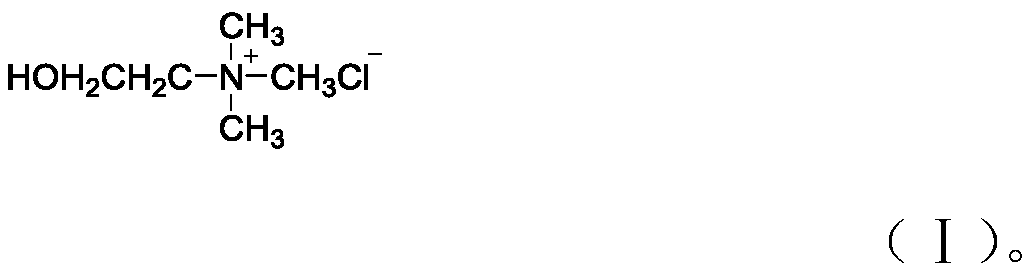Method for preparing acylferrocenyl hydrazinodithio acid ester Schiff base
The technology of acyl ferrocenyl hydrazino dithioate and acyl ferrocenyl hydrazino dithioformate is applied in the field of preparing acyl ferrocenyl hydrazino dithioate Schiff base, which can solve the problem of liquid The phase method has long reaction time, organic solvent pollutes the environment, catalyst cannot be recycled and other problems, and achieves the effect of high yield, short reaction time and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of N'-ferrocene methylene-hydrazino dithioformate methyl ester:
[0025]
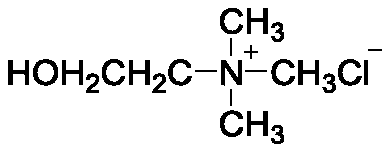
[0026] In the first step, 1 mol of choline chloride and 2 mol of methanesulfonic acid are added to the reaction vessel, and stirred at 80°C until fully dissolved to obtain a eutectic solvent;
[0027] In the second step, after cooling the reaction system to room temperature, add 1 mol of formyl ferrocene and 1 mol of methyl hydrazinodithioformate, slowly raise the temperature to 80°C, and monitor by TLC until the reaction is complete;
[0028] In the third step, the reaction liquid was cooled to room temperature, and solids were separated out, filtered with suction, and the solids were washed with a small amount of water to obtain the product with a yield of 91.2%, m.p. 145-147°C; the filtrate was recovered to obtain a eutectic solvent.
[0029] The structural characterization data of the product are:
[0030] IR(KBr)v:3122(v- NH- ), 3079 (v Unsaturated C-H ), 2963 (v Saturated C-H ...
Embodiment 2
[0032] Example 2 Preparation of N′-ferrocene methylene-hydrazinodithiocarbamate ethyl ester:
[0033]
[0034] In the first step, add 1 mol of choline chloride and 2 mol of methanesulfonic acid to the reaction vessel, and stir at 80°C until fully dissolved to obtain a eutectic solvent;
[0035] In the second step, after cooling the reaction system to room temperature, add 1 mol of formyl ferrocene and 1 mol of methyl hydrazinodithioformate, slowly increase the temperature to 80°C, and monitor by TLC until the reaction is complete;
[0036] In the third step, the reaction solution was cooled to room temperature, and solids were separated out, filtered with suction, and the solids were washed with a small amount of water to obtain the product with a yield of 93.1%, m.p. 126-128°C; the filtrate was recovered to obtain a eutectic solvent.
[0037] The structural characterization data of the product are:
[0038] IR(KBr)v:3115(v- NH- ), 2927(v Saturated C-H ), 1599(v- C=N ),1388(v- CH3 ),...
Embodiment 3
[0040] Example 3 Preparation of N′-ferrocene methylene-hydrazinodithiocarbamate benzyl ester:
[0041]
[0042] In the first step, add 1 mol of choline chloride and 2 mol of methanesulfonic acid to the reaction vessel, and stir at 80°C until fully dissolved to obtain a eutectic solvent;
[0043] In the second step, after cooling the reaction system to room temperature, add 1 mol of formyl ferrocene and 1 mol of methyl hydrazinodithioformate, slowly increase the temperature to 80°C, and monitor by TLC until the reaction is complete;
[0044] In the third step, the reaction solution was cooled to room temperature, and solids were separated out, filtered with suction, and the solids were washed with a small amount of water to obtain the product with a yield of 93.1%, m.p. 189-192°C; the filtrate was recovered to obtain a eutectic solvent.
[0045] The structural characterization data of the product are:
[0046] IR(KBr)v:3174(v- NH- ), 3084 (v Unsaturated C-H ), 2901(v Saturated C-H ),15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com