Organic phosphine-containing polymer carrier loaded Rh-based catalyst, preparation and applications thereof
A carrier-supported, catalyst technology, applied in the direction of organic compound/hydride/coordination complex catalyst, catalyst activation/preparation, physical/chemical process catalyst, etc., can solve the problems of cumbersome synthesis steps and low specific surface area of forming materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
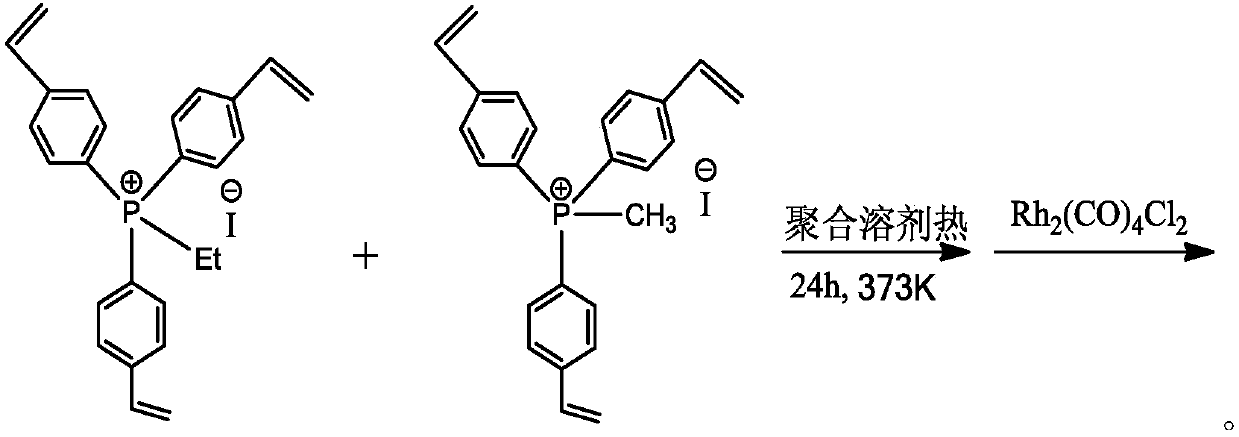
[0037] at 298K and N 2 Under a protective atmosphere, dissolve 5g of tris(4-vinylphenyl)phosphine ethyl iodide and 5g of tris(4-vinylphenyl)phosphine methyl iodide as monomers in 100.0ml of tetrahydrofuran solvent, and add 0.25 g of azobisisobutyronitrile free radical initiator, stirred for 2 hours. The stirred solution was transferred to a hydrothermal kettle, and solvothermally polymerized for 24 hours at 373K and nitrogen gas protection atmosphere. After the above polymerized solution is cooled to room temperature, the solvent is vacuumed out at room temperature to obtain an organic polymer carrier with a large surface area and a hierarchical porous structure. Then, at 298K and N 2 Under protective atmosphere, 0.0285g Rh 2 (CO) 4 Cl 2 Dissolve in 50ml of dichloromethane, then add 5g of the above polymer into it, stir at room temperature for 24h, wash with dichloromethane and suction filter, then vacuum the solvent to obtain a Rh-based catalyst supported by an organic p...
Embodiment 2
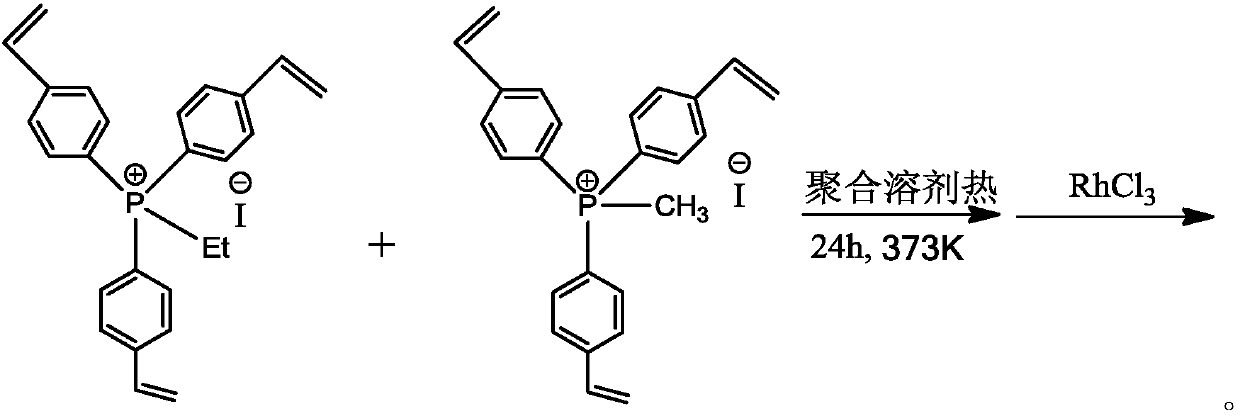
[0040] at 298K and N 2 Under a protective atmosphere, 5 g of tris (4-vinylphenyl) phosphine ethyl iodide and 5 g of tris (4-vinyl phenyl) phosphine methyl iodide as monomers were dissolved in 100.0 ml of CH 2 Cl 2 In the solvent, 0.25 g of azobisisobutyronitrile radical initiator was added to the above solution, and stirred for 2 hours. The stirred solution was transferred to a hydrothermal kettle, and solvothermally polymerized for 24 hours at 373K and nitrogen gas protection atmosphere. After the above polymerized solution is cooled to room temperature, the solvent is vacuumed out at room temperature to obtain an organic polymer carrier with a large surface area and a hierarchical porous structure. Then, at 298K and N 2 Under protective atmosphere, 0.050g RhCl 3 Dissolve in 50ml of tetrahydrofuran, then add 5g of the above polymer into it, stir at room temperature for 24h, wash with tetrahydrofuran and suction filter, and vacuum the solvent to obtain a Rh-based catalyst ...
Embodiment 3
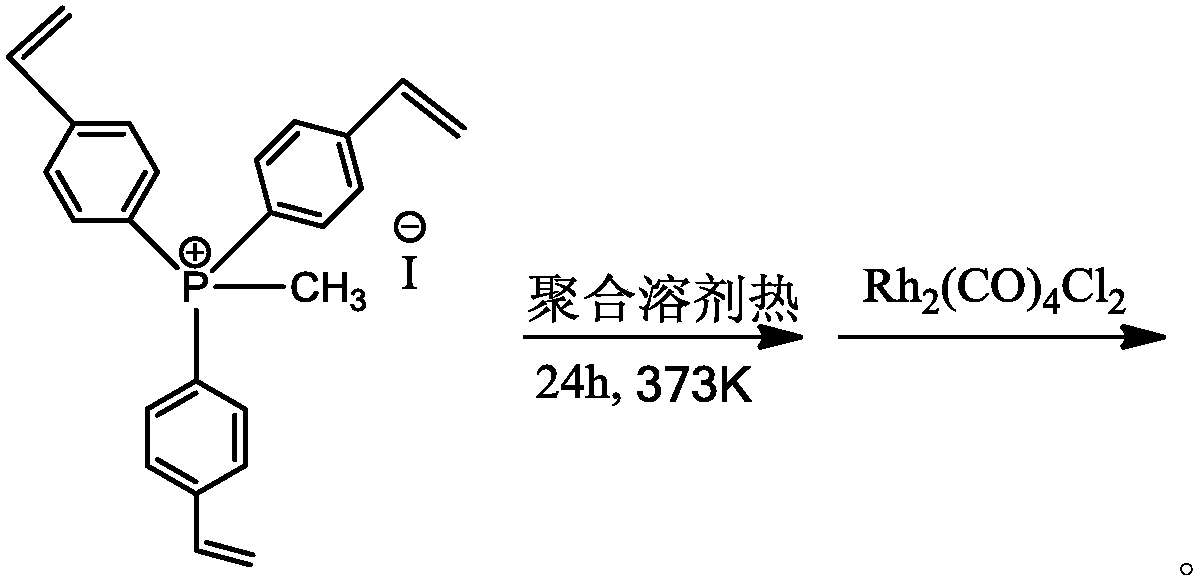
[0043] at 298K and N 2 Under a protective atmosphere, 10.0 g of tris(4-vinylphenyl)phosphine methyl iodide was dissolved in 100.0 ml of dimethylformamide solvent as a monomer, and 0.25 g of azobisisoheptanonitrile radical was added to the above solution Initiator, stirred for 2 hours. The stirred solution was transferred to a hydrothermal kettle, and solvothermally polymerized for 24 hours at 373K and nitrogen gas protection atmosphere. After the above polymerized solution is cooled to room temperature, the solvent is vacuumed out at room temperature to obtain an organic polymer carrier with a large surface area and a hierarchical porous structure. Then, at 298K and N 2 Under protective atmosphere, 0.0285g Rh 2 (CO) 4 Cl 2 Dissolve in 50ml CH 2 Cl 2 , and then 5 g of the above polymer was added therein, stirred at room temperature for 24 h, washed with dichloromethane and suction filtered, and vacuumed to remove the solvent to obtain a Rh-based catalyst supported by an ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com