Application of catalyst based on organic metal framework UiO-66 in cellulose hydrolysis
An organometallic, uio-66 technology, applied in organic compound/hydride/coordination complex catalysts, organic chemistry, physical/chemical process catalysts, etc., can solve the corrosion of reactant equipment, low conversion rate of cellulose hydrolysis, The problem of complex reaction operation, etc., achieves the effects of high reuse rate, improved hydrolysis conversion rate, and less dosage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
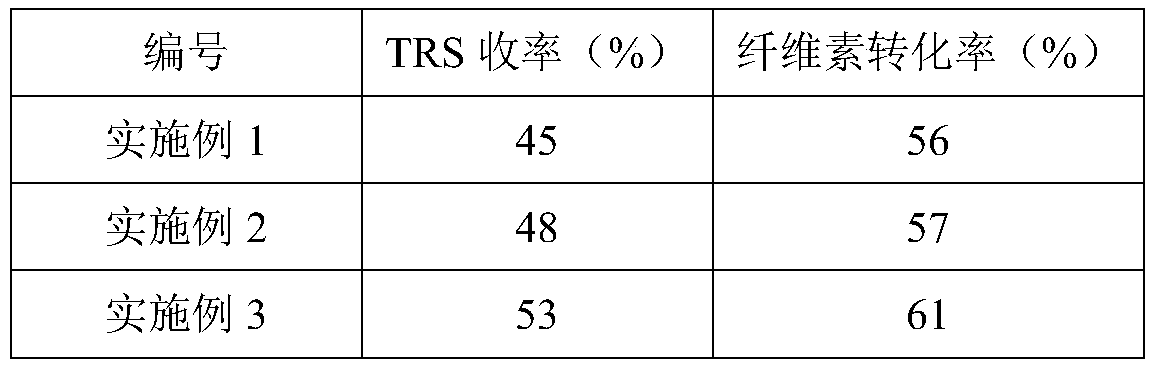
Embodiment 1
[0033] Preparation of UiO-66 catalyst: 2.93g ZrCl4 and 2.075g of 1,4-phthalic acid were dissolved in 60mL of dimethylformamide (DMF) solvent, then 1.0739mL of 37wt% hydrochloric acid was added, and the mixture was ultrasonically dissolved for 30min; then the mixture solution was placed in 100mL of polytetrafluoroethylene In an ethylene reactor, keep the constant temperature at 120°C for 24 hours. After the reaction, cool to room temperature and filter to remove the solvent, wash the precipitate with DMF and methanol, and then dry the washed sample in an oven at 80°C overnight to obtain UiO-66 catalyst.
[0034] Application of UiO-66 catalyst in cellulose hydrolysis: Weigh 0.5g cellulose, 0.1g UiO-66 catalyst and 5mL deionized water and mix them in a 25mL stainless steel hydrothermal reaction kettle lined with polytetrafluoroethylene, seal Then heat the temperature to 180°C and react for 3 hours to obtain a hydrolyzed solution.
Embodiment 2
[0036] Preparation of UiO-66-COOH catalyst: 2.93g ZrCl 4 and 2.627g of 1,2,4-benzenetricarboxylic acid were dissolved in 60mL of dimethylformamide (DMF) solvent, then 1.0739mL of 37wt% hydrochloric acid was added, and the mixture was ultrasonically dissolved for 30min; then the mixture solution was placed in 100mL of poly In the tetrafluoroethylene reactor, keep the constant temperature at 140°C for 12h. After the reaction, cool to room temperature, filter and remove the solvent, wash the precipitate with DMF and methanol, and then dry the washed sample in an oven at 85°C overnight to obtain UiO -66-COOH catalyst.
[0037] Application of UiO-66-COOH catalyst in cellulose hydrolysis: weigh 0.5g cellulose, 0.1g UiO-66-COOH catalyst and 5mL deionized water and mix in a 25mL stainless steel hydrothermal reaction lined with polytetrafluoroethylene In the kettle, heat the temperature to 180°C after sealing, and react for 3 hours to obtain a hydrolyzed solution.
Embodiment 3
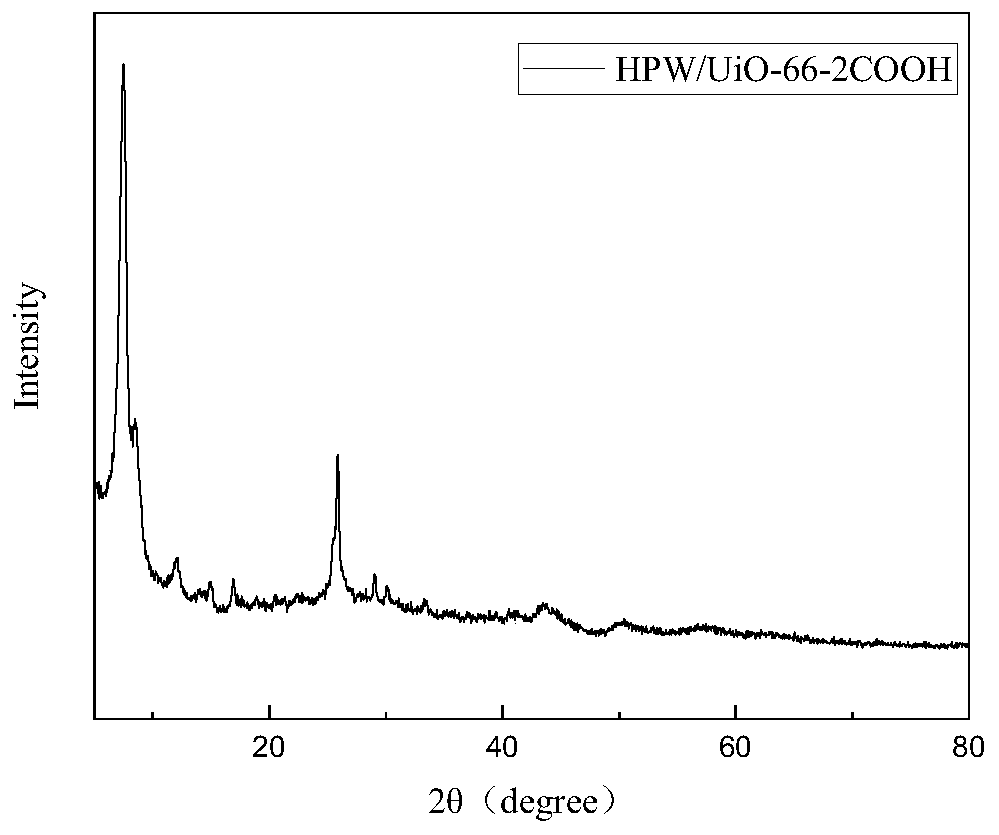
[0039] Preparation of UiO-66-2COOH catalyst: 2.93g ZrCl 4 and 3.177g of pyromellitic acid were dissolved in 60mL of dimethylformamide (DMF) solvent, then 1.0739mL of 37wt% hydrochloric acid was added, and the mixture was ultrasonically dissolved for 60min; then the mixture solution was placed in a 100mL polytetrafluoroethylene reactor In the process, the temperature was maintained at 100°C for 48h. After the reaction, cooled to room temperature and filtered to remove the solvent, the precipitate was washed with DMF and methanol, and then the washed sample was dried in an oven at 75°C overnight to obtain the UiO-66-2COOH catalyst. .
[0040] Application of UiO-66-2COOH catalyst in cellulose hydrolysis: Weigh 0.5g cellulose, 0.1g UiO-66-2COOH catalyst and 5mL deionized water and mix in a 25mL stainless steel hydrothermal reaction lined with polytetrafluoroethylene In the kettle, heat the temperature to 180°C after sealing, and react for 3 hours to obtain a hydrolyzed solution. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com