2-<18>F-fluoropropionic acid isomers, synthesis method and application thereof
A technology of S-18F-FPA and R-18F-FPA is applied in the application field of preparing positron emission tomography imaging agent drugs, which can solve the problem of difficult synthesis of chiral compounds, high difficulty of automatic synthesis, poor stereoselectivity, etc. problem, to achieve the effect of excellent pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
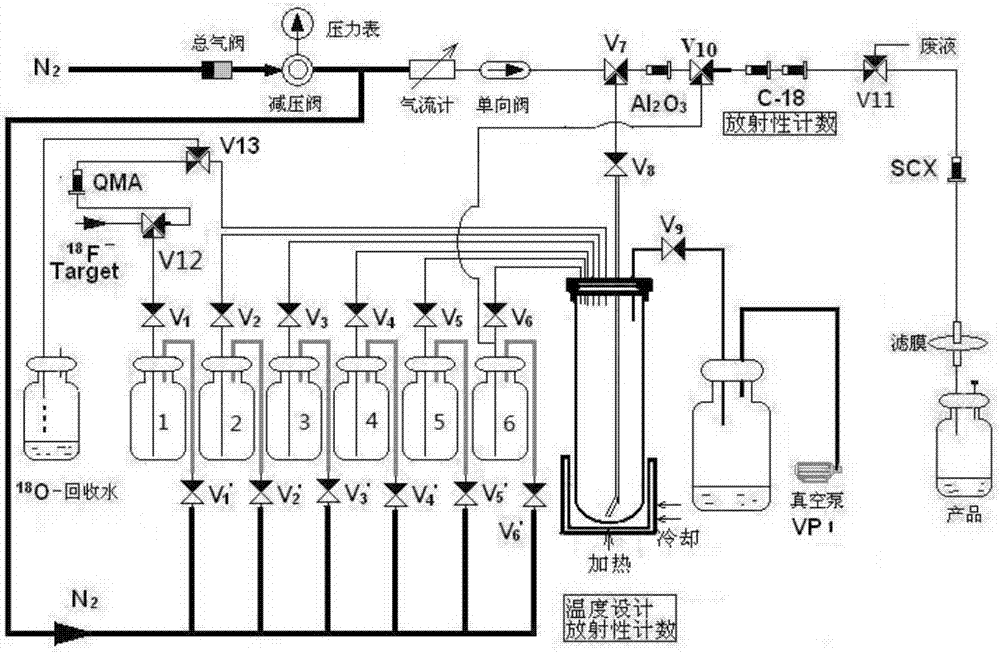
[0029] 1.1 Synthetic route
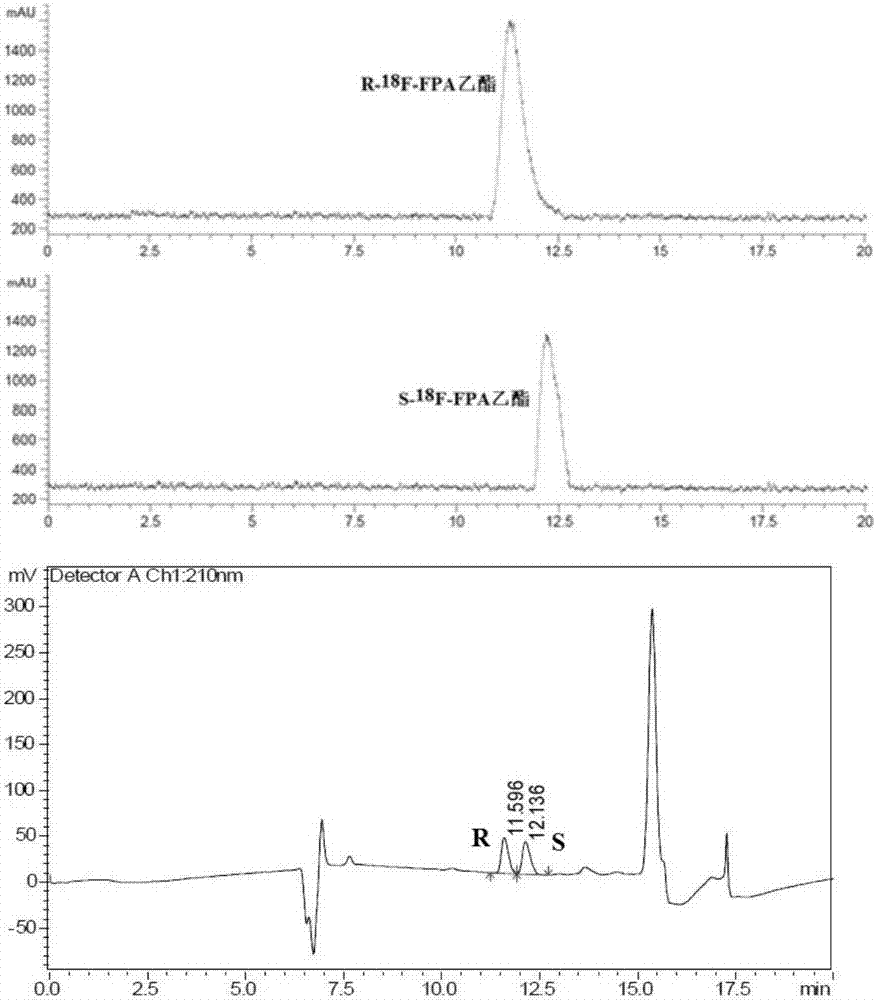
[0030] 2- of the present invention 18 F-fluoropropionic acid ( 18 Enantiomer of F-FPA): R-2- 18 F-fluoropropionic acid (R- 18 F-FPA) and S-2- 18 F-fluoropropionic acid (S- 18 F-FPA), using S- or R-2-trifluoromethanesulfonate ethyl propionate as a precursor respectively, can realize its fully automatic synthesis through two-step reactions of nucleophilic fluorination and column hydrolysis. S- or R-ethyl 2-trifluoromethanesulfonate propionate, in catalyst 4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8,8,8 ] Hexadecane (Kryptofix2.2.2, K222) under the action, and [K / K222] +18 f - A fluorination reaction occurs to generate the intermediate R-2- 18 F-Ethyl fluoropropionate or S-2- 18 F-Ethyl fluoropropionate. Dilute with water, pass through two Sep-Pak plus C18 columns, and the intermediate ethyl fluoropropionate is captured in the Sep-Pak plus C18 columns. Add sodium hydroxide solution to the small column, and the intermediate hydrolysis reacti...
Embodiment 2
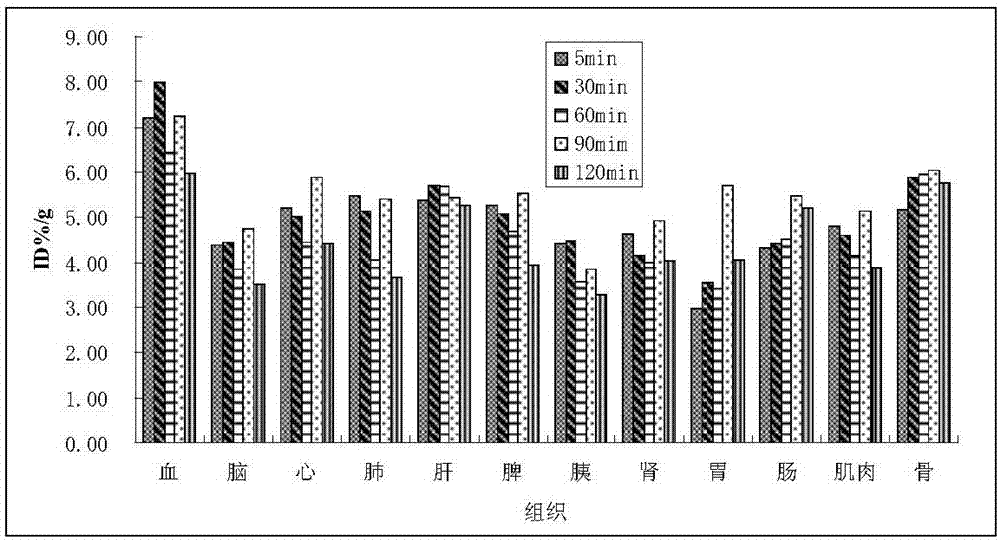
[0038] Example 2 R- 18 F-FPA and S- 18 F-FPA biodistribution test in vivo
[0039] 2.1 Biodistribution in vivo
[0040] Determination of R- 18 F-FPA and S- 18 Biodistribution of F-FPA in normal Kunming mice. Forty healthy Kunming mice weighing 20-25 g were randomly divided into 5 groups, with 4 mice at each time point. After intraperitoneal injection of 5% chloral hydrate (6mL / kg) to anesthetize mice, tail vein injection of 0.2mL containing 1.28-2.96MBq (40-80μCi) R- 18 F-FPA or S- 18 F-FPA injection was divided into groups at 5, 30, 60, 90 and 120 minutes after injection, and the mice were sacrificed by cervical dislocation after the eyeballs were removed to collect blood. After dissection, samples of tissues of interest such as brain, heart, lung, liver, spleen, pancreas, kidney, stomach, small intestine, right thigh muscle, and humerus were taken, weighed, and radioactive counts were measured with a gamma counter. All measured data were subtracted from the background...
Embodiment 3
[0043] Example 3 R- 18 F-FPA and S- 18 F-FPA small animal PET / CT imaging test
[0044] 3.1 Small animal PET / CT imaging
[0045] Screening of SPC-A-1 human lung adenocarcinoma model mice, small animal R- 18 F-FPA or S- 18 F-FPA PET-CT imaging. After injecting imaging agent (0.2mL, about 3.7MBq) through the tail vein for 10min, mice were anesthetized by intraperitoneal injection of 5% chloral hydrate (6mL / kg), fixed on a fixed board, and kept warm with a heating pad. After CT scanning, PET data were collected at different time points (30, 60, and 90 min). After attenuation correction, iterative reconstruction obtained cross-sectional, sagittal, coronal tomographic images and PET / CT fusion images. Use software (Inevon Research PET Workplace 4.1) to outline regions of interest (ROIs) such as tumor sites and muscles, and obtain ROIs by measuring the radioactive count and volume of tissue in the region of interest (the default density per gram of tissue is 1g / mL). Percent inje...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com