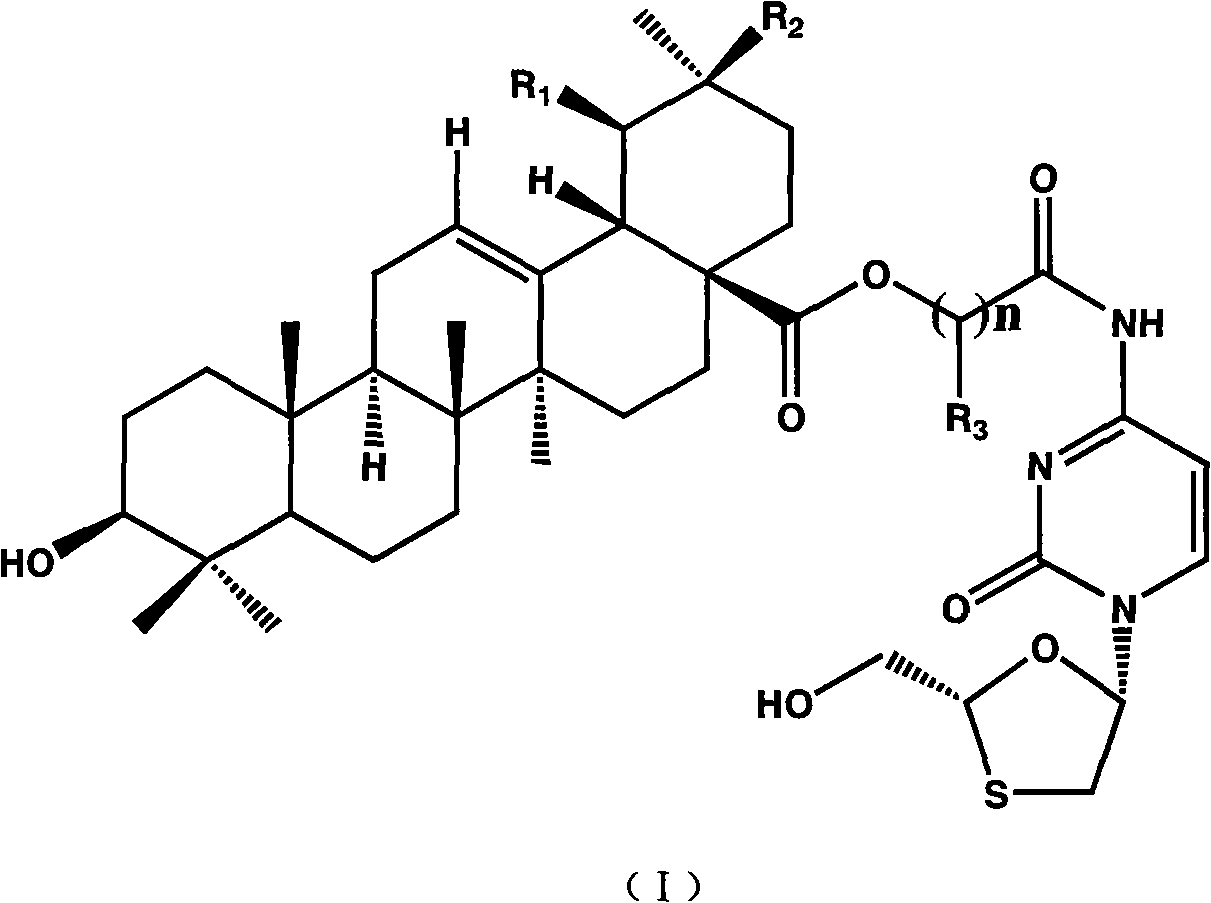
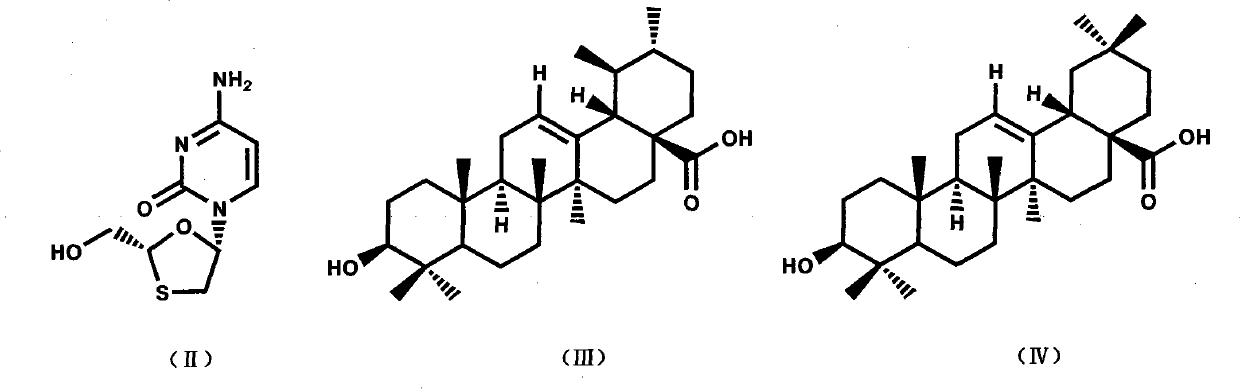
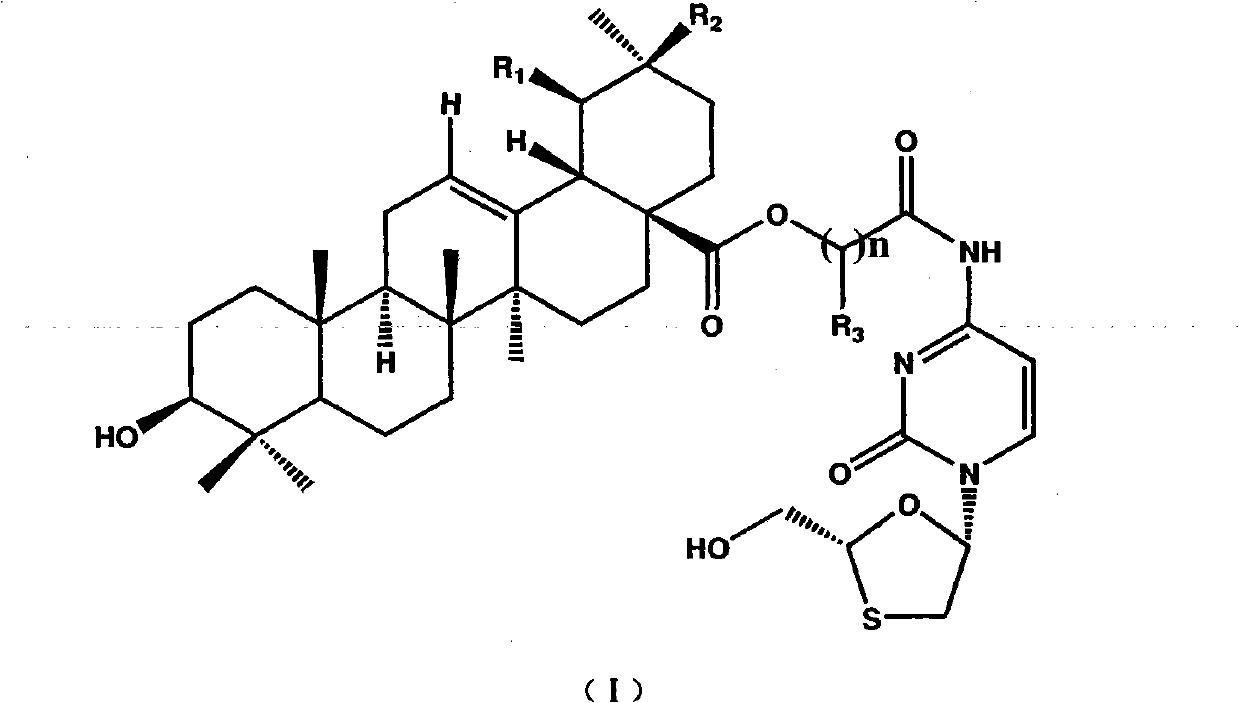
Preparation method and applications of lamivudine twin drug
A technology of lamivudine and twin drugs, applied in the application of hepatitis therapeutic drugs, the field of preparation of lamivudine twin drugs, can solve problems such as inability to effectively alleviate liver fibrosis in patients, achieve easy preparation and quality control, Effects that are less complex and easy to apply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 5 g of ursolic acid in 125 ml of DMF, add 1.17 ml of ethyl chloroacetate (or equal mol of ethyl bromoacetate), K 2 CO 3 4.5 g, stirred at room temperature for 5 hours. The insoluble solid was removed by filtration, the filtrate was concentrated under reduced pressure, and 30ml×3 toluene was used to carry out DMF to obtain a pale yellow clear oily liquid, which was the esterification product, which was directly used in the next reaction without purification.
[0024] Add 30ml of 4NNaOHaq, 50ml of methanol, and 75ml of THF to the above oily liquid, stir to obtain a pale yellow clear liquid, and react with stirring at room temperature for 18 hours. Concentrate under reduced pressure to obtain a pale pink solid, add 90ml of water, adjust the pH to 3 with 4N NaOHaq, the solid turns white and thicker, add 75ml of chloroform, stir until the solid dissolves, separate the liquids, and extract the water with 70ml of chloroform Layers were combined and the chloroform la...
Embodiment 2
[0026] Add 3 g of the above-mentioned hydrolyzed product into 30 ml of DMF, stir to dissolve, control the external temperature at -15°C to -20°C, add 0.81 ml of triethylamine, slowly add 1.2 g of isobutyl chloroformate dropwise, after the addition is complete, keep stirring for 20 minutes. A white solid precipitated and was used in the next step without treatment.
[0027] A solution of 1.337 g of lamivudine and 1.2 ml of triethylamine in 15 ml of DMF was prepared, and slowly added dropwise to the above reaction solution at an external temperature of -15°C to -20°C. After the dropwise addition was completed, the mixture was incubated and stirred for 1 hour, then raised to room temperature, and stirred for 1 hour. The insoluble matter was filtered off, the filtrate was concentrated under reduced pressure, and 30ml×4 toluene was used to carry out DMF, and a white paste was obtained, which was the crude product. Add 75 ml of chloroform to the above crude product, shake in a wate...
Embodiment 3
[0029] Operation is with embodiment 1,2;
[0030] Embodiment 4 is the lamivudine-ursolic acid gemini drug (LMX-3) of linking group with 3-chloro (bromo) ethyl propionate
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com