Catalyst and preparation method thereof, and preparation method of 1, 1, 4, 4-tetramethoxy-2-butene
A technology of tetramethoxy and catalysts, which is applied in the field of catalysts and preparations for the preparation of 1,1,4,4 tetramethoxy-2-butene, which can solve the problem of low conversion rate of raw materials and low product selectivity and reduced selectivity In order to achieve the effect of improving the conversion rate of process raw materials and product selectivity, improving the subsequent separation of products, and improving the utilization rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
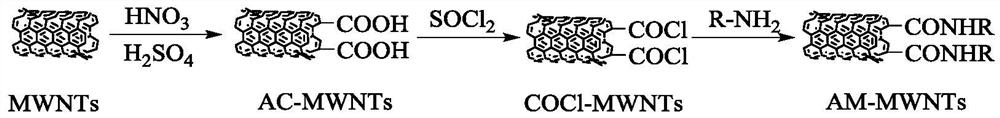
[0063] 50 g of multi-walled carbon nanotubes (MWNTs) were weighed and placed in 1000 g of mixed acid with a mass ratio of concentrated sulfuric acid and concentrated nitric acid of 3:1, and ultrasonically dispersed in an ultrasonic cleaner for 1 hour at room temperature. Transfer the ultrasonically dispersed sample into a single-necked flask, react in a constant temperature oil bath at 120°C for 2 hours, transfer it to a beaker after cooling to room temperature, and wash it by high-speed centrifugation (using absolute ethanol / distilled water alternately), and then vacuum-dry dry to obtain acid-modified multi-walled carbon nanotubes (AC-MWNTs).
[0064] Take 45g of acid-modified multi-walled carbon nanotubes (AC-MWNTs) and disperse them in 1800g of thionyl chloride, stir at 70°C for 20h, filter, and wash 3 times with anhydrous tetrahydrofuran to obtain acid chloride-modified multi-walled carbon nanotubes (COCl-MWNTs).
[0065] 40 g of acid chloride-modified multi-walled carbon...
Embodiment 2
[0069] Adjust the amount of carrier, trifluoromethanesulfonic acid, and acetylacetonylbis(ethylene)rhodium to 40g, 6.5g, and 3.84g respectively, and refer to Example 1 for the remaining conditions to prepare catalyst b. According to XPS analysis, its composition is carrier: trifluoromethanesulfonic acid: acetylacetonyl bis(ethylene)rhodium=100.0:15.0:8.0 (mass ratio).
[0070] Adjust the amount of carrier, trifluoromethanesulfonic acid, and acetylacetonyl bis(ethylene)rhodium to 40 g, 21.7 g, and 0.48 g, respectively, and refer to Example 1 for the rest of the conditions to prepare catalyst c. According to XPS analysis, its composition is carrier: trifluoromethanesulfonic acid: acetylacetonyl bis (ethylene) rhodium = 100.0:50.0:1.0 (mass ratio).
Embodiment 3
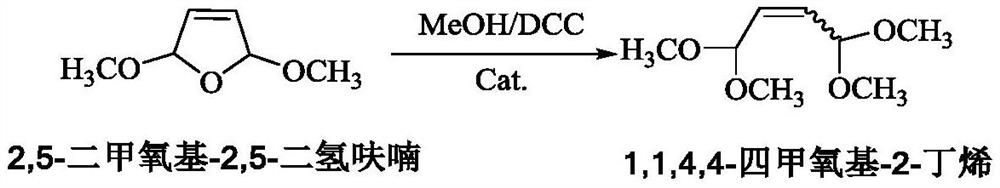
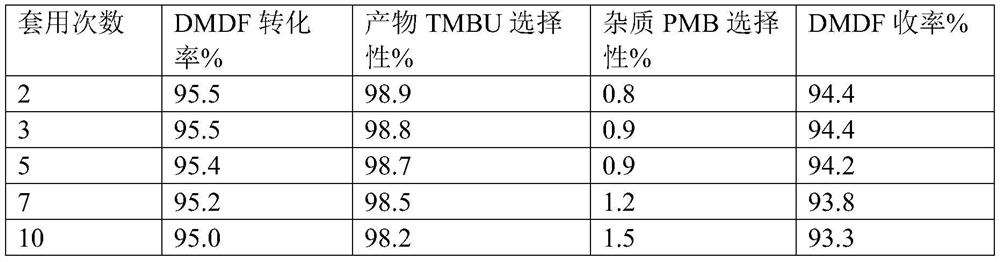
[0072] Add 96.1g (3mol) methanol, 165.1g (0.8mol) DCC and 130.1g (1mol) 2,5-dimethoxy-2,5-dihydrofuran (DMDF) to the three-necked flask, and use an oil bath to The bottle is heated. When it is heated to an internal temperature of 40°C, weigh 6.5g of catalyst a and add it into the three-necked flask to start the insulation reaction. When the reaction was carried out for 4.0 hours, samples were taken for gas phase analysis. The conversion rate of raw material DMDF was 95.5%, the selectivity of product TMBU was 98.9%, the selectivity of impurity PMB was 0.8%, and the yield of TMBU was 94.4%.
[0073] The catalyst is applied mechanically, and the experimental data are shown in Table 1 below:
[0074] Table 1 Catalyst a application data
[0075]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com