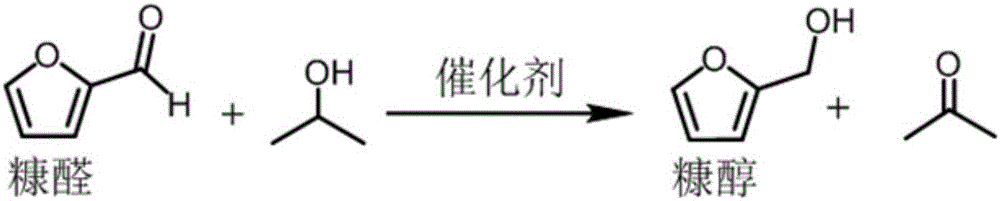
Method for preparing furfuryl alcohol by utilizing hydrogen transfer reaction to catalyze furfural
A hydrogen transfer and furfural technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic chemistry, etc., can solve problems such as hexavalent chromium environmental pollution, high energy consumption, and increased equipment investment costs, achieving low cost, Low energy consumption and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the heterogeneous magnetic hydroxyapatite catalyst used in the present invention, the steps are:
[0027] ① Weigh 2.64g (NH4) 2 HPO 4 , add 90mL distilled water to dissolve, then add concentrated ammonia (25-28wt.%) to adjust the pH to 11; ②Weigh 7.95g Ca(NO 3 ) 2 4H 2 O, add 90mL distilled water to dissolve, then add concentrated ammonia water (25-28wt.%) to adjust the pH to 11; ③at room temperature, put a suitable stirring bar into a 500mL two-necked flask, add 0.514g FeSO 4 ·7H 2 O and 1gFeCl 3 ·6H 2 O, pass nitrogen to exhaust the air in the flask, continue to pass nitrogen, add 30mL of distilled water at the same time, add 10mL of 26.5wt.% ammonia water, after stirring for 15 minutes, drop the two solutions obtained in steps ① and ② at the same time, and heat up to 90 ° C. After stirring for 2 hours, stop stirring and let stand overnight; ④ filter, wash 4 times with water, dry at 80°C, and calcined at 300°C for 3 hours to obtain a r...
Embodiment 1
[0031] A kind of method utilizing hydrogen transfer reaction catalysis furfural to prepare furfuryl alcohol, comprises the following steps:
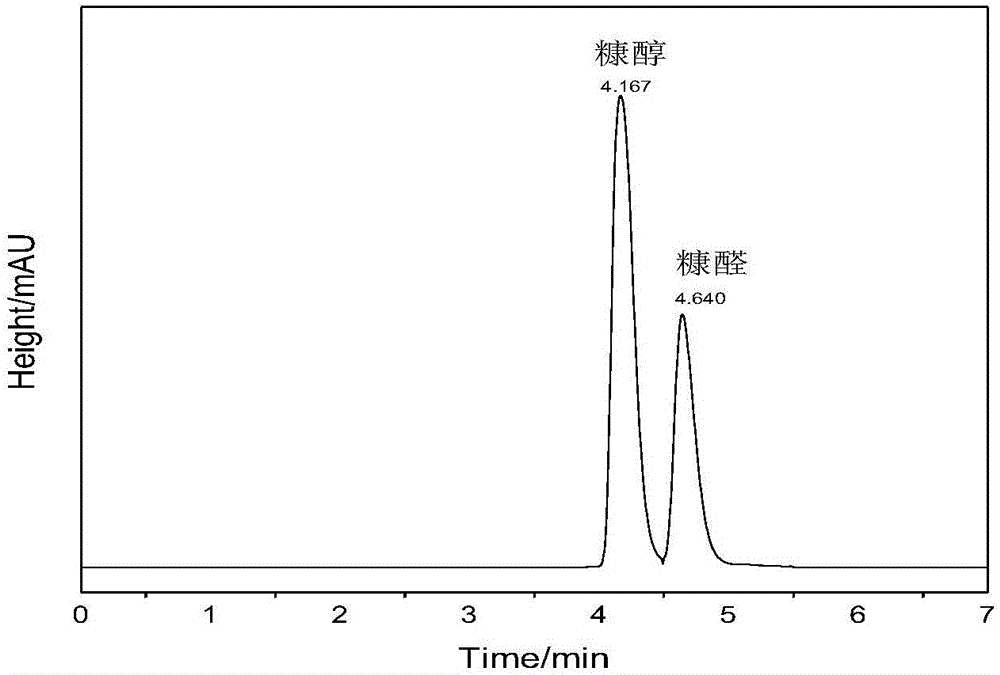
[0032] 120mg catalyst γ-Fe 2 o 3 Add @HAP to the lining of a clean autoclave, then add 1mmol furfural and 15mL methanol (both as a hydrogen donor and as a reaction solvent), seal the stainless steel autoclave, and use N 2 Change air three times, then fill with 10barN at room temperature 2 , react at 190°C for 10h, and detect the composition of the product by liquid chromatography after the reaction (such as figure 1 ), conversion rate 100%, productive rate 30.8%, selectivity 30.8%.
Embodiment 2-7
[0034] This embodiment provides the experiment of reducing furfural to furfuryl alcohol under different hydrogen donors;
[0035] A kind of method utilizing hydrogen transfer reaction catalysis furfural to prepare furfuryl alcohol, comprises the following steps:
[0036] 40mg catalyst γ-Fe 2 o 3 Add @HAP to the lining of a clean autoclave, add 1mmol furfural and 15mL of different alcohol compounds (both as a hydrogen donor and as a reaction solvent), seal the stainless steel autoclave and use N 2 Change air three times, then fill with 10barN at room temperature 2 , reacted at 180°C for 3h; using different alcohol compounds, the specific experimental results are shown in Table 1:
[0037] Table 1 Catalytic effects when using different hydrogen donors
[0038] Example Hydrogen Donor and Solvent Conversion rates(%) Yield(%) selectivity (%) 2 ethanol 64.0 45.4 70.9 3 n-propanol 65.7 53.6 81.5 4 Isopropanol 64.9 59.5 91.8 5 But...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com