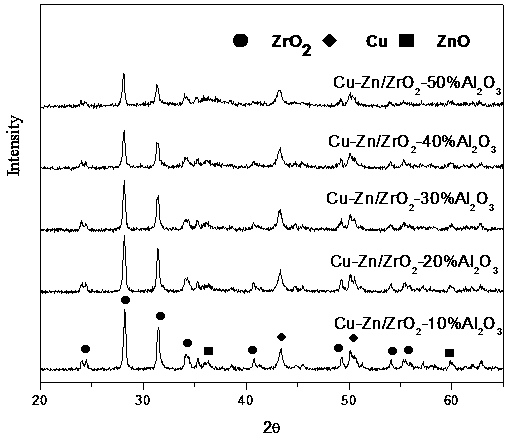
Copper-zinc zirconium oxide metal catalyst and method for catalytic continuous synthesis of gamma-valerolactone by catalyst
A technology of metal catalyst and zinc zirconia, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of harsh reaction conditions, affecting production reaction running time, etc., and achieves simple and good preparation Application prospects, the effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A preparation method of a copper-zinc-zirconia metal catalyst, the steps are as follows:
[0025] (1) Accurately weigh copper nitrate and zinc nitrate according to the stoichiometric ratio of molar ratio (2-6): 1, dissolve in distilled water, add excess sodium carbonate solution for co-precipitation, wash with water until neutral, and filter under reduced pressure. Copper-zinc carbonate precipitation is obtained after drying;
[0026] (2) Mix the copper-zinc carbonate precipitate obtained in step (1) with zirconia and alumina, stir evenly, and shape it with an extruder; the diameter of the strip-shaped catalyst is 2-3mm;
[0027] (3) Put the obtained strip-shaped catalyst into an electric heating constant temperature blast drying oven, and dry it at 100-120°C;
[0028] (4) Put the dried catalyst into a muffle furnace, heat up to 400-600°C and roast for 4-5 hours to obtain a copper-zinc oxide metal catalyst; copper oxide and zinc oxide in the catalyst account for 20-40...
Embodiment 2
[0033] A preparation method of a copper-zinc-zirconia metal catalyst, the steps are as follows:
[0034] (1) Accurately weigh copper nitrate and zinc nitrate according to the stoichiometric ratio of 2:1, dissolve them in distilled water, add excess sodium carbonate solution for co-precipitation, wash with water until neutral, filter under reduced pressure, and dry to obtain copper zinc carbonate precipitation;
[0035] (2) Mix the copper-zinc carbonate precipitate obtained in step (1) with zirconia and alumina, stir evenly, and shape it with an extruder; the diameter of the strip-shaped catalyst is 2-3mm;
[0036] (3) Put the obtained strip-shaped catalyst into an electric heating constant temperature blast drying oven, and dry it at 100°C;
[0037] (4) Put the dried catalyst into a muffle furnace, heat up to 400°C and roast for 4 hours to obtain a copper-zinc oxide metal catalyst; copper oxide and zinc oxide in the catalyst account for 20% of the total mass of the catalyst. ...
Embodiment 3
[0040] A preparation method of a copper-zinc-zirconia metal catalyst, the steps are as follows:
[0041] (1) Accurately weigh copper nitrate and zinc nitrate according to the stoichiometric ratio of 2:1, dissolve them in distilled water, add excess sodium carbonate solution for co-precipitation, wash with water until neutral, filter under reduced pressure, and dry to obtain copper zinc carbonate precipitation;
[0042] (2) Mix the copper-zinc carbonate precipitate obtained in step (1) with zirconia and alumina, stir evenly, and shape it with an extruder; the diameter of the strip-shaped catalyst is 2-3mm;
[0043] (3) Put the obtained strip-shaped catalyst into an electric heating constant temperature blast drying oven, and dry it at 100°C;
[0044] (4) Put the dried catalyst into a muffle furnace, heat up to 400°C and roast for 4 hours to obtain a copper-zinc oxide metal catalyst; copper oxide and zinc oxide in the catalyst account for 20% of the total mass of the catalyst. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com