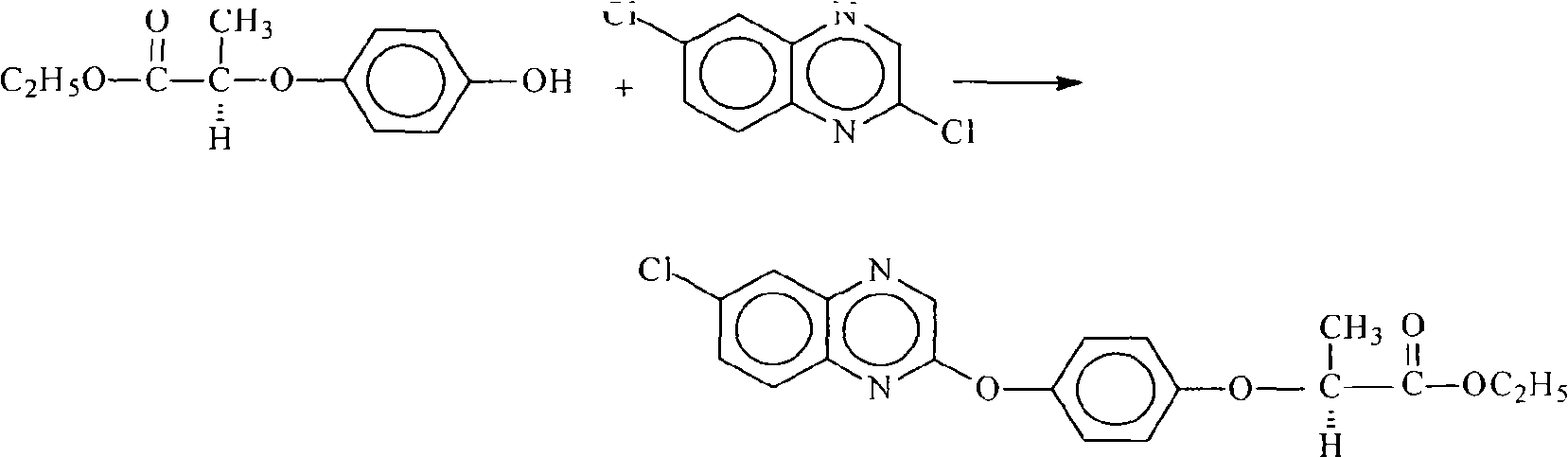
Method for preparing quizalofop-p-ethyl
A technology for quizalofop-p-ethyl and dichloroquinoxaline is applied in the field of preparation of herbicide quizalofop-p-ethyl, which can solve the problems of refractory hydroquinone, low optical purity of quizalofop-p-ethyl, etc. , The effect of avoiding hydroquinone-containing sewage and relieving environmental pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
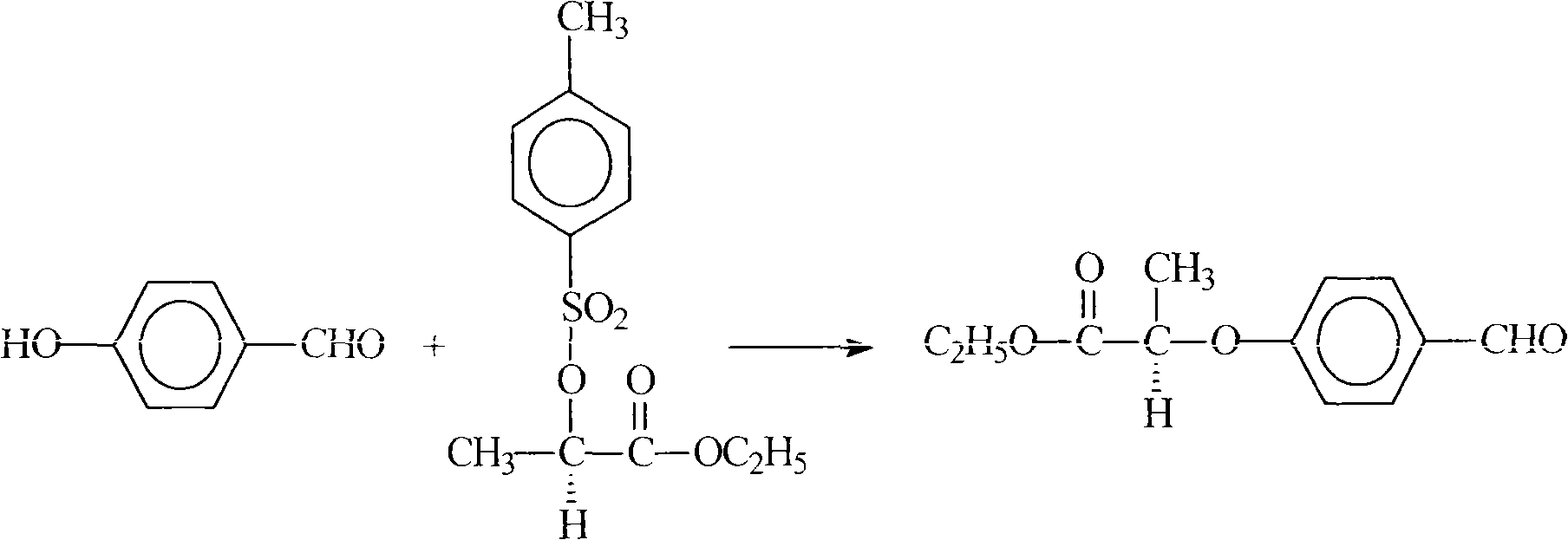
[0030] Preparation of ethyl R(+)-2-(p-formylphenoxy)propionate
[0031] In the flask that thermometer, condenser, stirrer are housed, add 100ml acetonitrile, then add 12.2g (0.1mol) p-Hydroxybenzaldehyde, 40.8g (0.15mol) S (-)-ethyl p-toluenesulfonyl lactate, 25g of anhydrous potassium carbonate, heated to reflux reaction for 5h under stirring, cooled to room temperature, filtered, distilled out acetonitrile from the filtrate under reduced pressure, added toluene and water, separated the organic layer, then distilled under reduced pressure, collected at 130-140°C / 1mmHg fraction, obtain product 21.5g, content 98.3%, yield 95.2% (in p-hydroxybenzaldehyde).
Embodiment 2
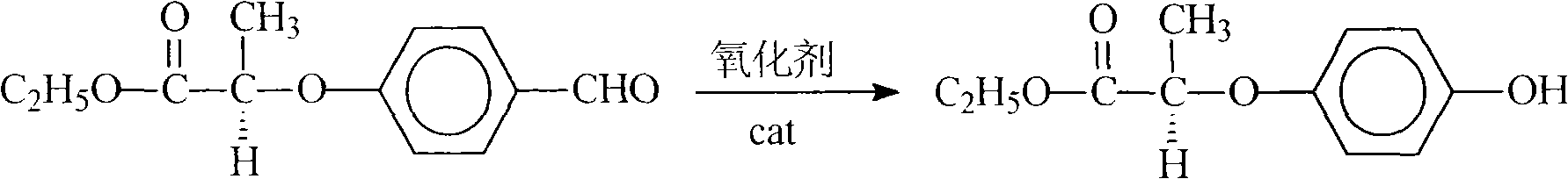
[0033] Preparation of R(+)-2-(p-hydroxyphenoxy)ethyl propionate
[0034] In a flask equipped with a thermometer, a condenser and a stirrer, add 80ml of toluene, then add 22.2g (0.1mol) of R(+)-2-(p-formylphenoxy) ethyl propionate, 0.5g of molybdenum phosphate acid, add 32.5g of 35% peracetic acid dropwise under stirring at room temperature, after the addition, slowly raise the temperature to 50°C and stir for 2h, add 5g of water and stir for 1h, cool to room temperature, add 30g of water to wash, separate layers, and distill under reduced pressure The toluene was extracted to obtain 21 g of the product, the content was 93.8%, and the yield was 94.3%.
Embodiment 3
[0036] Preparation of R(+)-2-(p-hydroxyphenoxy)ethyl propionate
[0037] Operation is the same as Example 2, hydrogen peroxide replaces peracetic acid, the content is 93.2%, and the yield is 92.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com