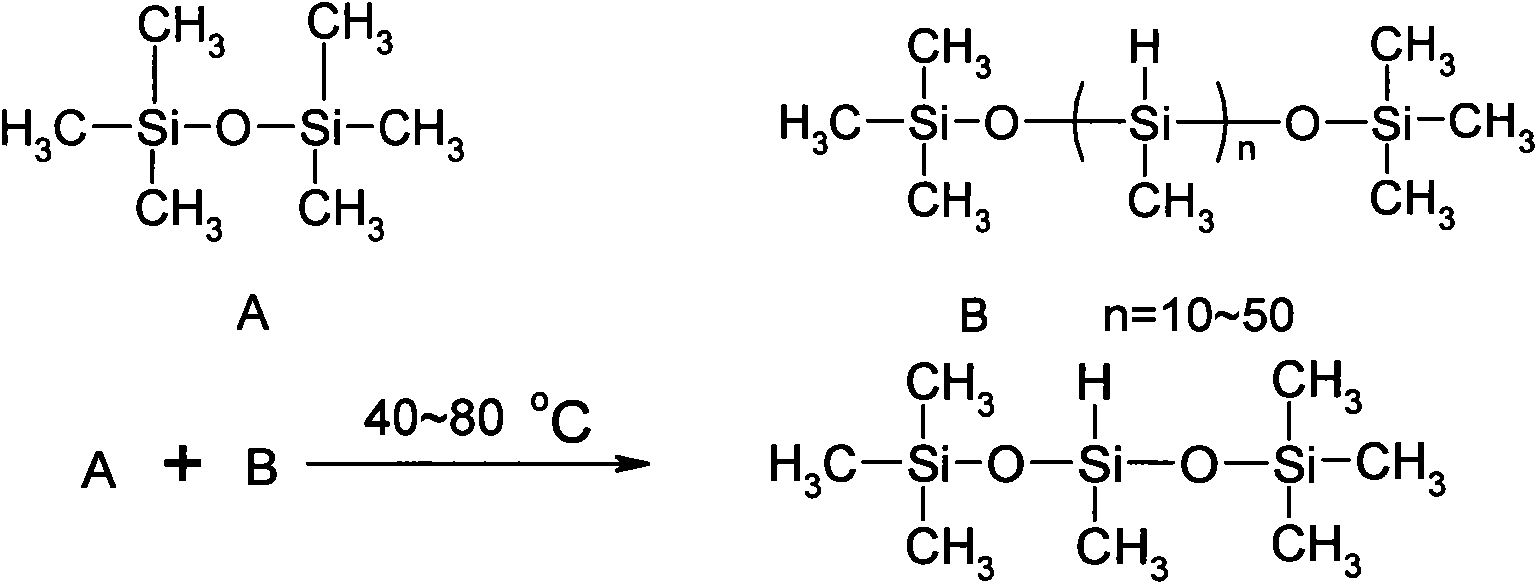
The invention relates to a method for synthesizing 1,1,1,3,5,5,5-heptamethyltrisiloxane by continuous catalysis of a solid phase catalyst. Hexamethyldisiloxane and straight chain methyl hydrogen polysiloxane in a molar ratio of 3:1-2:1 are taken as raw materials, strongly acidic ion exchange resin is taken as the solid phase catalyst, the reaction temperature is 40 to 80 DEG C, a fixed bed reactor is adopted, the raw materials pass through the reactor filled with the ion exchange resin at a certain flow rate, the retention period is 5 to 10 hours, the conversion rate of the raw materials is ensured by the retention period of the reaction through controlling the flow rate of the reaction product, the continuous reaction production is realized, and the reaction product is subjected to low fraction and high fraction removal rectification to form the 1,1,1,3,5,5,5-heptamethyltrisiloxane product with the purity of over 99 percent. The method has the advantages of readily available raw materials, no three-waste emission, simple process, continuous production, high-efficiency and stable reaction, simple separation, recyclable catalyst, conversion per pass of the raw material straight chain methyl hydrogen polysiloxane of over 32 percent, good product quality and low production cost.
Owner:JIANGXI HITO CHEM
Method for preparing acylferrocenyl hydrazinodithio acid ester Schiff base
InactiveCN109970817AShort reaction timeImprove efficiencyChemical recyclingMetallocenesRecyclable catalystFiltration
The invention discloses a method for preparing an acylferrocenyl hydrazinodithio acid ester Schiff base. The method comprises the following steps: adding formula amounts of choline chloride (A mol) and methanesulfonic acid (B mol) into a reaction vessel, and performing stirring at 80 DEG C until complete dissolving in order to obtain a eutectic solvent; adding formula amounts of acylferrocene (C mol) and hydrazinodithio acid ester (D mol), slowly heating the obtained solution to 80 DEG C, performing TLC monitoring until a reaction is finished (1 h), cooling the obtained reaction solution to room temperature to precipitate a solid, carrying out suction filtration, washing the solid with a small amount of water, and drying the washed solid to obtain the product with a yield of 90% or more; and recovering the obtained filtrate to obtain the eutectic solvent, wherein a ratio of A:B:C:D is 1:(1-4):(1-1.5):(1-1.5). The method has the advantages of short reaction time, high efficiency, high yield, high purity of the product, recyclable catalyst, greenness, environmental protection and cost reduction.
Owner:SHAANXI UNIV OF SCI & TECH
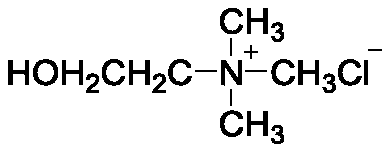
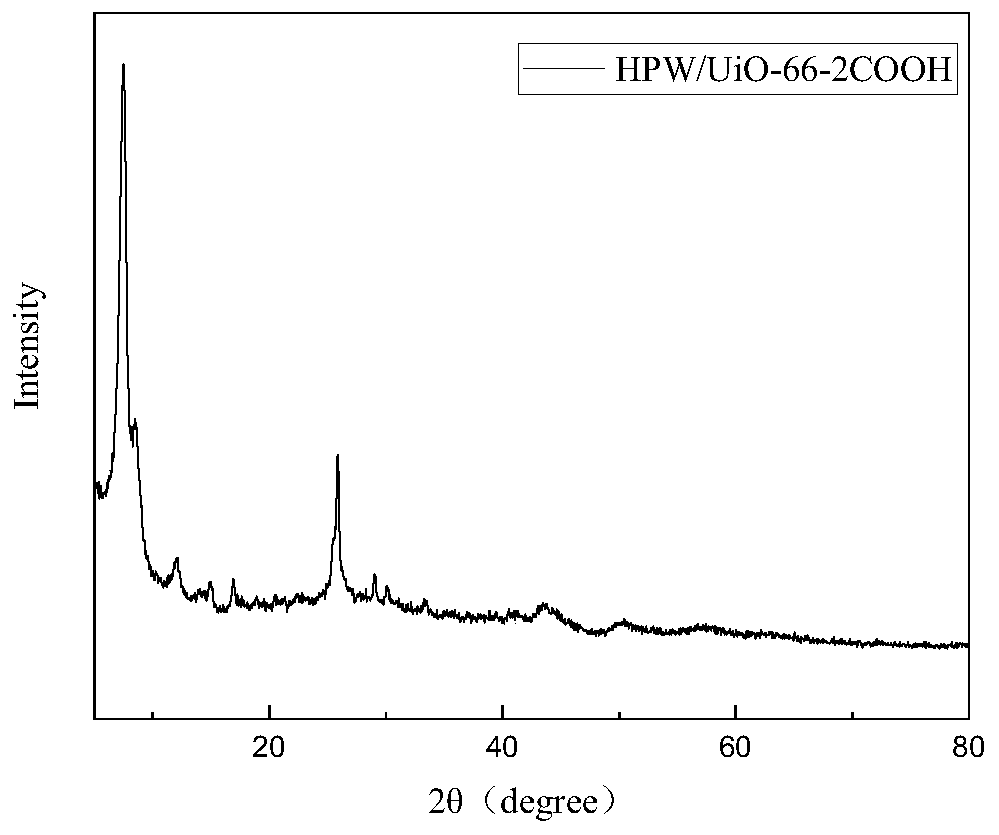
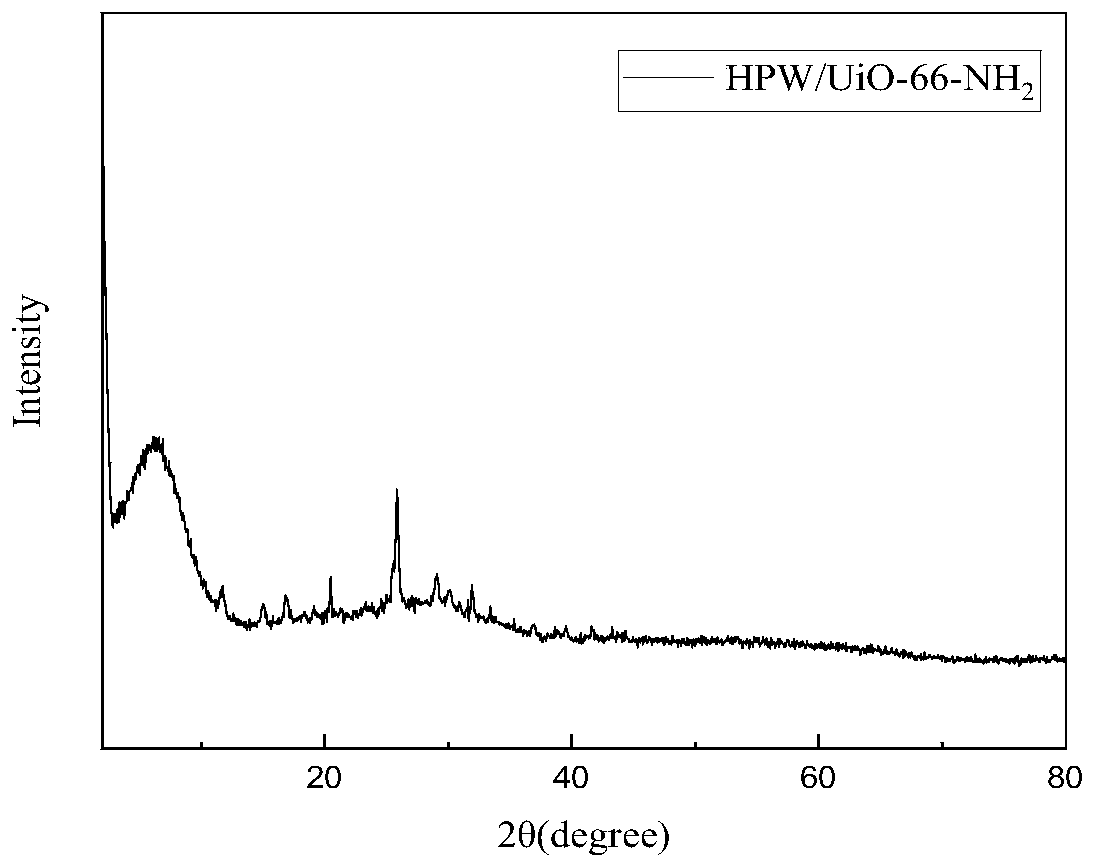
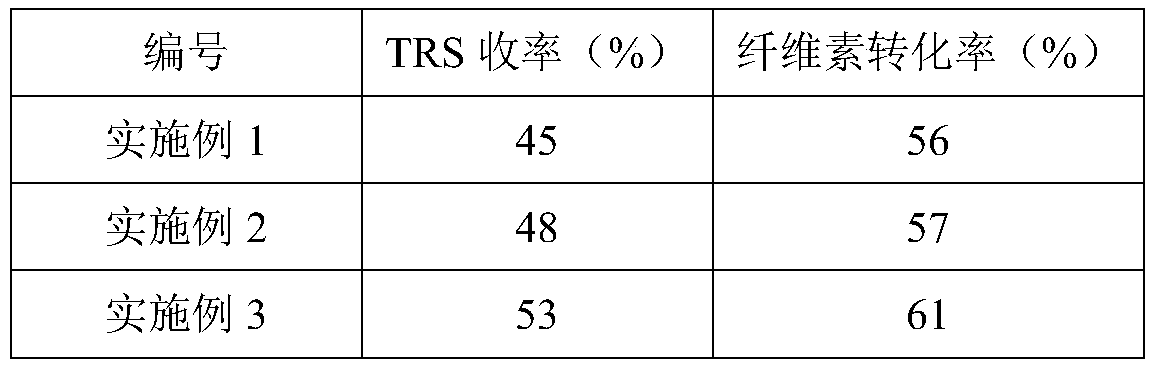
Application of catalyst based on organic metal framework UiO-66 in cellulose hydrolysis
ActiveCN111359663AImprove the conversion rate of hydrolysisEasy to separateSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsCelluloseRecyclable catalyst
The invention relates to the technical field of preparation of cellulose hydrolysis catalysts., and discloses an application of a catalyst based on an organic metal framework UiO-66 in cellulose hydrolysis. The catalyst is one of UiO-66, UiO-66-X, HPW / UiO-66 and HPW / UiO-66-X, wherein -X is one of-NH2, -COOH and -2COOH; the UiO-66 and the UiO-66-X are prepared from ZrCl4 and an organic ligand through a reaction; and the HPW / UiO-66 and the HPW / UiO-66-X are UiO-66 and UiO-66-X loaded with phosphotungstic acid. When the catalyst is applied to cellulose hydrolysis, the mass ratio of the catalyst tocellulose is 1:(5-10). The organic-inorganic hybrid recyclable catalyst based on the organic metal framework UiO-66 prepared in the invention has excellent catalytic performance when being used in cellulose hydrolysis reaction.
Owner:ZHEJIANG UNIV OF TECH
Method for synthesizing dimethyl methylphosphonate
ActiveCN102964382AAvoid oxidative hydrolysis reactionsIncrease distillation timeGroup 5/15 element organic compoundsChemical recyclingRecyclable catalystDimethyl methylphosphonate
A method for synthesizing dimethyl methylphosphonate. Under protection of inert gas, A raw material trimethyl phosphite and a proper amount of benzene sulfonic acid catalyst are mixed and stirred; and a the mixture is subjected to a rearrangement reaction through a pressurized heating way, so as to synthesize a target product dimethyl methylphosphonate with yield up to 93% and purity higher than 99%. The invention has advantages of easily available raw materials, short synthesis time, simple production control, cheap and recyclable catalyst and little pollutant emission.
Owner:HUBEI XINGFA CHEM GRP CO LTD +1
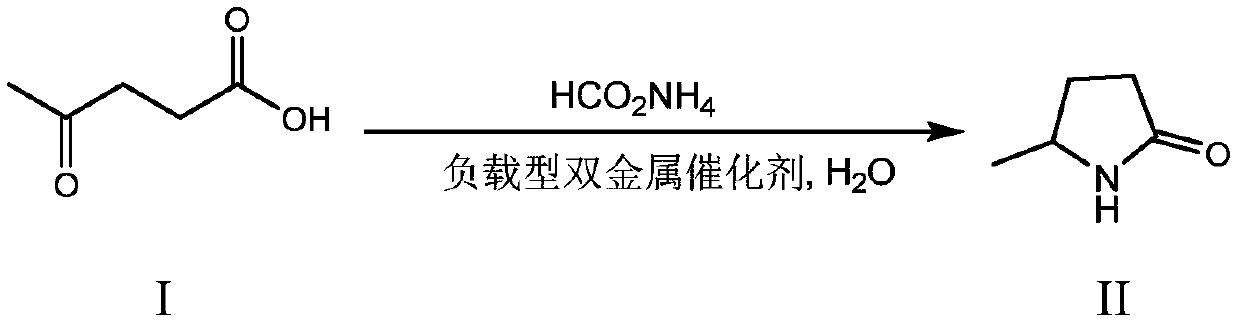
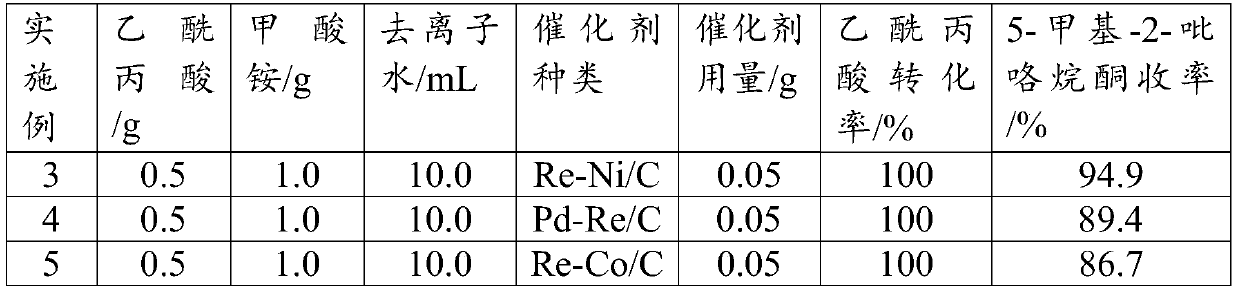
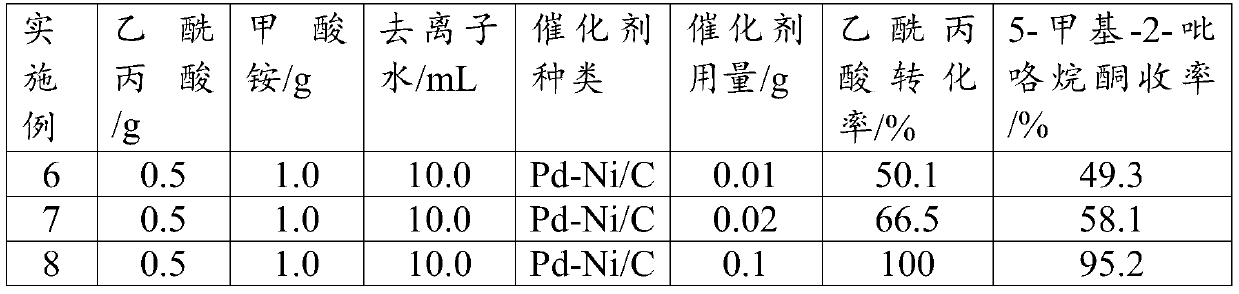
Synthesis method of 5-methyl-2-pyrrolidone
InactiveCN110615754ASimple processOperational securityOrganic chemistryChemical recyclingRecyclable catalystPropanoic acid
The invention discloses a preparation method of 5-methyl-2-pyrrolidone. A biomass derivative levulinic acid is used as an initial raw material; ammonium formate is used as a hydrogen source and a nitrogen source; a supported bimetallic catalyst is used as a hydrogenation catalyst; and the 5-methyl-2-pyrrolidone is synthesized in water by adopting a one-pot method. The supporting metal of the supported bimetallic catalyst is bimetallic composed of two precious metals, bimetallic composed of one precious metal and one non-precious metal A or non-precious metal B, or bimetallic composed of one non-precious metal A and one non-precious metal B. According to the method, the conversion rate of the levulinic acid can reach 100%, and the yield of the 5-methyl-2-pyrrolidone can reach 94% or above.The method provided by the invention has the advantages of environment-friendly process, simple operation, recyclable catalyst, high reaction selectivity, high product yield, and obvious industrial production advantages.
Owner:ZHEJIANG UNIV OF TECH
Selective liquid phase air oxidation of toluene catalysed by composite catalytic system
InactiveUS20030187304A1High selectivityMinimise the loss of expensive brominePreparation by oxidation reactionsOrganic compound preparationRecyclable catalystZinc bromide
The present invention provides an improved process for the production of benzaldehyde with 40-50% selectivity by the catalytic liquid phase air oxidation of toluene using a composite catalytic system comprising salts of iron, cobalt, manganese, molybdenum or nickel as recyclable catalyst, salts of manganese or copper as recyclable co-catalyst and cobalt bromide, sodium bromide, sodium chloride and zinc bromide as promoter.
Owner:COUNCIL OF SCI & IND RES
Reaction method for selectively synthesizing aromatic aldehyde or aromatic carboxylic acid
ActiveCN111960936ATo achieve selective regulationAtom economyOrganic compound preparationCarbonyl compound preparationRecyclable catalystPtru catalyst
The invention provides a reaction method for selectively synthesizing aromatic aldehyde or aromatic carboxylic acid. Toluene aromatic hydrocarbon without substituent or with substituent on a benzene ring is used as a raw material, an inorganic salt of ferric iron is used as a catalyst, air or oxygen is used as an oxidizing agent, a mixed solution of acetonitrile and water is used as a solvent, theraw material is oxidized by adjusting the dosage of the catalyst to obtain aromatic aldehyde or aromatic carboxylic acid, and the aromatic aldehyde or aromatic carboxylic acid is irradiated by ultraviolet light for 10-16 hours. Aromatic carboxylic acid obtained under the condition that the dosage of the catalyst is 5-50% mol of aromatic hydrocarbon is used as a main product, wherein the use amount of the catalyst is 70-200% mol of aromatic hydrocarbon. The reaction method provided by the invention has the characteristics of atom economy and high selectivity, uses the metal iron salt with richearth content for catalysis, and has the advantages of mild conditions, recyclable catalyst and solvent and the like.
Owner:NANJING UNIV OF TECH
Recyclable catalysts methods of making and using the same
InactiveUS20050075504A1Extend your lifeImprove thermal stabilitySilicon organic compoundsOrganic compound preparationRecyclable catalystPtru catalyst
Organometallic complexes are provided, which include a catalyst containing a transition metal, a ligand and a component having the formula GArF. ArF is an aromatic ring system selected from phenyl, naphthalenyl, anthracenyl, fluorenyl, or indenyl. The aromatic ring system has at least a substituent selected from fluorine, hydrogen, hydrocarbyl or fluorinated hydrocarbyl, G is substituted or unsubstituted (CH2)n or (CF2)n, wherein n is from 1 to 30, wherein further one or more CH2 or CF2 groups are optionally replaced by NR, PR, SiR2, BR, O or S, or R is hydrocarbyl or substituted hydrocarbyl, GArF being covalently bonded to either said transition metal or said ligand of said catalyst, thereby rendering said cationic organometallic complex liquid. The catalyst of the organometallic complex can be [CpM(CO)2(NHC)Lk]+A−, wherein M is an atom of molybdenum or tungsten, Cp is substituted or unsubstituted cyclopentadienyl radical represented by the formula [C5Q1Q2Q3Q4Q5], wherein Q1 to Q5 are independently selected from the group consisting of H radical, GArF C1-20 hydrocarbyl radical, substituted hydrocarbyl radical, substituted hydrocarbyl radical substituted by GArF, halogen radical, halogen-substituted hydrocarbyl radical, —OR, —C(O)R′, —CO2R′, —SiR′3 and —NR′R″, wherein R′ and R″ are independently selected from the group consisting of H radical, C1-20 hydrocarbyl radical, halogen radical, and halogen-substituted hydrocarbyl radical, wherein said Q1 to Q5 radicals are optionally linked to each other to form a stable bridging group, NHC is any N-heterocyclic carbene ligand, L is either any neutral electron donor ligand, wherein k is a number from 0 to 1 or L is an anionic ligand wherein k is 2, and A− is an anion. Processes using the organometallic complexes as catalysts in catalytic reactions, such as for example, the hydrosilylation of aldehydes, ketones and esters are also provided.
Owner:BROOKHAVEN SCI ASSOCS
Treatment method for electroplating wastewater and sludge
InactiveCN111018208AEfficient removalAchieve recyclingWater contaminantsCatalyst activation/preparationRecyclable catalystPtru catalyst
The invention discloses a treatment method for electroplating wastewater and sludge. The treatment method comprises the following steps: (1) evaporating and concentrating electroplating wastewater, discharging condensed water or recycling the condensed water to an electroplating process, and mixing and slurrying the concentrated solution, electroplating sludge and a carbon source; (2) carrying outmicrowave hydrothermal treatment on slurry under the conditions that a temperature is 130-250 DEG C and a pressure is 2-10 MPa so as to allow a carbon source and metal elements in the slurry to reactin situ to generate a carbon-based catalyst; (3) introducing an oxidant, carrying out catalytic wet oxidation on the slurry having undergone hydro-thermal treatment, carrying out solid-liquid separation after the reaction is finished, carrying out drying treatment on a solid phase, and recycling the treated solid phase as a catalyst; and (4) carrying out electric flocculation on a filtrate obtained by solid-liquid separation, wherein solid slag of electric flocculation is partially recycled to the electroplating sludge. According to the method, deep harmless and reduction treatment of the electroplating wastewater and the electroplating sludge is realized, and meanwhile, the recyclable catalyst for wet oxidation wastewater treatment is obtained through resource utilization.
Owner:ZHEJIANG QICAI ECO TECH CO LTD
Imide bond grafted NHPI catalyst, preparation method and application thereof
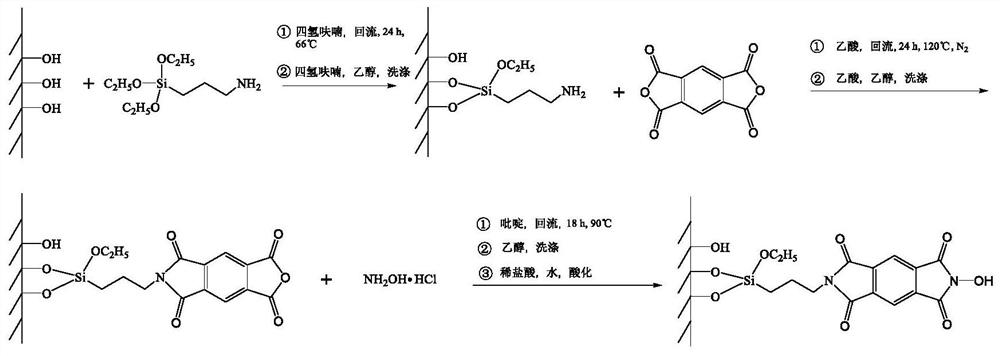
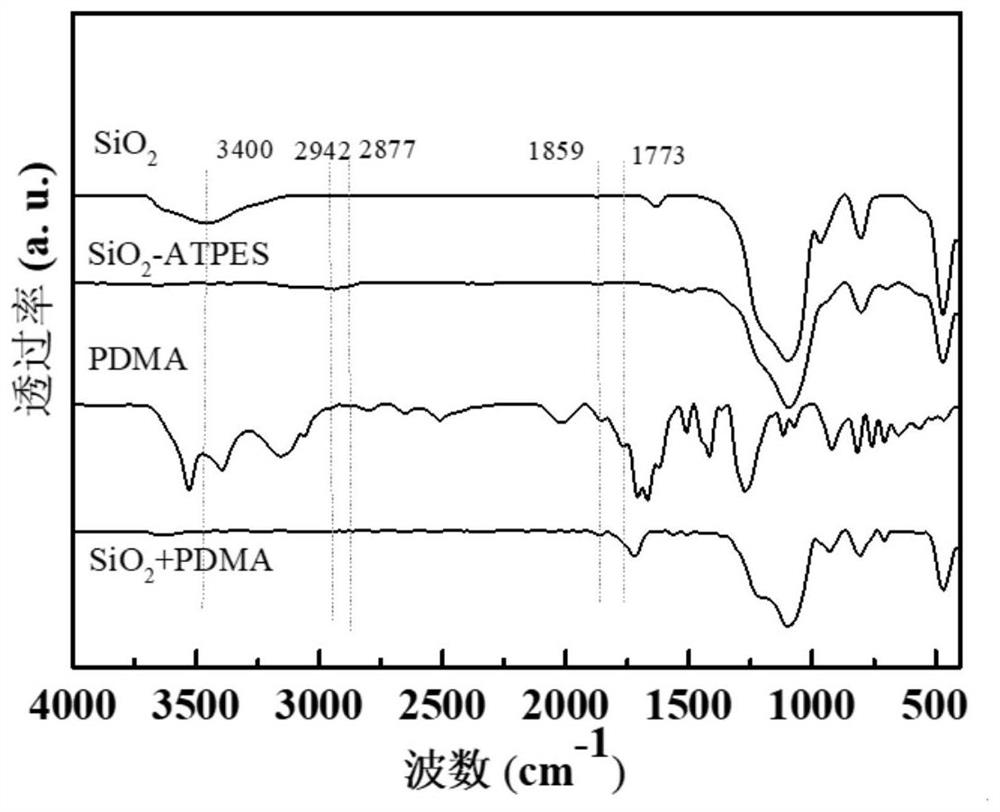
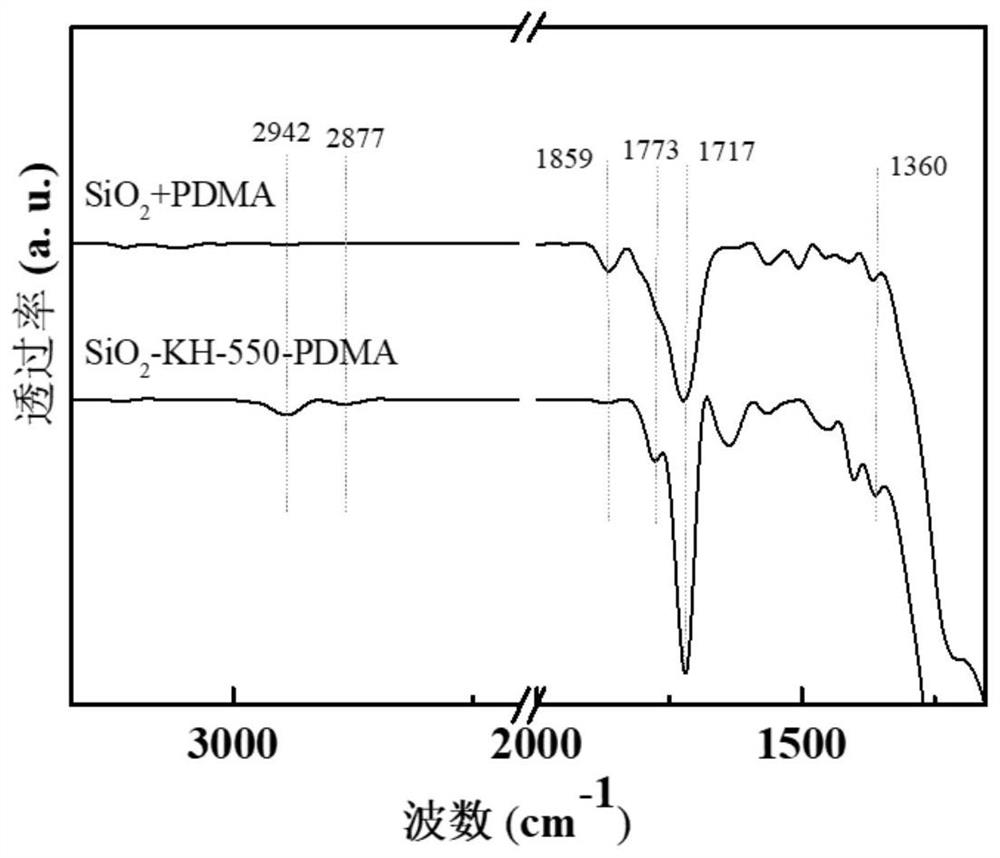
ActiveCN111790440AImprove stabilityAchieve recyclabilityOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsRecyclable catalystPolymer science
The invention discloses an NHPI catalyst grafted by utilizing imide bonds, a preparation method and application thereof. The catalyst comprises a SiO2 carrier and N-hydroxyphthalimide grafted on the SiO2 carrier, wherein the N-hydroxyphthalimide is grafted on the SiO2 carrier through imide bonds. The preparation method of the catalyst comprises the following steps: (1) modifying the surface of a carrier SiO2 by using aminopropyltriethoxysilane to obtain a surface ammoniation carrier SiO2-APTES; (2) dissolving cyclized dianhydride and the surface ammoniation carrier SiO2-APTES in a solvent, andgrafting through imide covalent bonds to prepare grafted anhydride; and (3) carrying out imidization treatment on the grafted anhydride to prepare the grafted NHPI catalyst, wherein the catalyst canbe used as a recyclable catalyst to be applied to preparation of acetophenone. The catalyst has the advantages of no loss of active components in the catalytic reaction, good stability, high active site density and good catalytic effect, and realizes the cyclic utilization of the catalyst.
Owner:YANGZHOU UNIV
Green method for catalytically synthesizing 2'-aminobenzothiazolyl-arylmethyl-2-naphthol
InactiveCN103360339AHigh acid densityHigh catalytic activityOrganic chemistryChemical recyclingChemical synthesisRecyclable catalyst
The invention provides a green method for catalytically synthesizing 2'-aminobenzothiazolyl-arylmethyl-2-naphthol, belonging to the field of organic chemical synthesis. The method comprises the following steps: carrying out synthetic reaction, wherein the aromatic aldehyde:2-aminobenzothiazole:beta-naphthol mol ratio is 1:1:1, the bissulfonate acidic ion liquid catalyst accounts for 5-8 mol% of the aromatic aldehyde, the reaction temperature is 80-95 DEG C, the reaction time is 3-10 minutes, and the volume (ml) of the reaction solvent water is 3-5 times of the molar weight (mmol) of the aromatic aldehyde; and after the reaction is finished, cooling to room temperature, carrying out vacuum filtration, recrystallizing the filter residue with 95% ethanol, and carrying out vacuum drying to obtain the 2'-aminobenzothiazolyl-arylmethyl-2-naphthol.Compared with other synthesis methods, the invention has the characteristics of high catalytic activity, low catalyst consumption, recyclable catalyst, low environmental pollution in the whole process, simple and convenient after-treatment and the like.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Preparation method of (S)-(+)-ethyl mandelate by microbial transformed ethyl benzoylformate
InactiveCN102719496ACost-effective synthetic routeFacilitate reuseMicroorganism based processesOn/in organic carrierRecyclable catalystMicrobial transformation
The invention provides a preparation method of (S)-(+)-ethyl mandelate by using biocatalytic ethyl benzoylformate and taking saccharomyces cerevisiae with CGMCC No.2266 as a biocatalyst. The method comprises the following steps of: performing a conversion reaction for 8 to 120 hours at a temperature of between 20 and 35 DEG C in a water and n-hexane two-phase system by using ethyl benzoylformate as a substrate and the saccharomyces cerevisiae with CGMCC No.2266 as a biocatalyst; and separating and purifying the conversion solution to obtain (S)-(+)-ethyl mandelate. The microbial conversion method has the advantages of mild reaction condition, friendly environment, high product optical purity, high substrate conversion rate, simple separation and purification process and recyclable catalyst, and is suitable for industrial production.
Owner:杭州联豪科技有限公司
Hdrogenation synthesis method for preparing 2-methyl allyl alcohol by using recyclable catalyst
InactiveCN102167657B"Atom Economy"Simple post-processingOrganic compound preparationHydroxy compound preparationRecyclable catalystSynthesis methods
The invention discloses a method for synthesizing 2-methylacrolein into 2-methyl allyl alcohol by using a catalytic hydrogenation method, belonging to the technical field of synthesis of organic intermediates. A catalyst used in the method is recyclable. The synthesis method comprises the following steps of: (1) adding 2-methylacrolein, a catalyst, a polymerization inhibitor and a high-boiling-point solvent into a high-pressure kettle and introducing hydrogen for reacting; (2) after reacting, exhausting excessive hydrogen and rectifying reaction liquid to obtain 2-methylacrolein; and (3) adding the 2-methylacrolein and the solvent into residual liquid in a rectifying kettle, transferring into the high-pressure kettle and undergoing a reaction for preparing 2-methylacrolein through hydrogenation to realize the recycling of the catalyst. The method has the advantages of atom economy, environmental friendliness, high yield, simple post-treatment and the like.
Owner:武汉凯森化学有限公司
A kind of heterogeneous catalyst and the method for using it to prepare cyclocarbonate
ActiveCN107715918BAvoid churnAvoid lossOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsAlkanePtru catalyst
The invention provides a heterogeneous catalyst and a method for preparing cyclic carbonate using the heterogeneous catalyst. The heterogeneous catalyst is composed of a main catalyst and a cocatalyst, and the main catalyst is filled in a reactor in a regular manner. The main catalyst is a cross-linked polymer containing quaternary ammonium salt groups. The active sites in the catalyst are quaternary ammonium salt groups, which are chemically bonded to the cross-linked polymer and have the characteristics of adjustable quantity. ; The molecular formula of the cocatalyst is M x L y , is a halogenated metal salt. The application of heterogeneous catalysts makes CO 2 The method for preparing cyclic carbonate by reacting with alkylene oxide has the advantages of convenient product separation, high yield of cyclic carbonate, and recyclable catalyst.
Owner:JIANGSU SOBUTE NEW MATERIALS +1
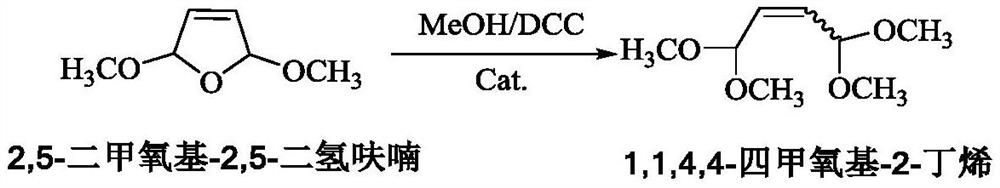
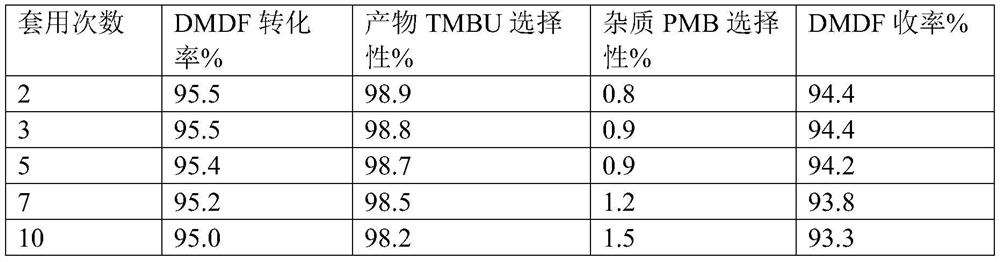
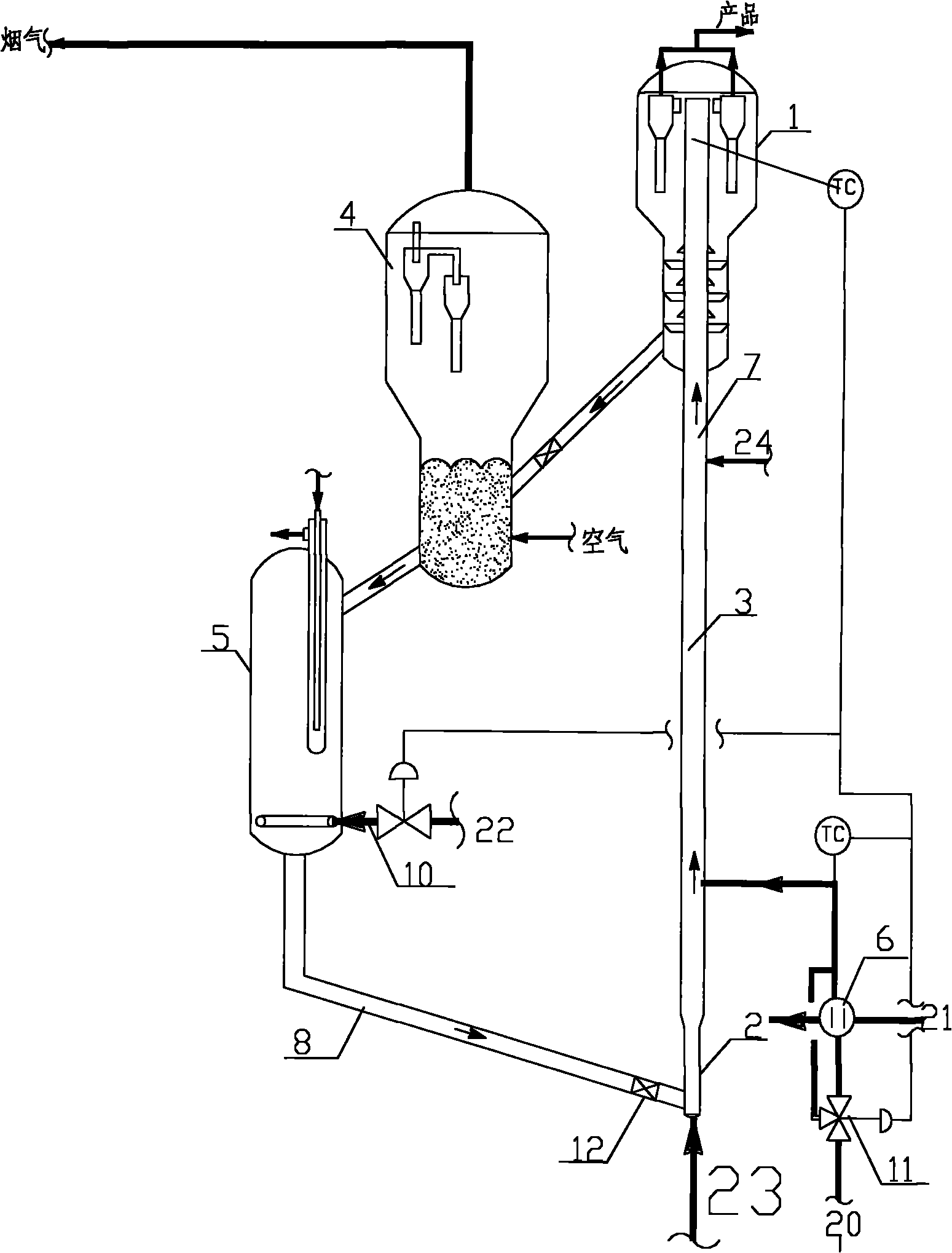
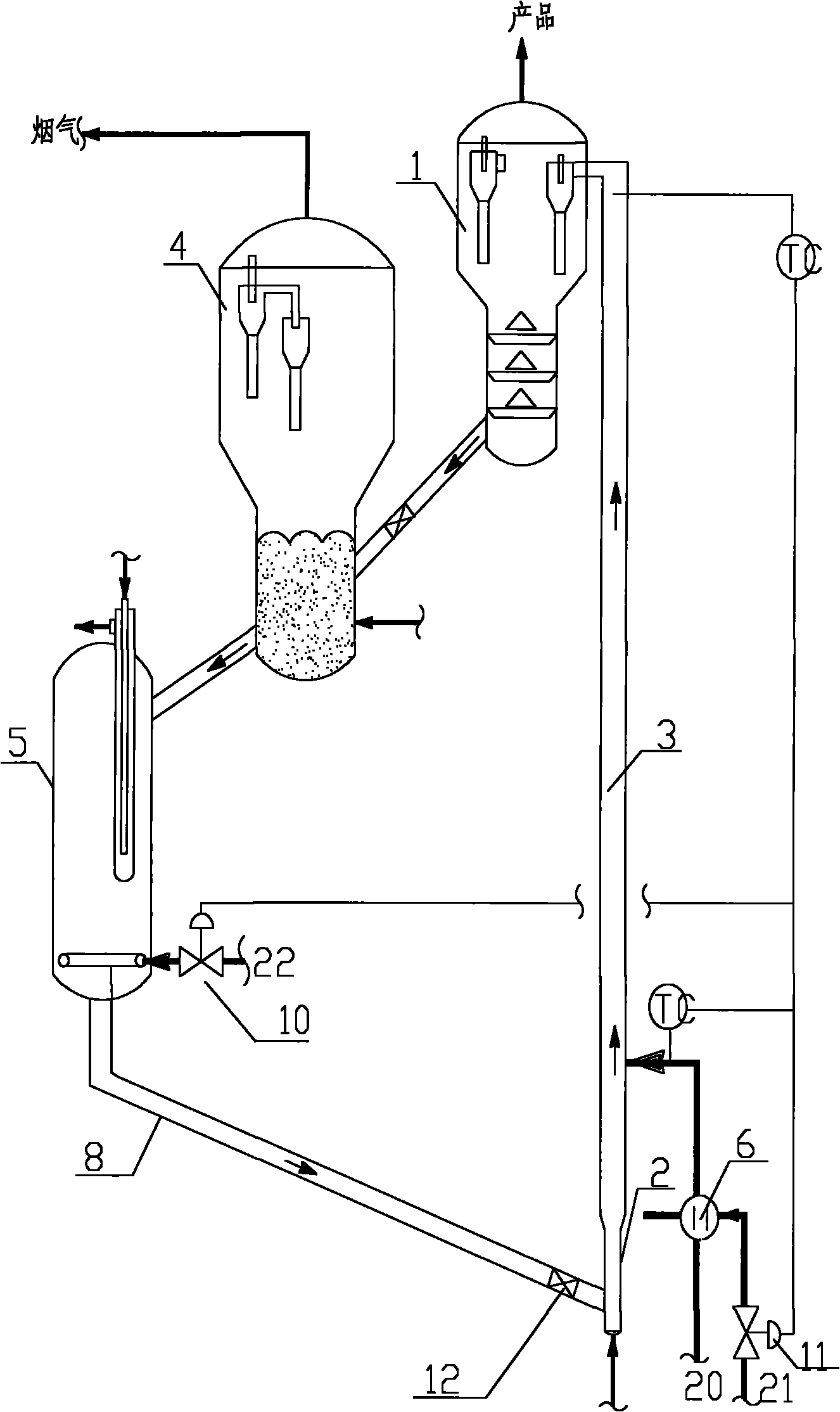
Catalyst and preparation method thereof, and preparation method of 1, 1, 4, 4-tetramethoxy-2-butene
ActiveCN112121859AHigh catalytic activityIncrease the number ofOrganic chemistryOrganic compound preparationRecyclable catalystFuran
The invention provides a catalyst and a preparation method thereof, and a preparation method of 1, 1, 4, 4-tetramethoxy-2-butene. The catalyst is prepared from the following components by mass: 100 parts of carrier aromatic ring primary amine modified multi-walled carbon nanotubes; 10-60 parts of an organic strong acid; and 1-10 parts of a rhodium complex or a palladium complex. The method comprises the following steps: under the action of the catalyst, carrying out acetalation reaction on 2, 5-dimethoxy-2, 5-dihydrofuran and methanol by taking a dehydrating agent as an auxiliary agent to prepare 1, 1, 4, 4-tetramethoxy-2-butene; the process has the advantages of high substrate conversion rate, high product selectivity, low environmental pollution and recyclable catalyst, and can overcomethe defects of low reaction yield, serious equipment corrosion and the like in the existing process.
Owner:WANHUA CHEM GRP CO LTD
Fluid catalytic conversion feeding preheating and reaction temperature control method
ActiveCN101838545AEasy to operateThe number of active centers is stableCatalytic crackingHydrocarbon from oxygen organic compoundsRecyclable catalystReaction temperature
The invention provides a petroleum fluid catalytic conversion control method. After being heated, raw oil enters a reactor and carries out catalytic conversion reaction after being mixed with recyclable catalyst which enters the reactor. The method is characterized in that the preheating temperature of the raw oil needs to be regulated when controlling the reaction temperature of the reactor. The control method overcomes the defects in the prior art, enhances the selectivity and the conversion rate of the fluid catalytic conversion reaction, improves the product distribution, enhances the yield of light distillate and has the characteristics of having reliable running and flexible regulation and saving energy and investment.
Owner:李群柱
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com