Method for preparing ethyl levulinate based on solid superacid catalysis and furfuryl alcohol alcoholysis
A technology of ethyl levulinate and solid super acid, applied in the field of ethyl levulinate, can solve the problems of non-conformity with green chemistry, difficult separation of products, severe reaction conditions, etc., achieves good application prospects, convenient post-processing, and reaction Simple system effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
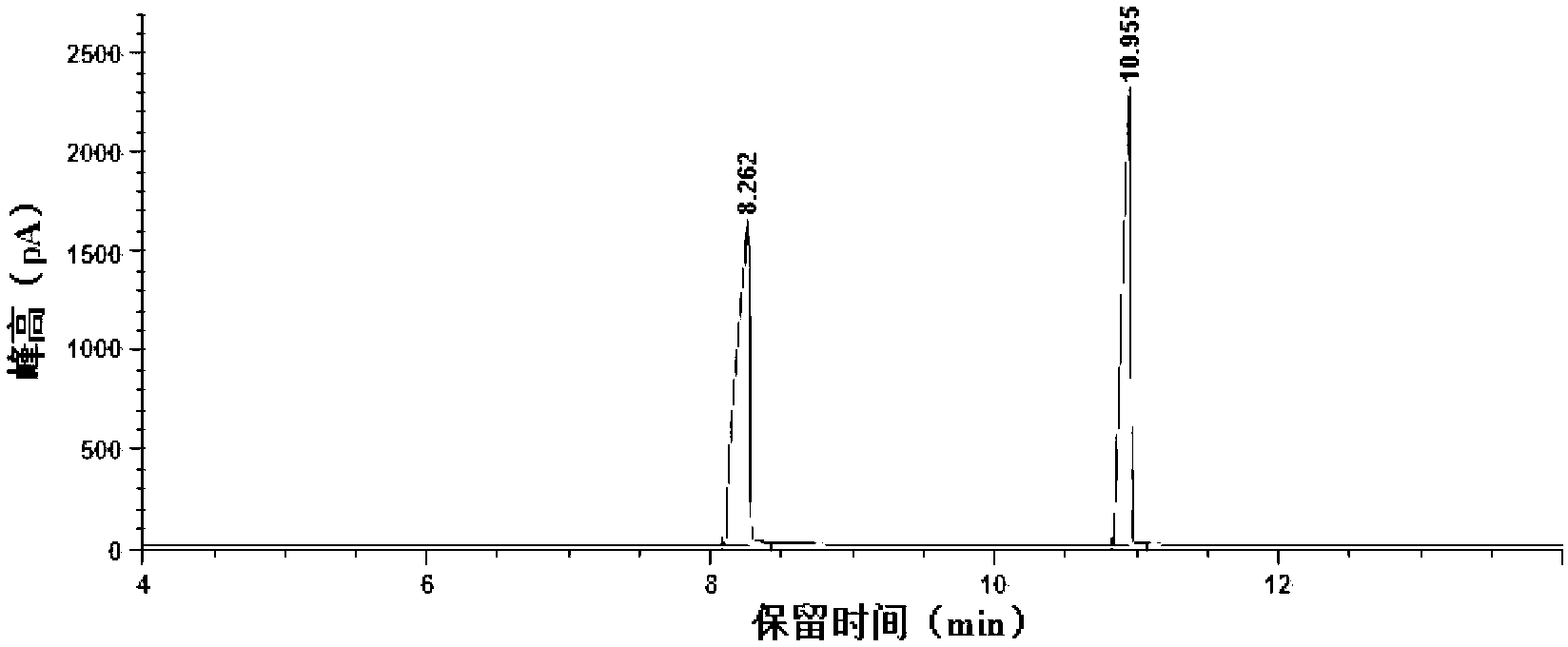
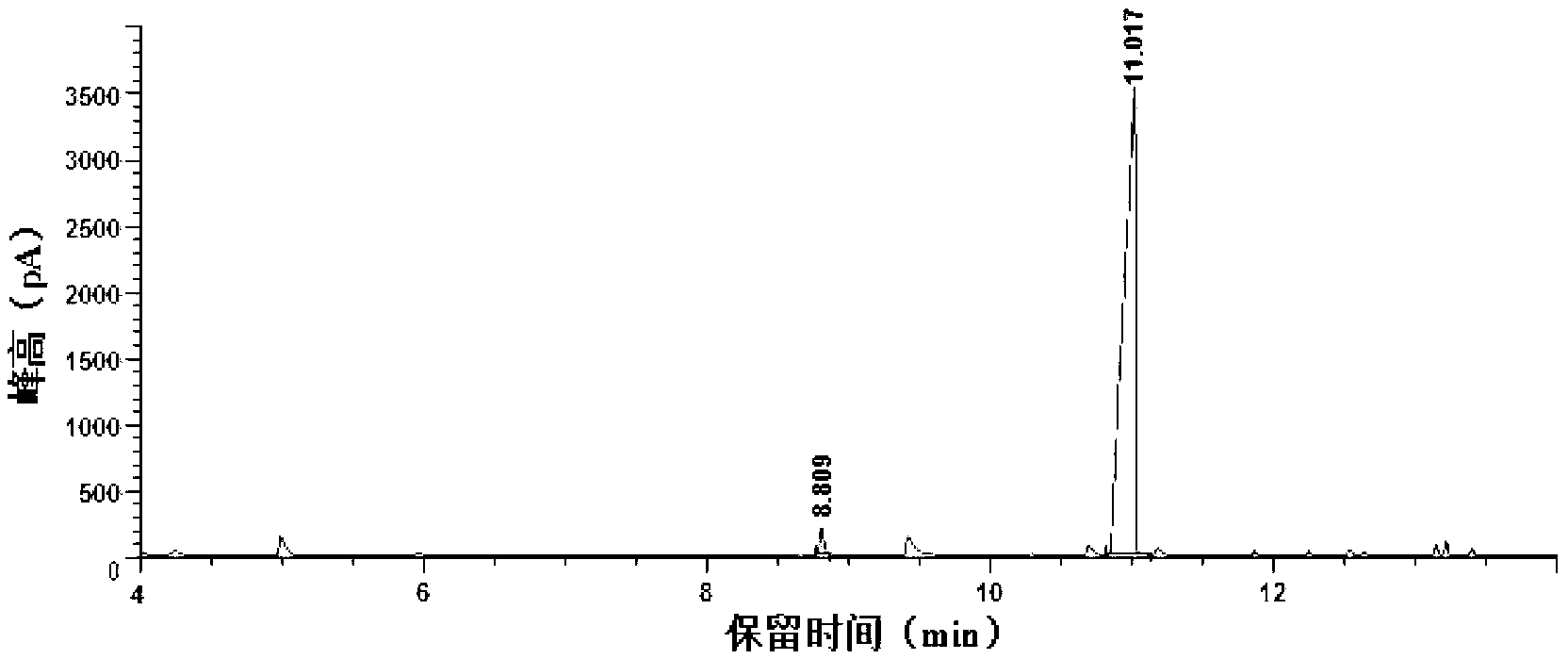
[0025] 1mL furfuryl alcohol, 0.5g solid superacid SO 4 2– / TiO 2 Add 20mL ethanol into a 50mL reactor, seal it and start the reaction, the stirring speed is 500rpm, start timing when the temperature rises to 125°C, keep the temperature for 2 hours, after the reaction, immediately immerse the reactor in cold water Cool down to room temperature. The reaction solution was centrifuged at 10000rpm, and the supernatant was taken for analysis and detection. After gas chromatography analysis, it was compared with the gas chromatography standard spectrum of furfuryl alcohol and ethyl levulinate (see figure 1 and figure 2 ) After comparison, it is calculated that the yield of the product ethyl levulinate can reach 68.3%.
Embodiment 2
[0027] 1mL furfuryl alcohol, 0.5g solid superacid SO 4 2– / ZrO 2 Add 20mL ethanol into a 50mL reactor, seal it and start the reaction, the stirring speed is 500rpm, start timing when the temperature rises to 125°C, keep the temperature for 2 hours, after the reaction, immediately immerse the reactor in cold water Cool down to room temperature. The reaction solution was centrifuged at 10,000 rpm, and the supernatant was taken for analysis and detection. The yield of ethyl levulinate was calculated by gas chromatography to reach 64.3%.
Embodiment 3
[0029] 1mL furfuryl alcohol, 0.5g solid superacid S 2 o 8 2- / SnO 2 Add 20mL ethanol into a 50mL reactor, seal it and start the reaction, the stirring speed is 500rpm, start timing when the temperature rises to 125°C, keep the temperature for 2 hours, after the reaction, immediately immerse the reactor in cold water Cool down to room temperature. The reaction solution was centrifuged at 10,000 rpm, and the supernatant was taken for analysis and detection. The yield of ethyl levulinate calculated by gas chromatography analysis could reach 65.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com