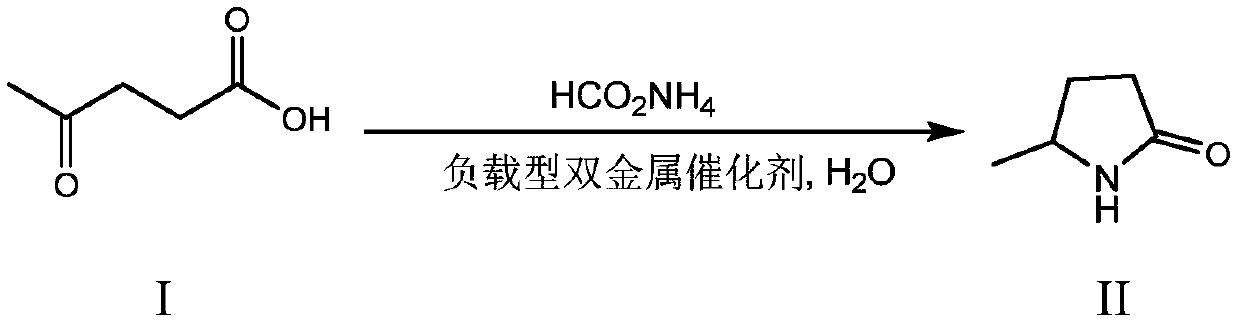
Synthesis method of 5-methyl-2-pyrrolidone
A pyrrolidone and synthesis method technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, organic chemistry, etc., can solve the problem of large amount of Raney nickel catalyst, unstable skeleton structure, and reduced catalyst activity and other issues, to achieve the effect of improving safety and environmental protection, high selectivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Get 0.5g levulinic acid and 0.27g ammonium formate in the beaker, add 10mL water, dissolve, add the solution in the 25mL autoclave, add the Pd-Ni / C catalyst (Pd, Ni of The mass ratio is 1:1), purging five times with nitrogen gas, and reacting for 3 hours at a reaction temperature of 120°C to obtain 5-methyl-2-pyrrolidone with a yield of 65.6%.
Embodiment 2
[0036]Get 0.5g levulinic acid and 1g ammonium formate in the beaker, add 10mL water, dissolve, solution is added in the 25mL autoclave, add the Pd-Ni / C catalyst (the mass of Pd, Ni of 0.05g loading capacity of 5wt%) The ratio is 1:1), purging five times with nitrogen gas, and reacting for 3 hours at a reaction temperature of 180°C to obtain 5-methyl-2-pyrrolidone with a yield of 94.5%.
Embodiment 3-5
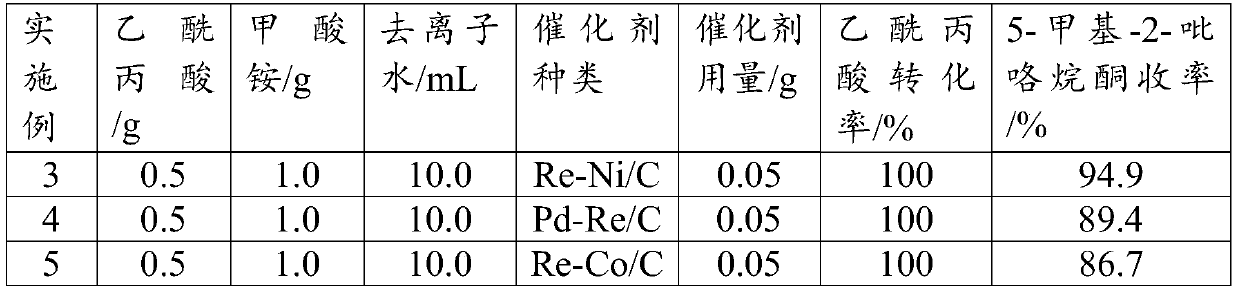
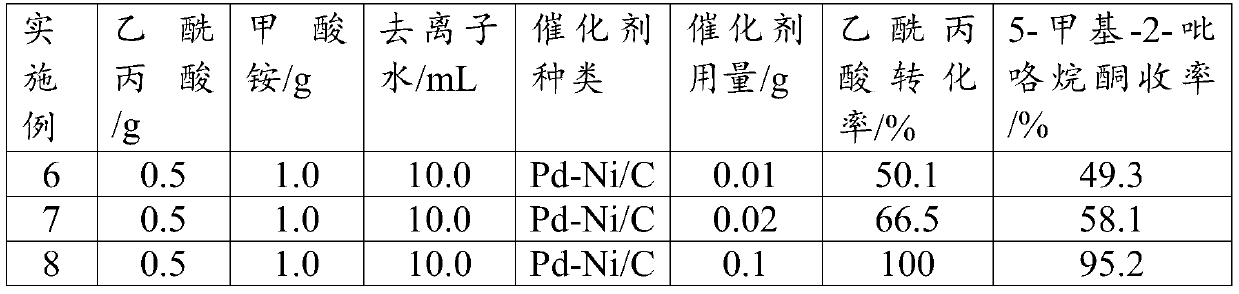
[0038] Other operations are the same as in Example 2, changing the type of supported bimetallic catalyst (the metal loading is 5wt%, and the mass ratio of bimetal is 1:1), to obtain the following reaction results (Table 1):
[0039] Table 1
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com