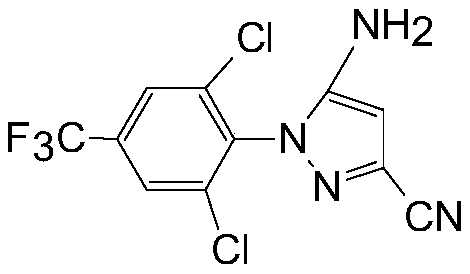
Production method of aryl pyrazole nitrile
An arylpyrazole nitrile technology and a production method, which are applied in directions such as organic chemistry, can solve problems such as low yield, potential safety hazards, and difficulty in handling the three wastes, and achieve high yield, reduced side reactions, and shortened residence time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0033] Reactor I is a horizontal tubular reactor made of DN32 glass-lined seamless steel pipe. The straight pipe part is equipped with a sleeve pipe that can be connected to frozen brine or circulating water for cooling. It has a built-in PTFE SK type static mixer. The rooms are connected by elbows, with an effective length of 60 meters and an effective volume of 40 liters. There are three thermocouples and a pH meter, and nitrite electrodes are installed in the middle of the reactor and before the outlet. The reactor can be divided into three sections and cooled with cooling media of different temperatures, so as to control different temperatures. The front end of each section is equipped with a feeding interface, and sodium nitrite or acid ethanol can be replenished when sodium nitrite or acid value is insufficient.
[0034]Add about 30% of commercially available acid ethanol (hydrogen chloride ethanol) into anhydrous ethanol to prepare 7-12% acid ethanol; add 98% 2,6-dichlo...
example 2
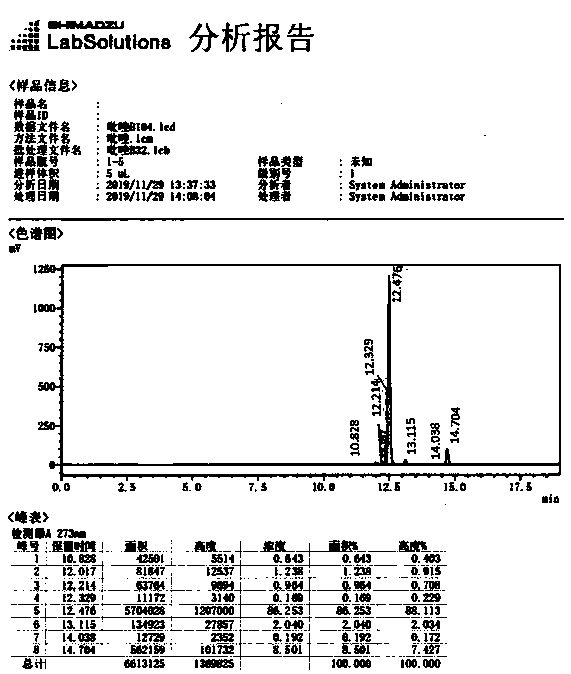
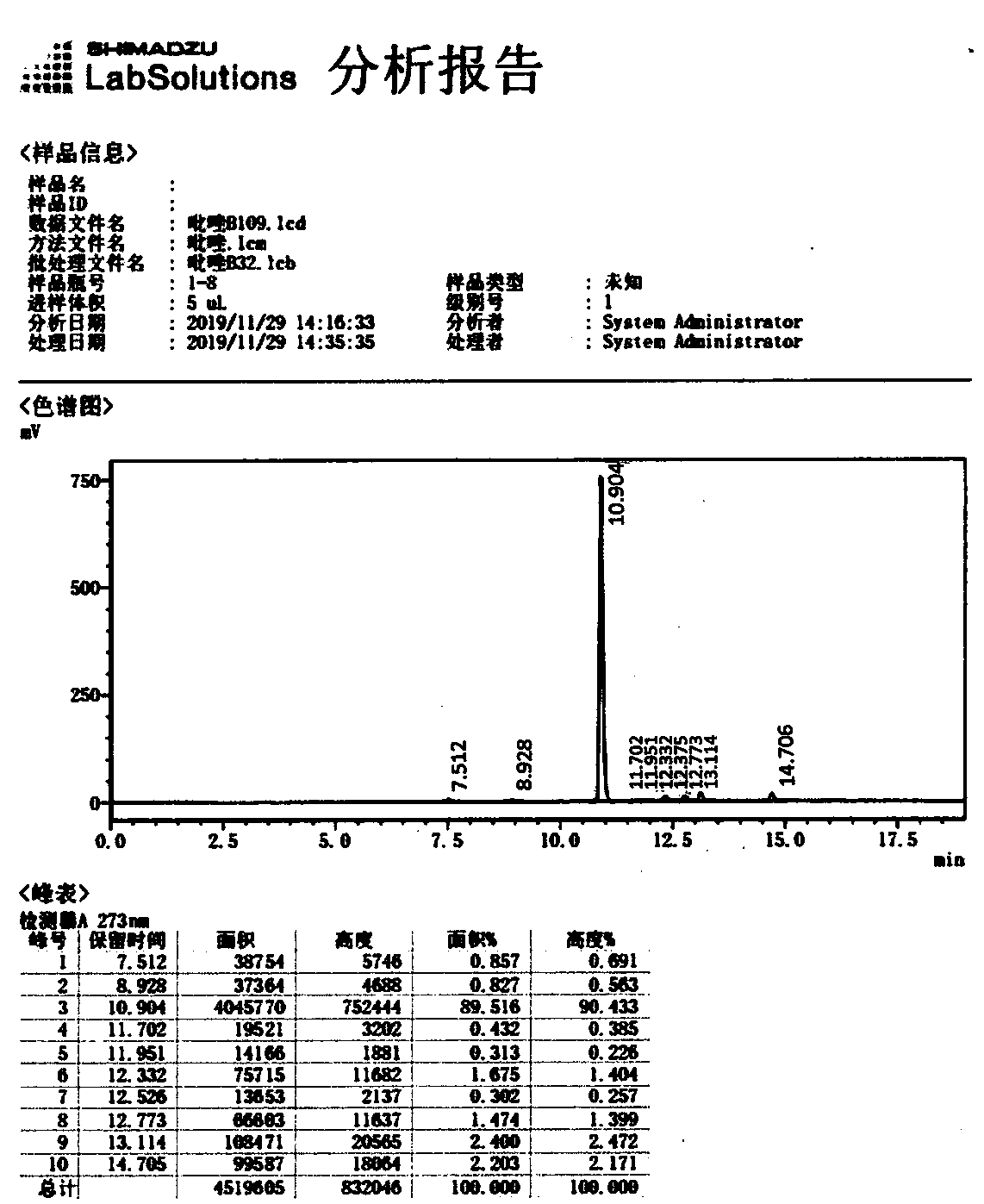
[0038] Frozen brine below 0°C was passed into the reactor jacket of the above example 1, and the flow rate of the chilled brine was reduced after normal circulation. The feed solution was then pumped into the reactor described in Example 1 at a flow rate of 1.00 liters / minute, while the suspension described in Example 1 was pumped into the reactor described in Example 1 at a flow rate of 0.20 liters / minute. The flow rate of frozen brine was adjusted to keep the temperature of the material in the reactor between 10 and 15°C. Monitor pH value and nitrous acid concentration, fine-tune the flow rate of the suspension, and control the nitrite concentration at the outlet of the reactor to be 0.1-0.5% and the pH value to be 1-5. The 2,6-dichloro-4-trifluoromethylaniline content was detected by sampling at the outlet of the reactor every 15 minutes, and the reaction was continued for 2 hours. The outlet of the reactor was 2,6-dichloro-4-trifluoromethylaniline. The average content was...
example 2~9
[0040] The experimental process of Example 2 is repeated with different flow rates, and the content of the 2,6-dichloro-4-trifluoromethylaniline material at the outlet of the reactor is as shown in Table 1:
[0041] Table 1 Diazo-coupling reaction at different flow rates at 10~15℃
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com