Imide bond grafted NHPI catalyst, preparation method and application thereof
The technology of imide and catalyst is applied in the field of NHPI catalyst grafted by imide bond and its preparation field, which can solve the problems of loss of NHPI active components, influence on catalytic effect, cleavage of graft bond, etc., and achieve recyclable The effect of utilization, good catalytic effect and high active site density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
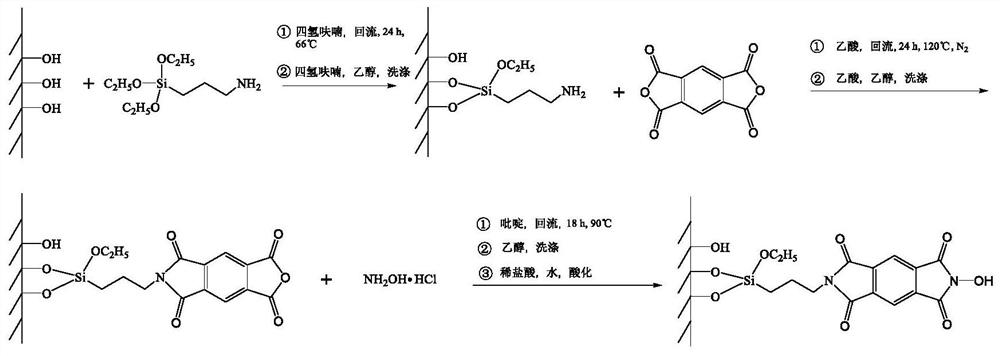
[0041] The synthesis route of this embodiment is as follows figure 1 Shown:
[0042] (1) Use γ-aminopropyltriethoxysilane APTES to modify SiO 2 : Add 2g SiO2 to a 250mL single-necked round bottom flask 2 , 10g γ-aminopropyltriethoxysilane APTES and 100mL tetrahydrofuran THF, move the flask into an oil bath, set up a reflux device, stir, heat the oil bath to 70°C and keep a constant temperature, stop heating and stirring after 24 hours of reaction and reflux, When the temperature dropped to room temperature, remove the reflux device, filter the reaction solution, wash the filter cake once with 60mL THF, and wash twice with 60mL absolute ethanol, and put the obtained white solid powder in an oven at 70°C for 24 hours to prepare SiO 2 -APTES.
[0043] (2) Using pyromellitic dianhydride and SiO 2 -Imidation reaction of primary amines on APTES support to prepare SiO 2 -APTES-PDMA: Take 1g pyromellitic dianhydride PDMA, 1g SiO prepared in the first step 2 -APTES and 100mL of a...
Embodiment 2
[0050] (1) Use γ-aminopropyltriethoxysilane APTES to modify SiO 2 : Add 2g SiO2 to a 250mL single-necked round bottom flask 2, 6g of γ-aminopropyltriethoxysilane APTES and 100mL of tetrahydrofuran THF, move the flask into an oil bath, set up a reflux device, stir, heat the oil bath to 80°C and keep it at a constant temperature, react and reflux for 48 hours, stop heating and stirring, When the temperature dropped to room temperature, remove the reflux device, filter the reaction solution, wash the filter cake once with 60mL THF, and wash twice with 60mL absolute ethanol, and put the obtained white solid powder in an oven at 70°C for 24 hours to prepare SiO 2 -APTES.
[0051] (2) Using 3,3',4,4'-biphenyltetracarboxylic dianhydride BDP and SiO 2 -Imidation reaction of primary amines on APTES support to prepare SiO 2 -APTES-BDP: Take 1g 3,3',4,4'-biphenyltetracarboxylic dianhydride BDP, 2g SiO prepared in the first step 2 -APTES and 100mL of dimethylacetamide DMAC were placed...
Embodiment 3
[0056] The difference between this embodiment and embodiment 2 is that in step 2, 1,4,5,8-naphthalene tetracarboxylic dianhydride NTDA and SiO 2 -Imidation reaction of primary amines on APTES support to prepare SiO 2 -APTES-NTDA, the amount of 1,4,5,8-naphthalene tetracarboxylic dianhydride NTDA used is 1g, and the product in step 3 is SiO 2 -APTES-NTDA–NOH with a grafted N-hydroxyphthalimide concentration of 0.75 mmol / g.
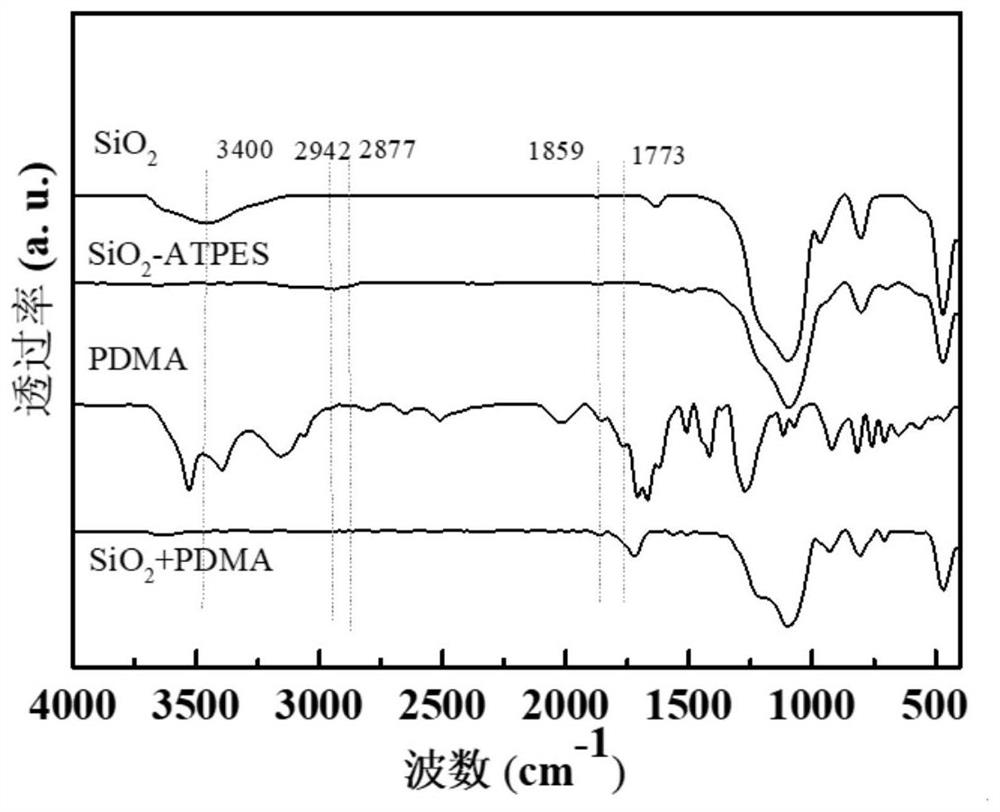
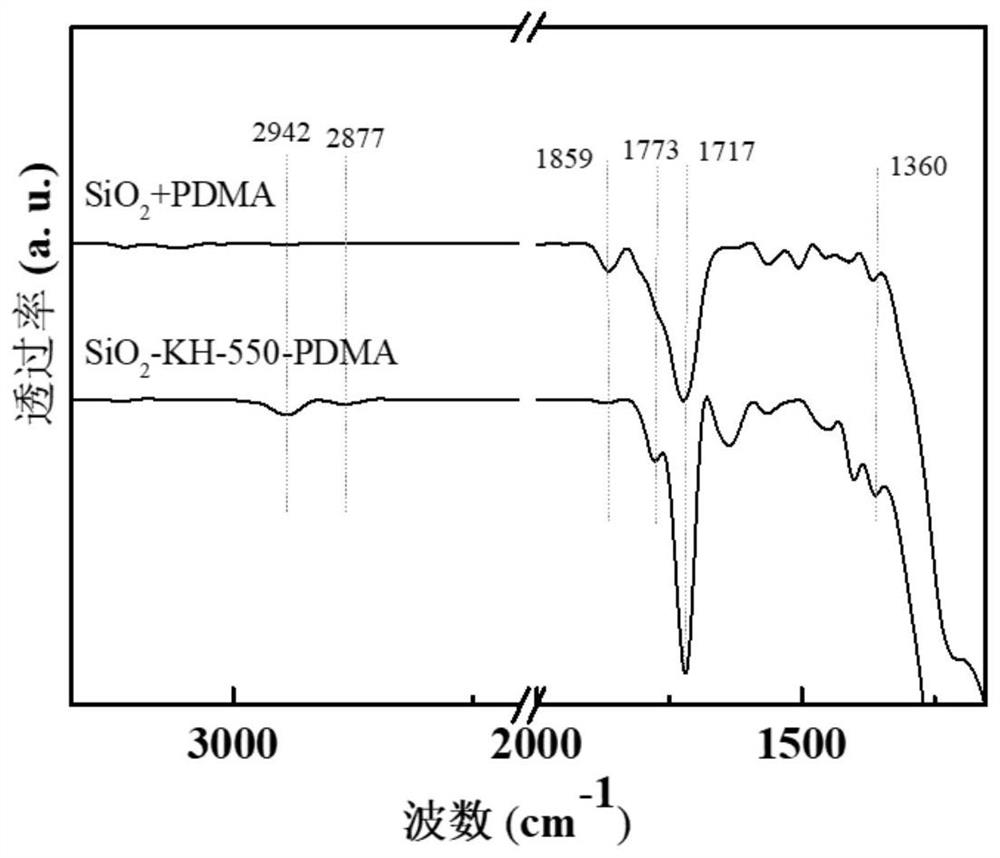
[0057] From Figure 6 It can be seen that NTDA and SiO 2 The asymmetric stretching vibration absorption peak of anhydride in +NTDA appears at 1774cm -1 Sample SiO after grafting reaction 2 -APTES-NTDA and SiO 2 -APTES-NTDA-NOH at 1774cm -1 Absorption peak disappears at 1666, 1712cm -1 Symmetrical and asymmetrical stretching vibration absorption peaks of imide group carbonyl appeared, 1345cm -1 The stretching vibration absorption peak of O=C-N-C=O on the imide ring appeared, indicating that NTDA and SiO 2 -APTES grafting was successful.
[0058] Fr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com