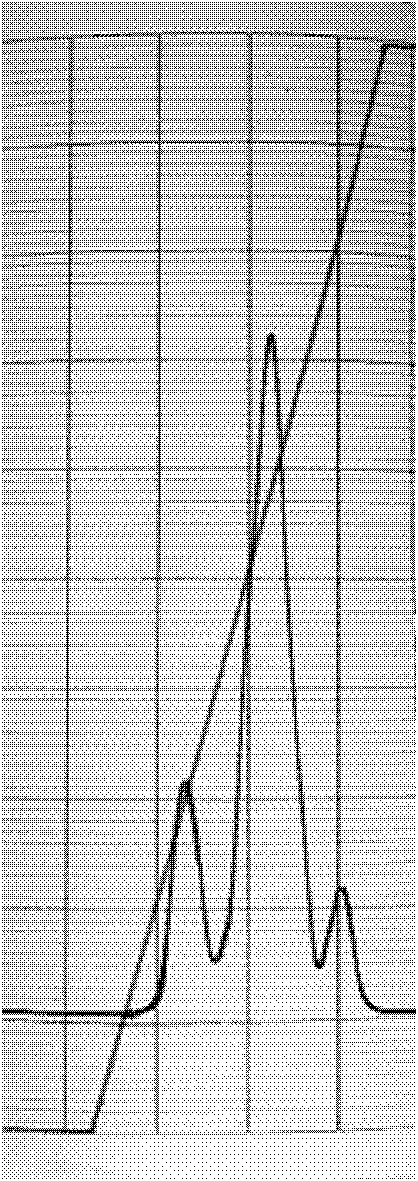Patents
Literature
226 results about "G-csf therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
G-CSF was first trialled as a therapy for neutropenia induced by chemotherapy in 1988. The treatment was well tolerated and a dose-dependant rise in circulating neutrophils was noted. A study in mice has shown that G-CSF may decrease bone mineral density.
G-csf site-specific mono-conjugates
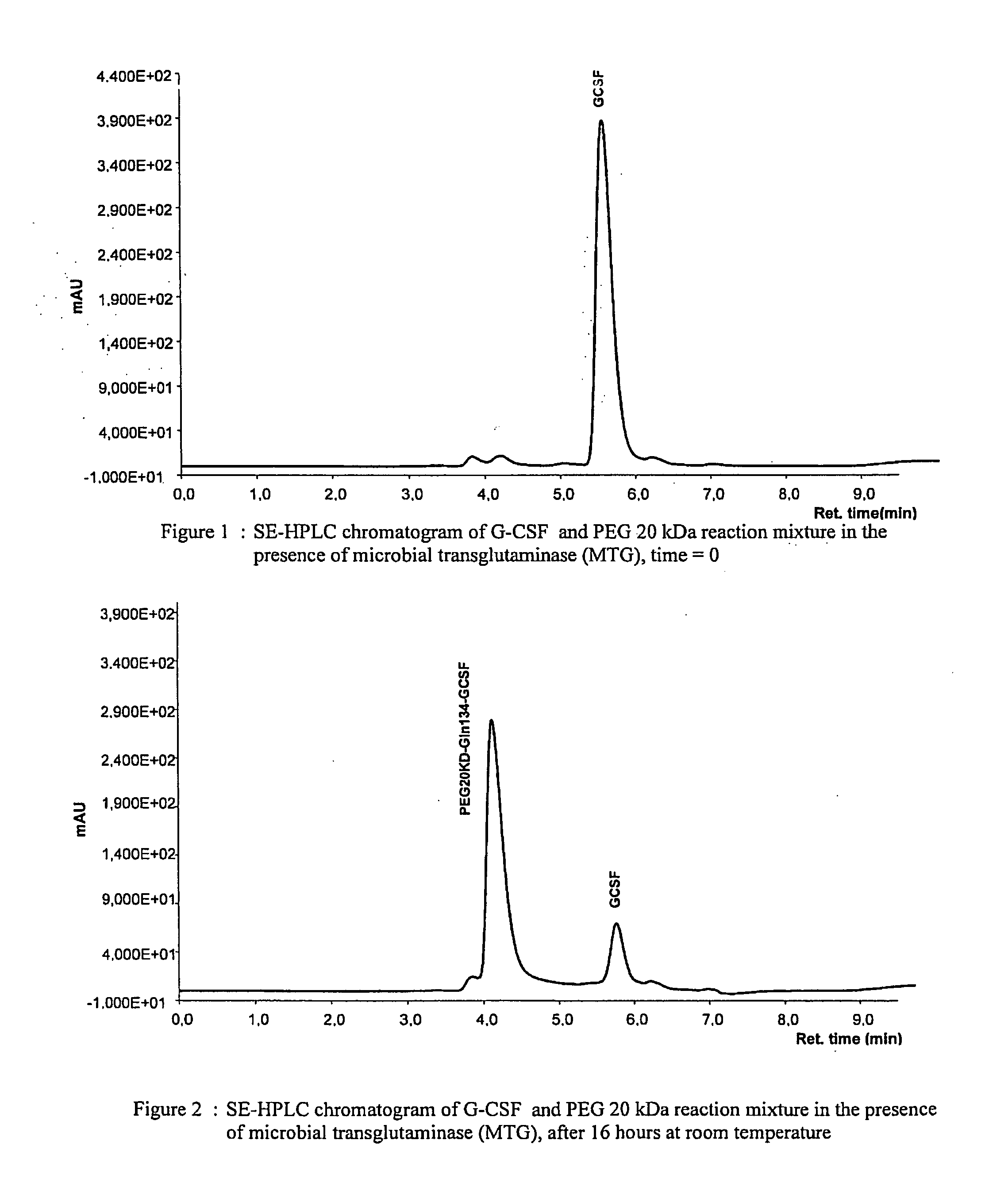
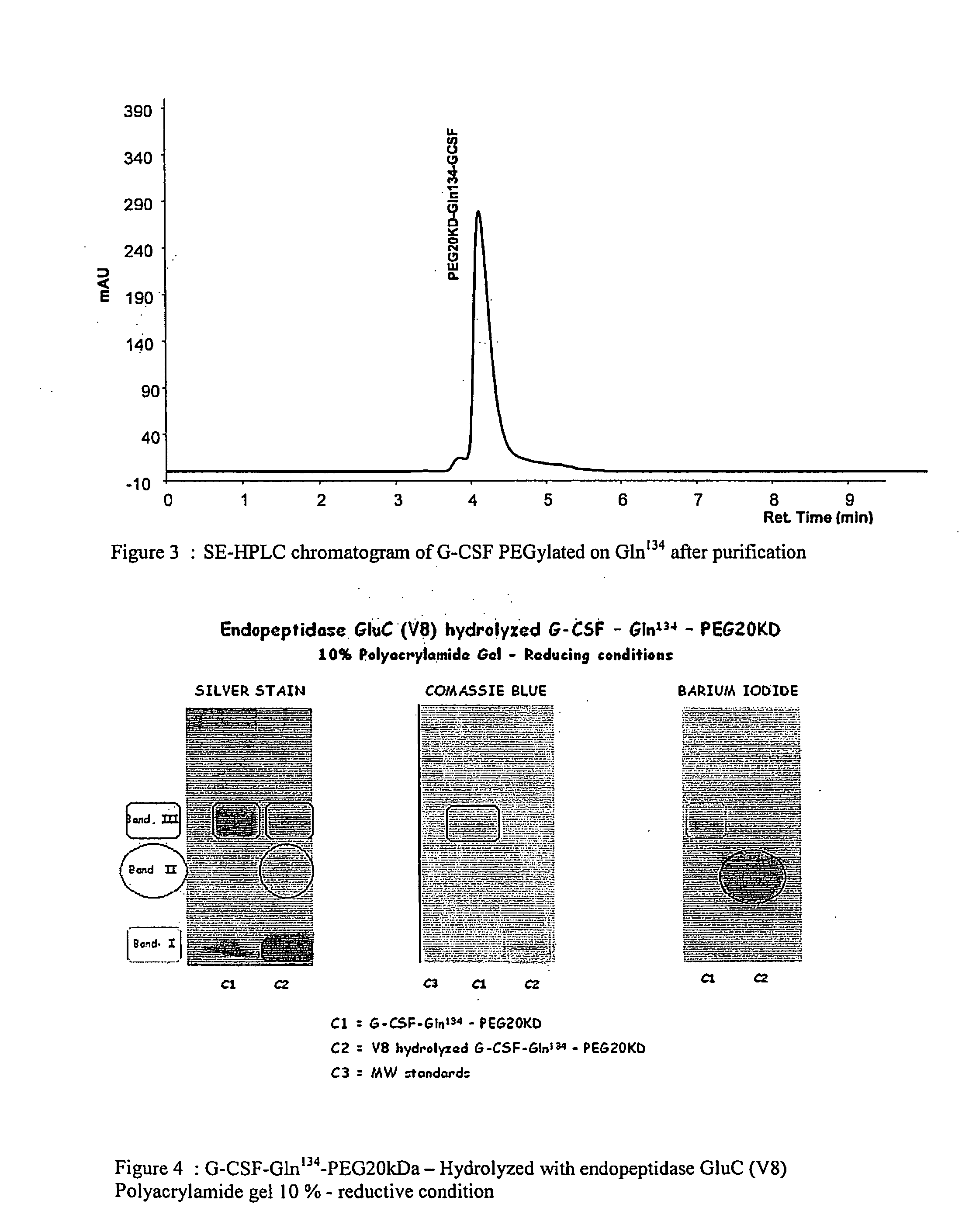
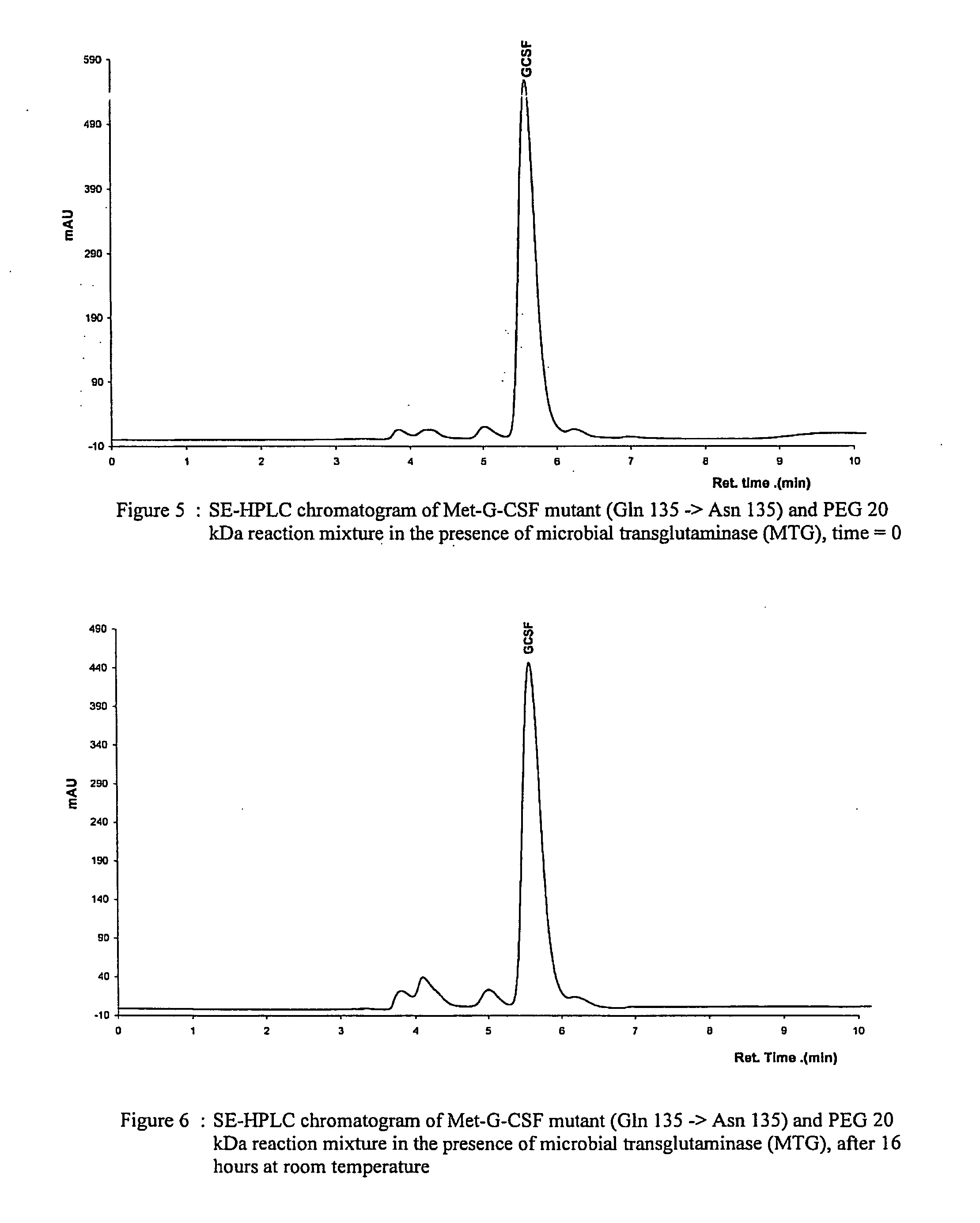
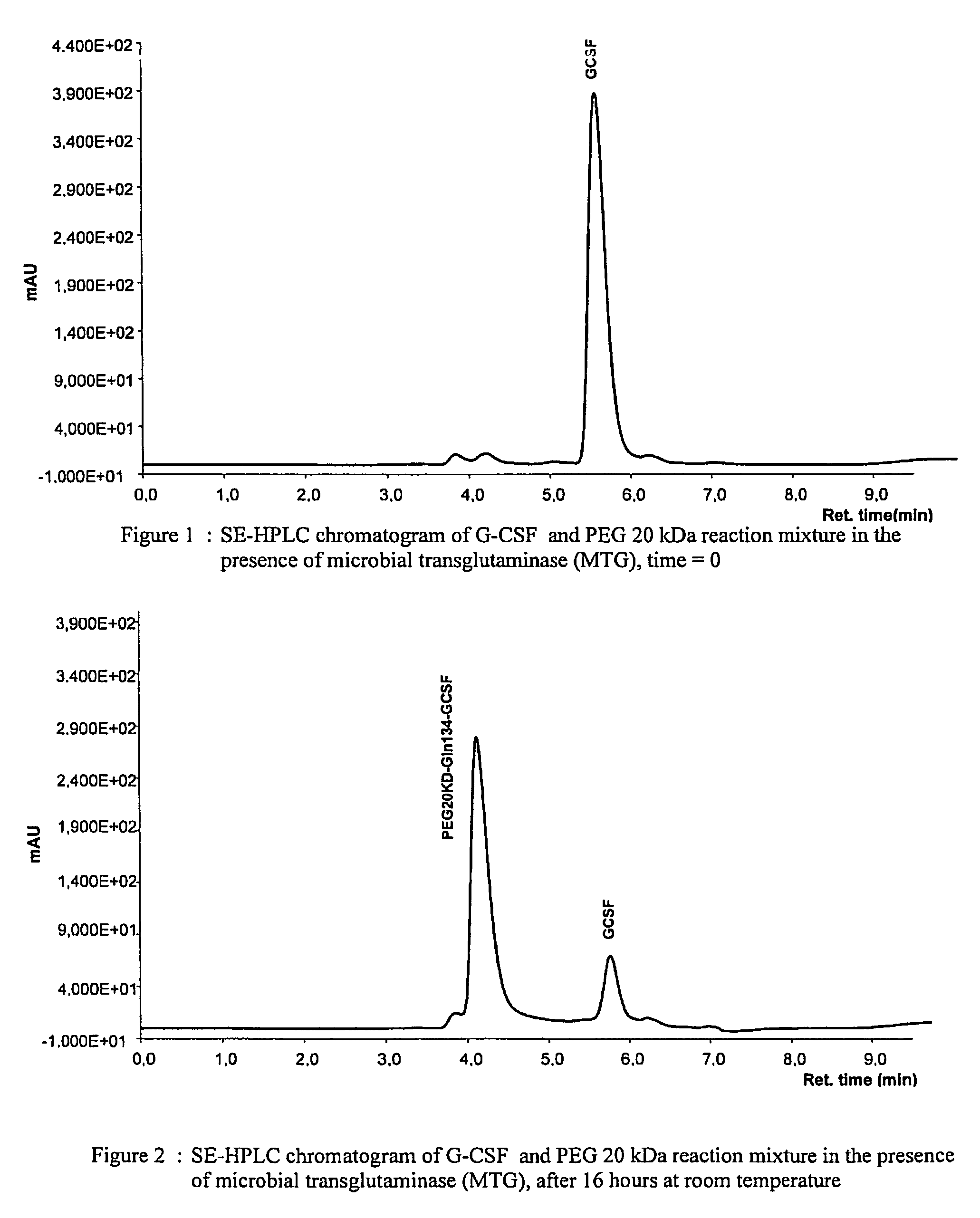
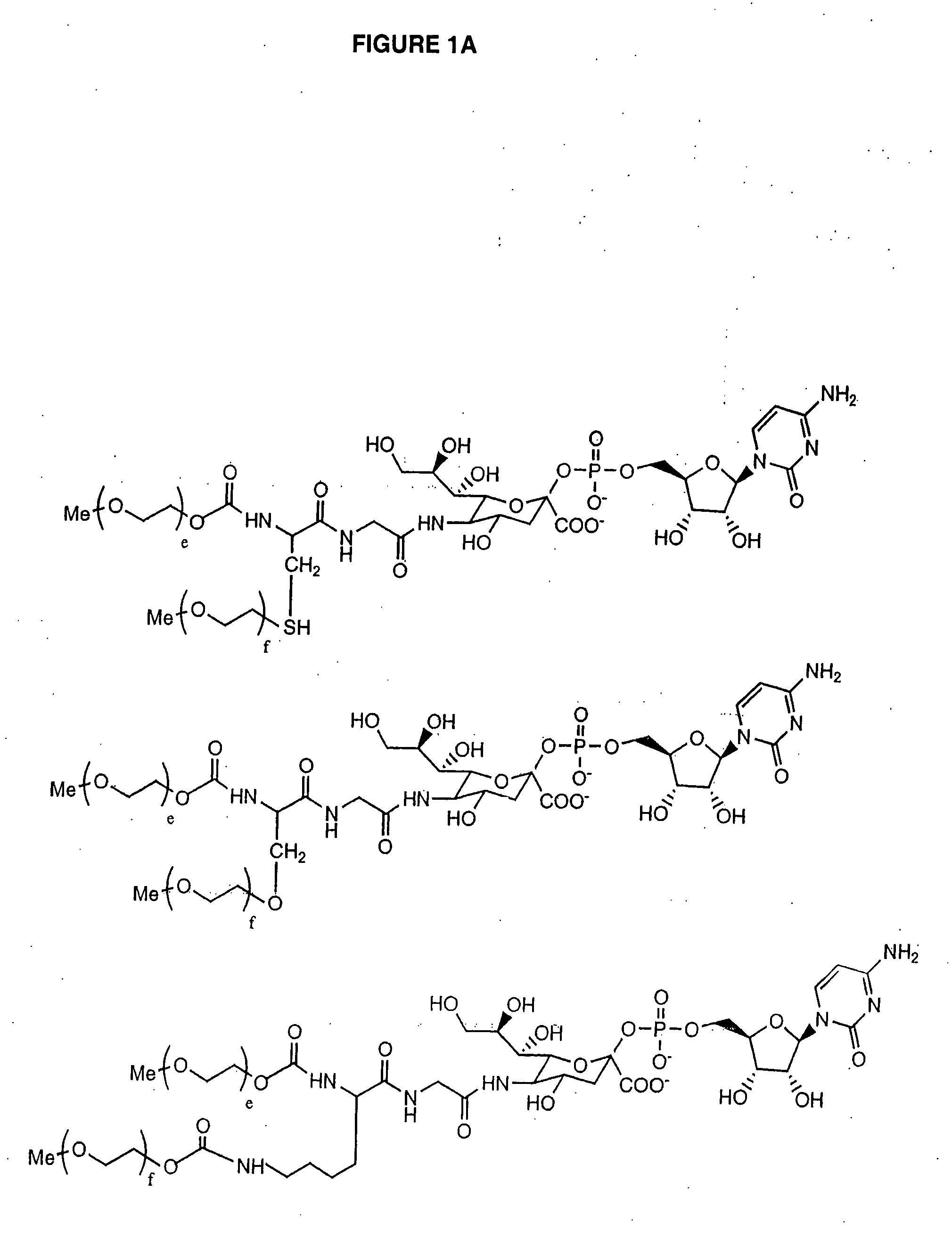
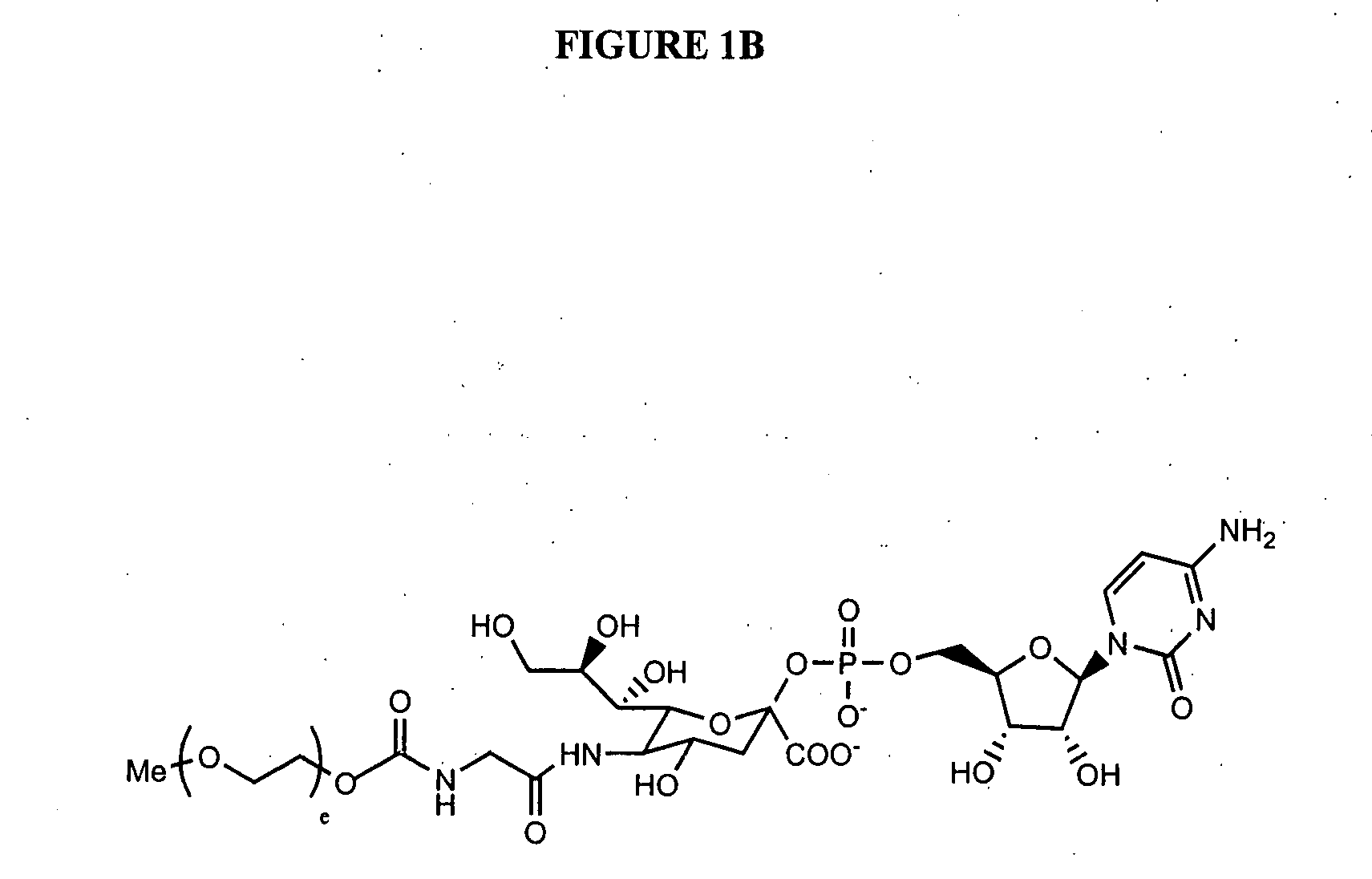
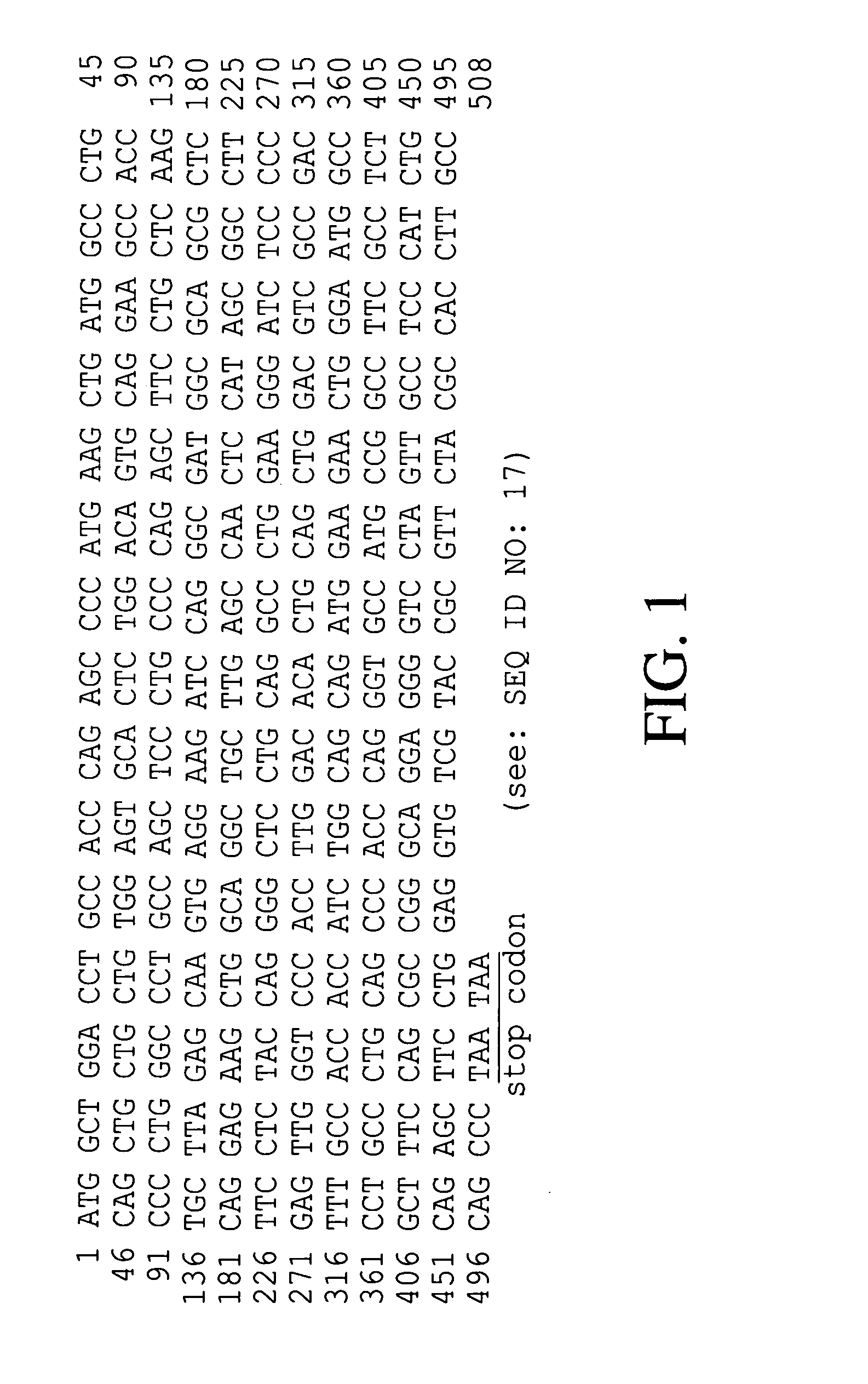
Novel site-specific mono-conjugates of Granulocyte Colony Stimulating Factor (G-CSF) are hereby described, with analogues and derivatives thereof, which stimulate proliferation and differentiation of progenitor cells to mature neutrophiles. These conjugates have been obtained using transglutaminase to covalently and site-specifically bind a hydrophilic, non-immunogenic polymer to a single glutamine residue of the human G-CSF native sequence and analogues thereof. These novel site-specific mono-conjugated derivatives are recommended for therapeutic use since they are stable in solution and exhibit significant biological activity in vitro and a longer bloodstream half-life, as compared to the non-conjugated protein, with a consequent prolonged pharmacological activity.
Owner:BIO KER
G-CSF site-specific mono-conjugates
Owner:BIO KER SRL
Methods of using G-CSF mobilized C-Kit+ cells in the production of embryoid body-like cell clusters for tissue repair and in the treatment of cardiac myopathy
InactiveUS20050186182A1Enhance mobilizationInhibition of differentiationBiocideMammal material medical ingredientsMyopathyTissue repair
The present invention relates to methods of using granulocyte colony stimulating factor (G-CSF) polypeptide, alone and in conjunction with stromal cell derived factor (SDF-1) polypeptide, to increase the mobilization of c-Kit+ stem cells in the blood, bone marrow, tissue, heart or other organs for the subsequent production of embryoid body-like cell clusters. These embryoid body-like cell clusters can be used for cell replacement therapy, for the treatment of cardiac myopathy and other diseases and disorders, and for screening agents that drive or inhibit differentiation and proliferation.
Owner:AMGEN INC
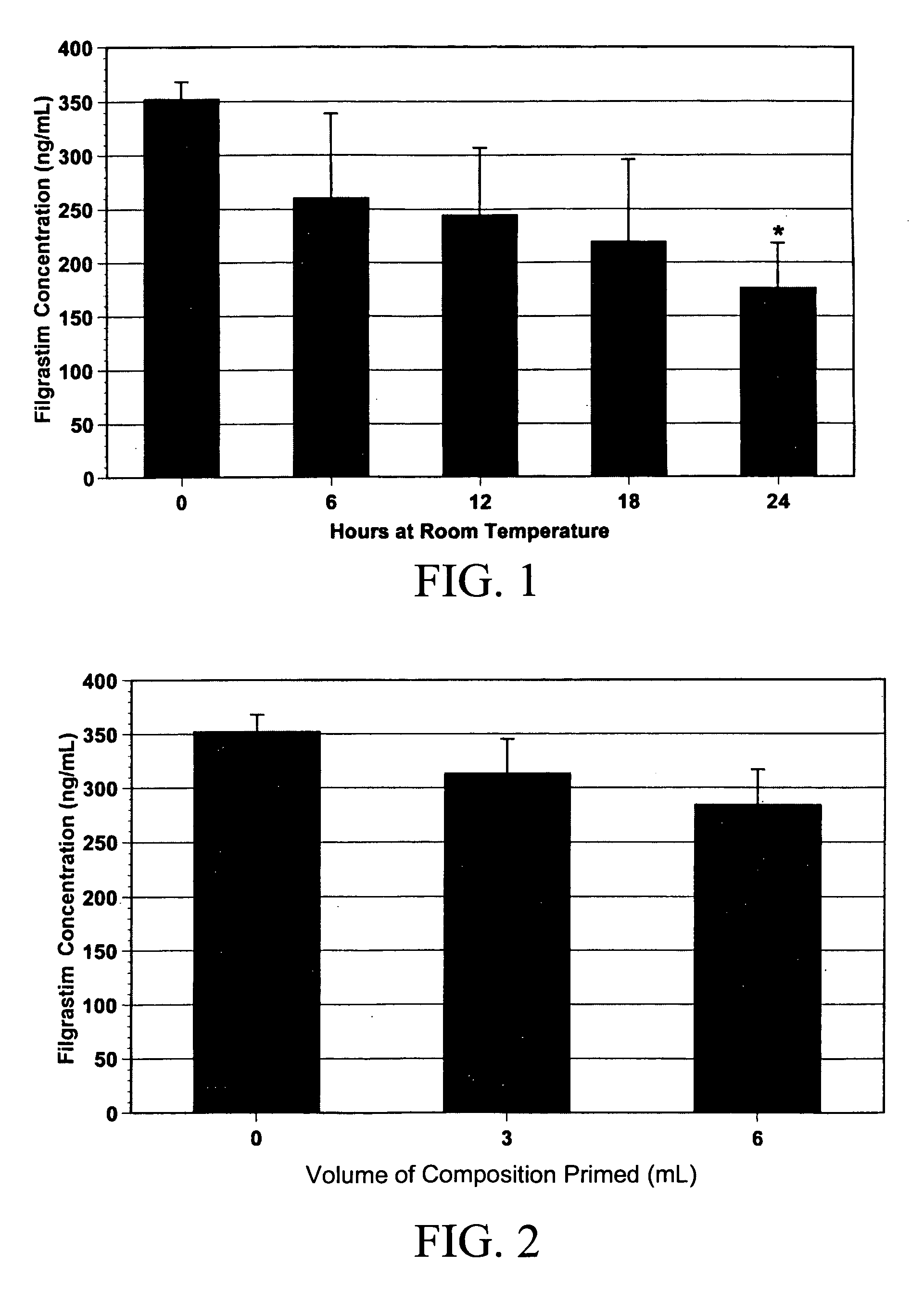
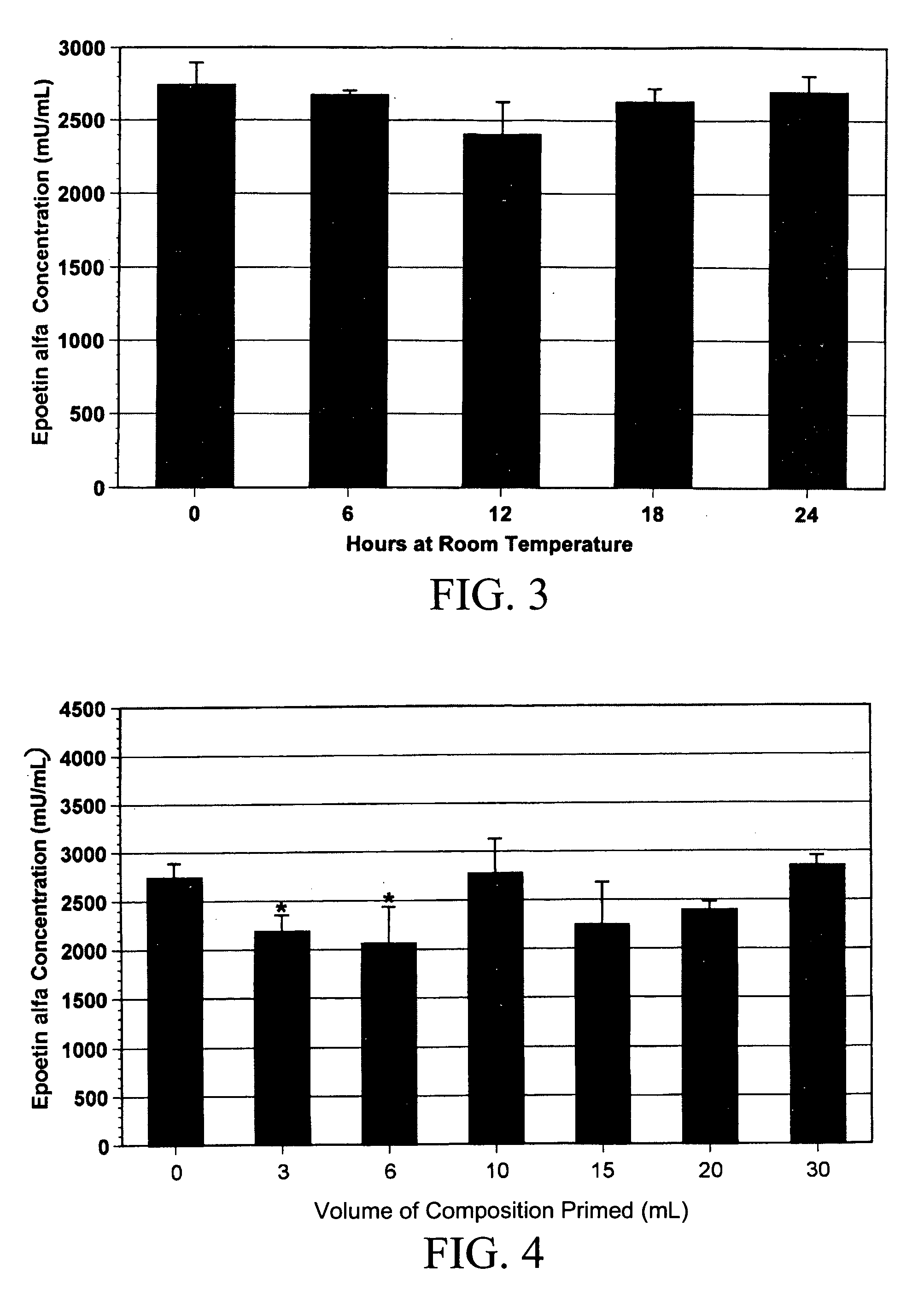
Liquid Formulation of G-CSF Conjugate
ActiveUS20090143292A1Prevents acid hydrolysisImprove solution stabilityPeptide/protein ingredientsPharmaceutical delivery mechanismG-csf therapySuccinic acid
The present invention relates to a liquid pharmaceutical composition comprising a granulocyte colony stimulating factor polypeptide conjugated with a polymer. In various embodiments, the composition has a pH value in the range of 4.5 to 5.5. Exemplary compositions further comprise a surfactant and optionally one or more other pharmaceutically acceptable excipients. The invention provides, inter alia, formulations free from tartaric acid or salts thereof and / or from succinic acid and salts thereof as buffering agents. Exemplary formulations are essentially devoid of not amino acids as stabilizers. The composition has good storage stability and is especially useful for the prophylaxis and treatment of disorders and medical indications where granulocyte colony stimulating factor preparations are considered as useful remedies.
Owner:RATIOPHARM GMBH
Method of isolating and culturing mesenchymal stem cell derived from umbilical cord blood
InactiveUS20070092967A1Increase success rateArtificial cell constructsSkeletal/connective tissue cellsInterleukin 6G-csf therapy
The present invention relates to a method of isolating and culturing mesenchymal stem cells using umbilical cord blood that is most ideal for cell therapy. The method comprises adding an anti-coagulant to umbilical cord blood having a volume of more than 45 ml per unit, which is obtained within 24 hours after parturition; diluting the resulting mixture of the anti-coagulant and umbilical cord blood with an αMEM (alpha-minimum essential medium), followed by centrifugation to harvest monocytes; and subjecting the obtained monocytes into suspension culture in the αMEM containing Stem Cell Factor, GM-CSF (granulocyte-macrophage colony-stimulating factor), G-CSF (granulocyte colony-stimulating factor), IL-3 (interleukin-3) and IL-6 (interleukin-6).
Owner:HAN HOON
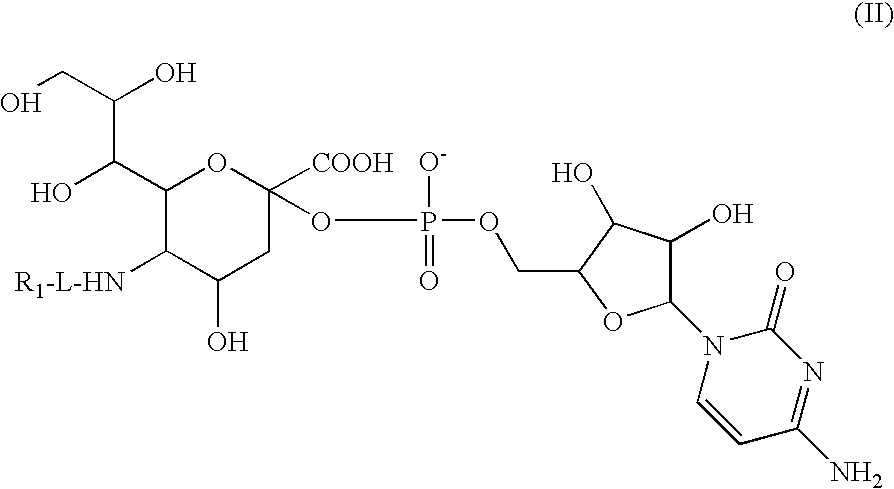
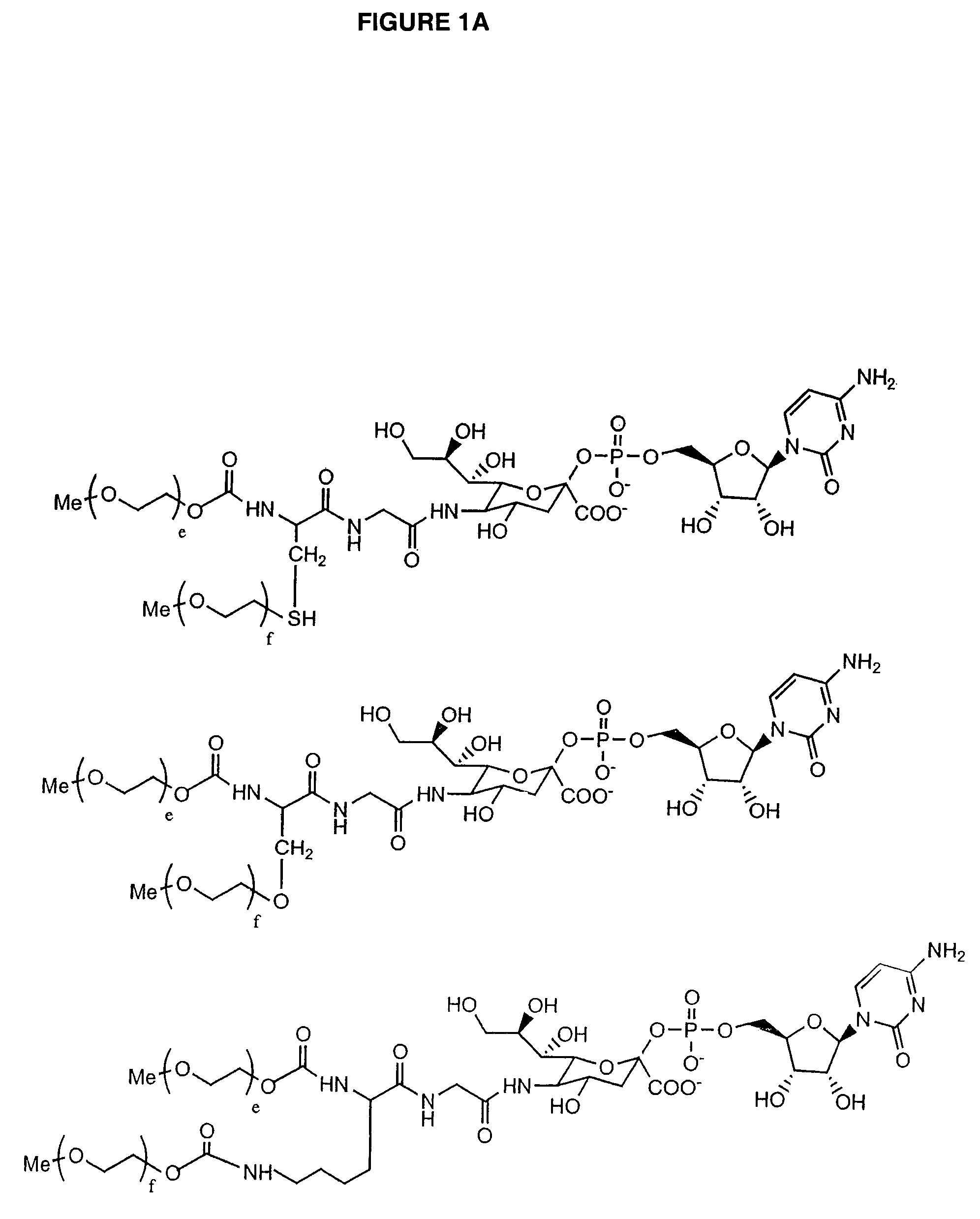
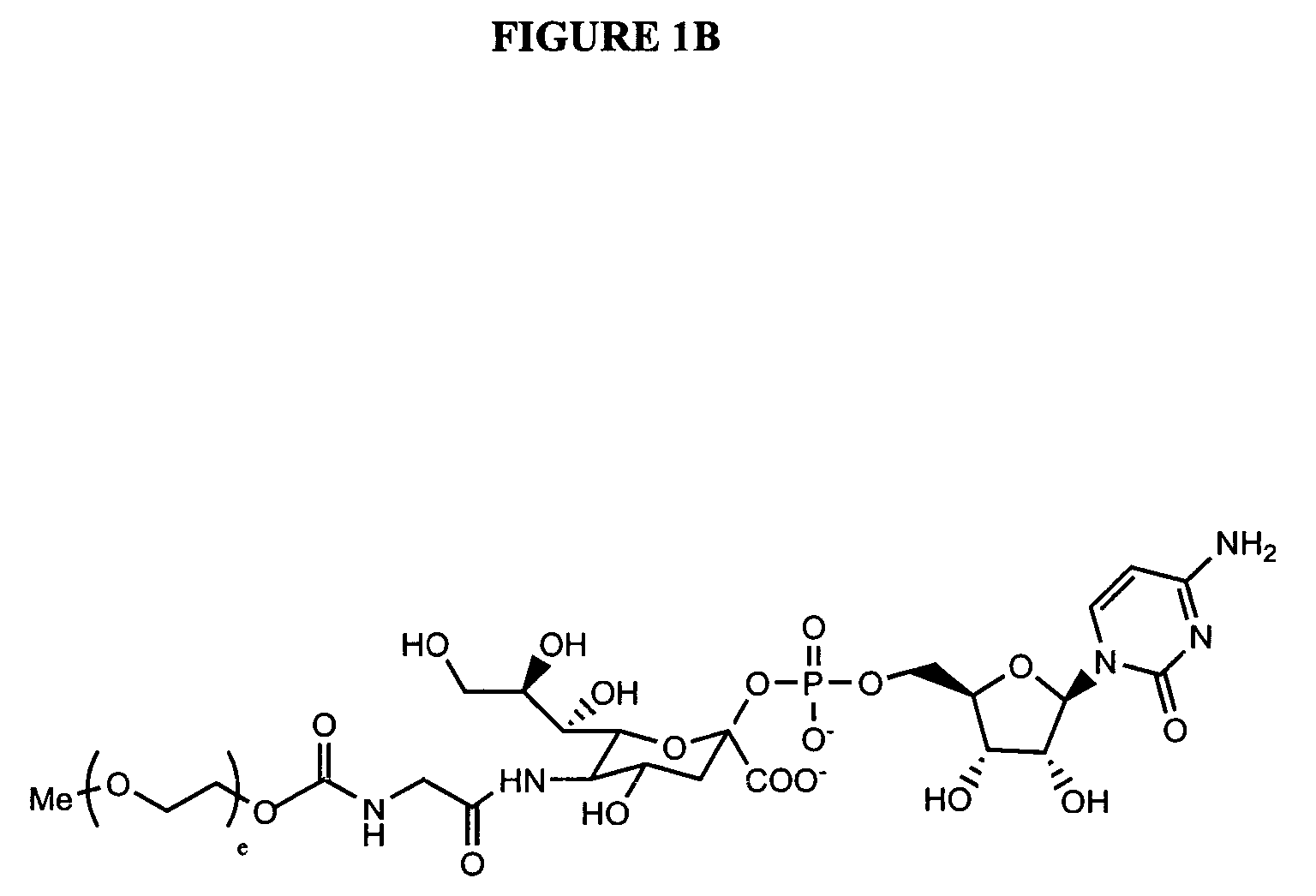
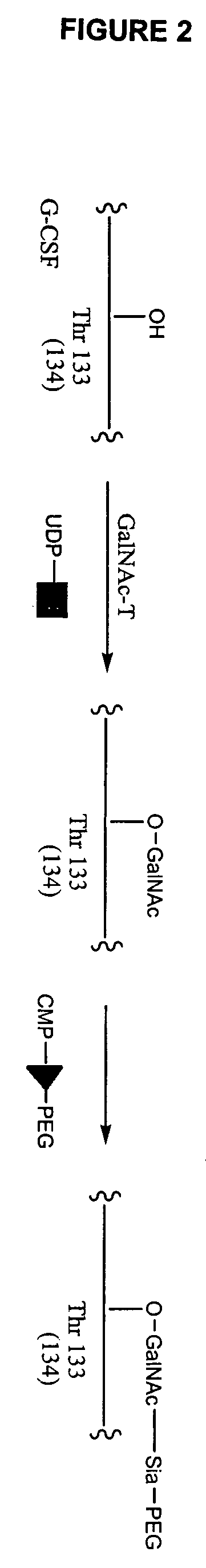
Glycopegylated Granulocyte Colony Stimulating Factor
InactiveUS20090203579A1Improved pharmacokinetic propertiesPeptide/protein ingredientsPeptide preparation methodsColony-stimulating factorG-csf therapy
The present invention provides conjugates between Granulocyte Colony Stimulating Factor and PEG moieties. The conjugates are linked via an intact glycosyl linking group that is interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from both glycosylated and unglycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto either an amino acid or glycosyl residue on the peptide. Also provided are pharmaceutical formulations including the conjugates. Methods for preparing the conjugates are also within the scope of the invention.
Owner:NOVO NORDISK AS
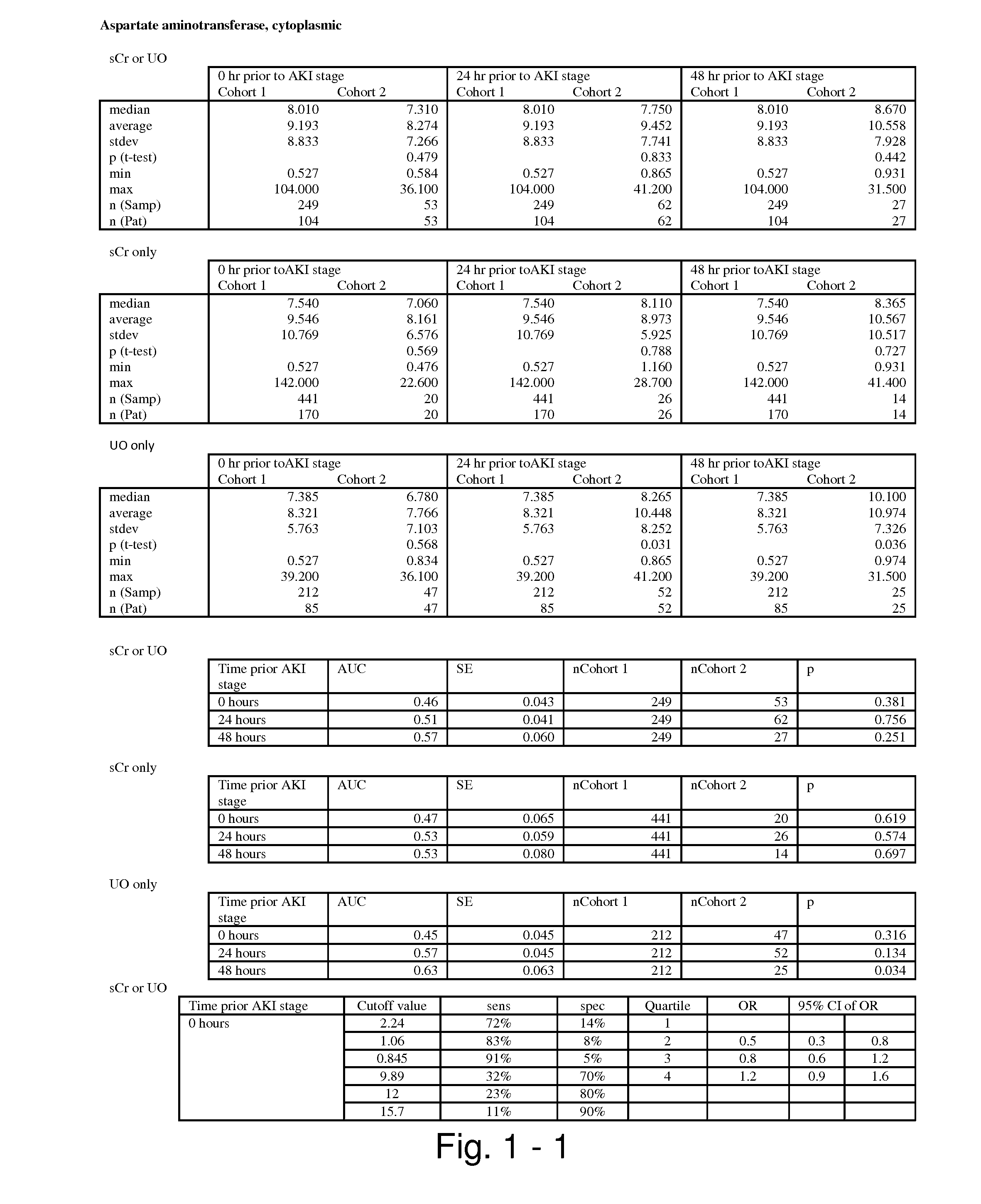
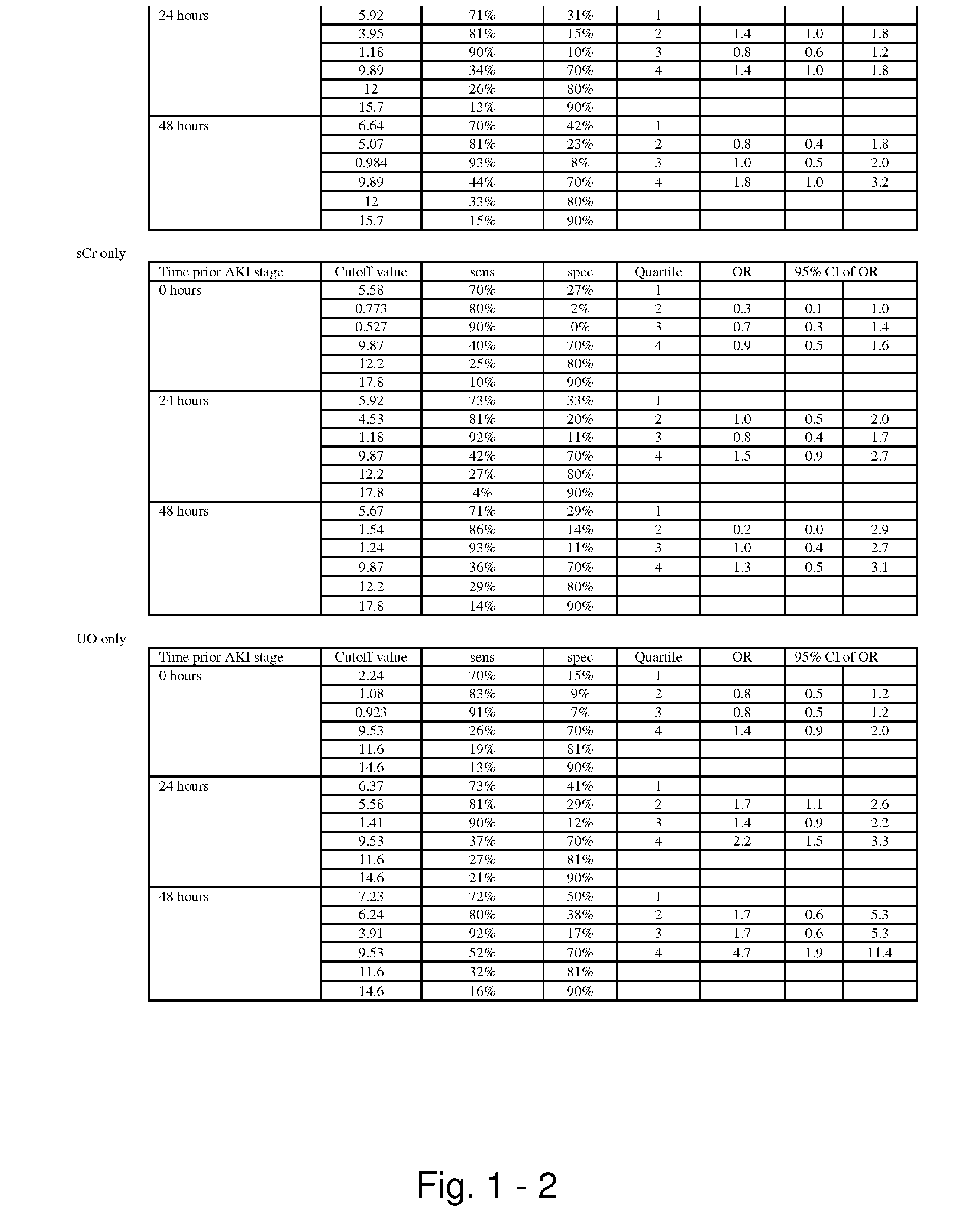
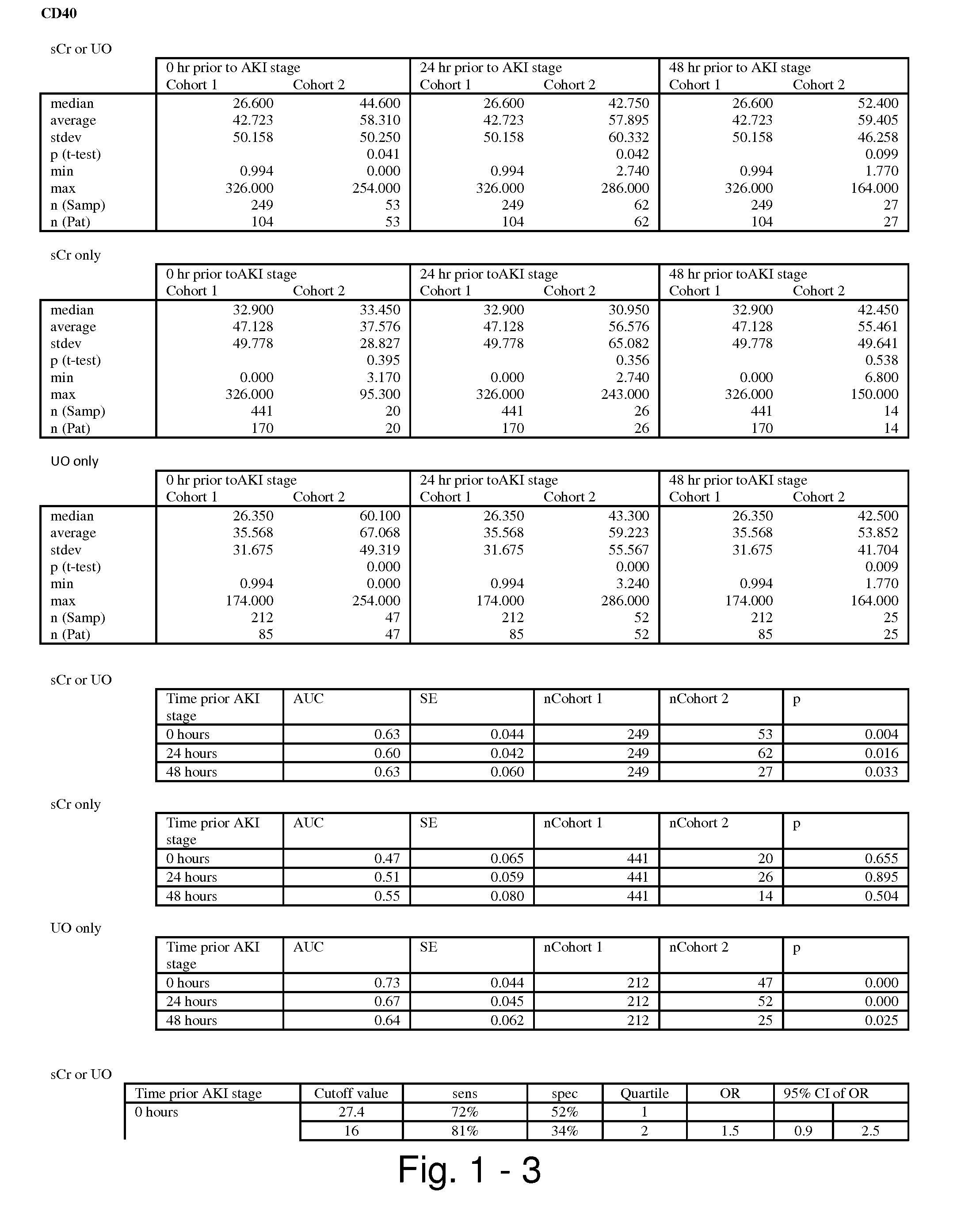
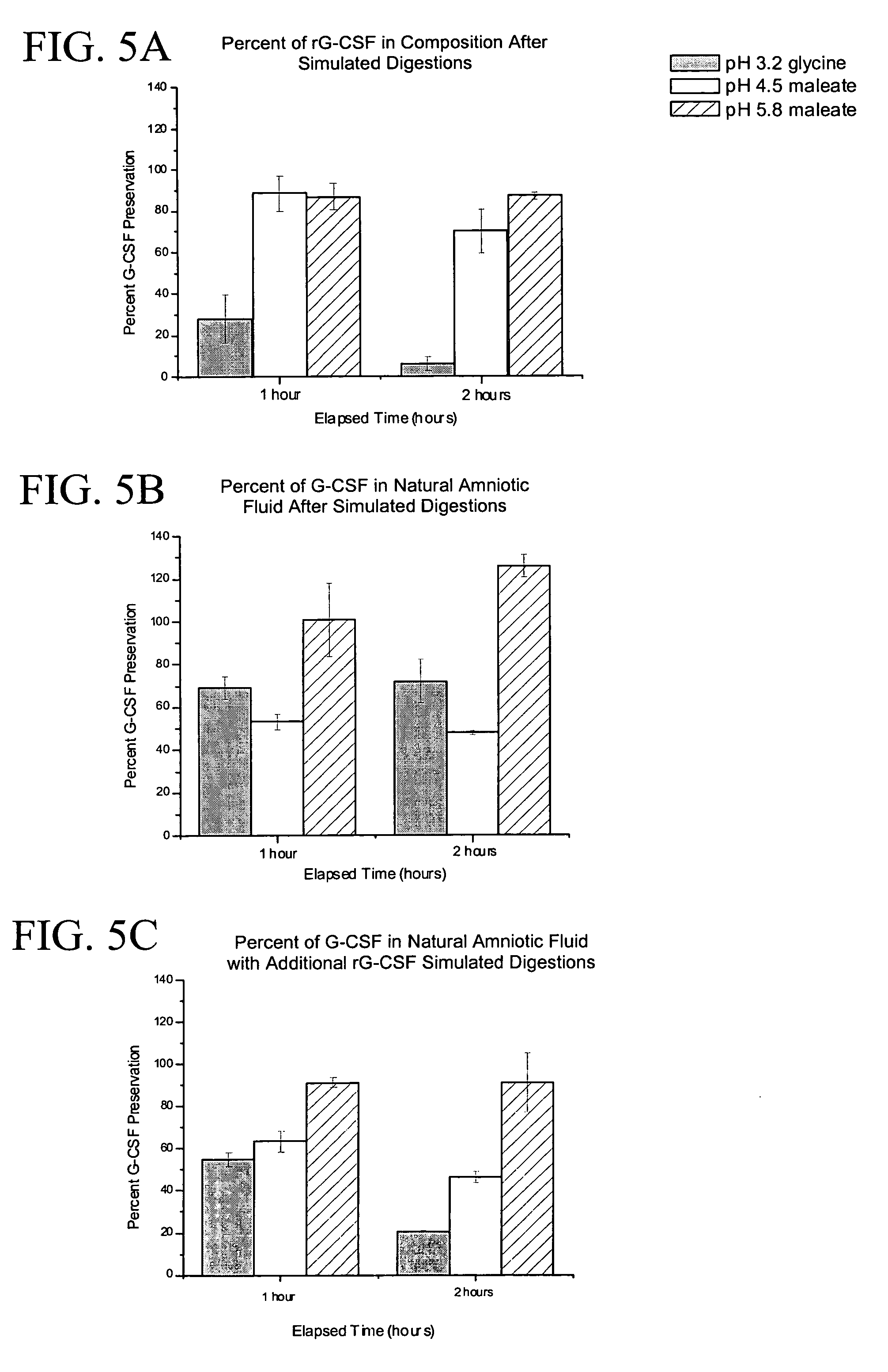
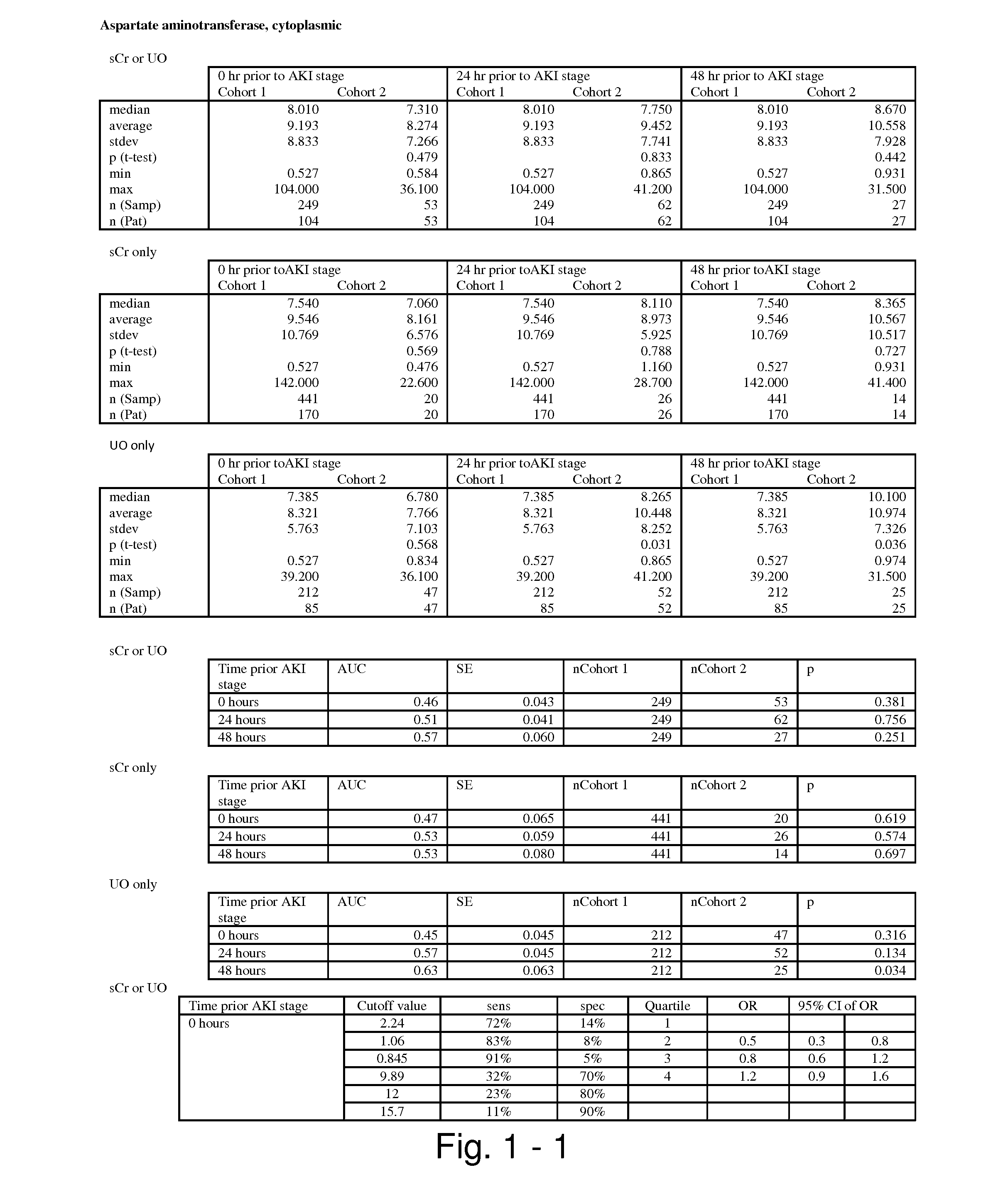
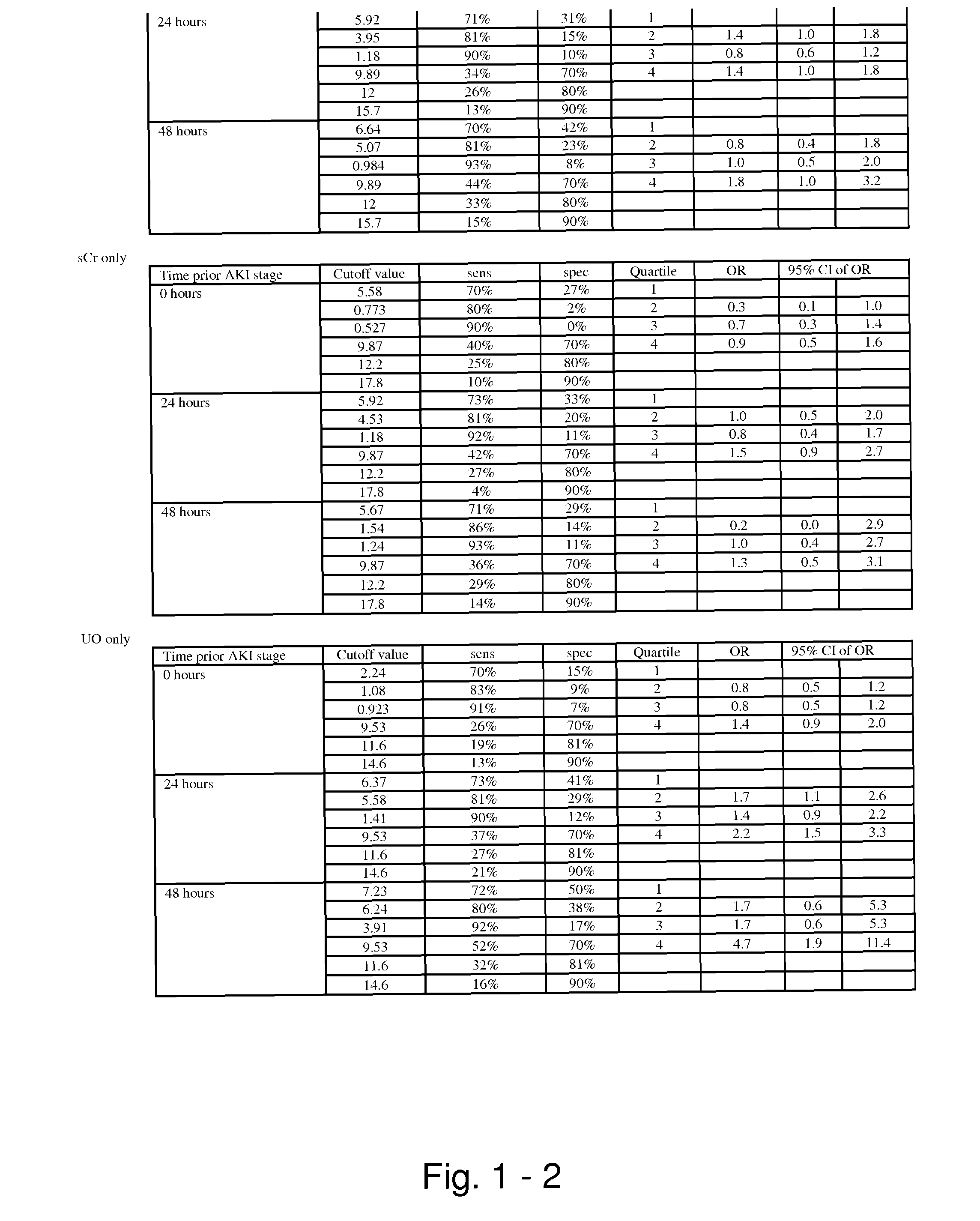
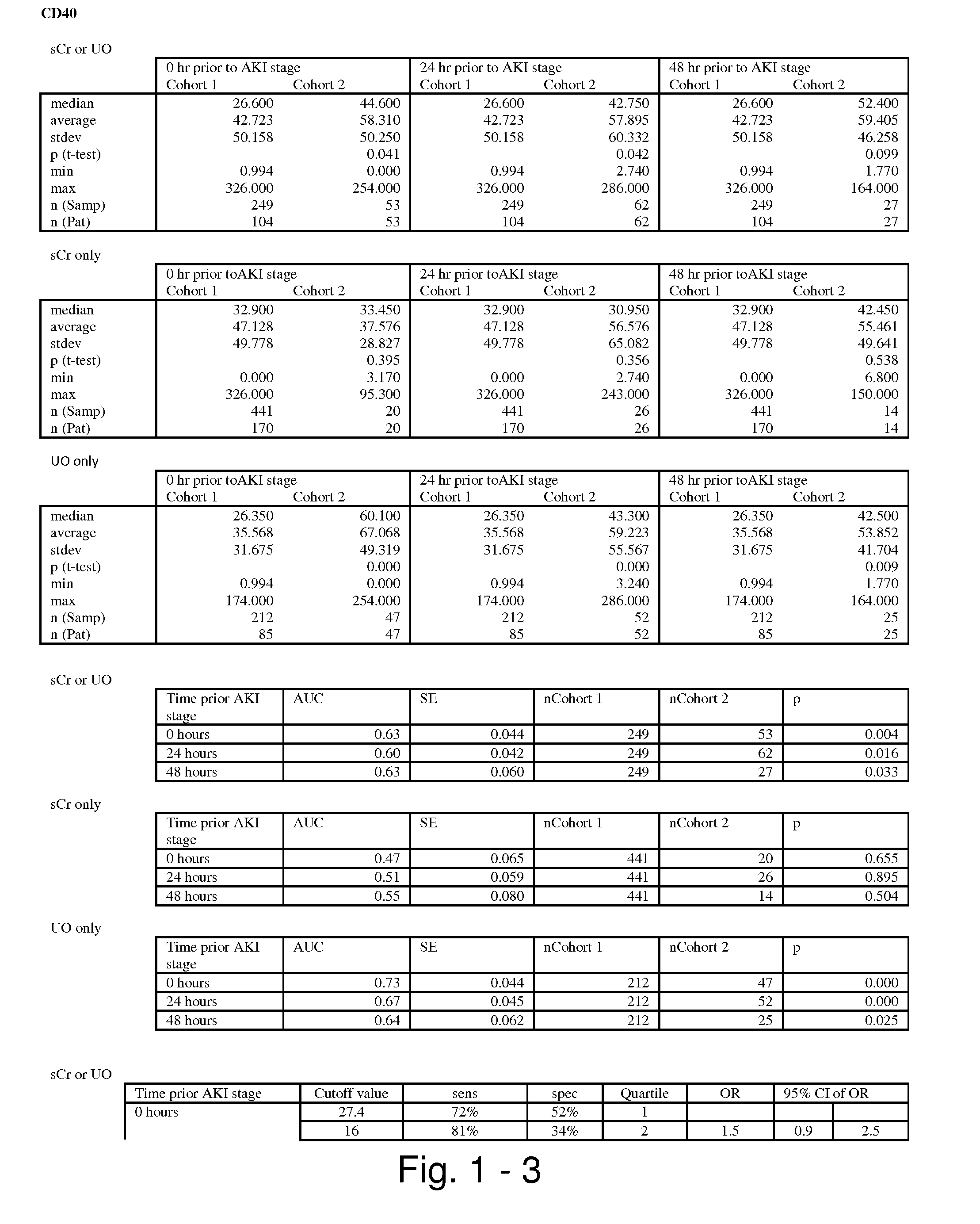
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS20110201038A1Easy to adaptMicrobiological testing/measurementDisease diagnosisInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Glycopegylated granulocyte colony stimulating factor
ActiveUS7956032B2Improved pharmacokinetic propertiesPeptide/protein ingredientsFermentationColony-stimulating factorG-csf therapy
The present invention provides conjugates between Granulocyte Colony Stimulating Factor and PEG moieties. The conjugates are linked via an intact glycosyl linking group that is interposed between and covalently attached to the peptide and the modifying group. The conjugates are formed from both glycosylated and unglycosylated peptides by the action of a glycosyltransferase. The glycosyltransferase ligates a modified sugar moiety onto either an amino acid or glycosyl residue on the peptide. Also provided are pharmaceutical formulations including the conjugates. Methods for preparing the conjugates are also within the scope of the invention.
Owner:NOVO NORDISK AS
Glycosylated G-CSF
InactiveUS20070243165A1Altered phenotypeVirusesPeptide/protein ingredientsG-csf therapyEvolutionary biology
Granulocyte colony stimulating factor obtained from eggs laid by transgenic avians having newly described G-CSF glycosylation patterns.
Owner:GEORGIA RESERACH FOUND INC UNIV OF +1
Liquid formulation of G-CSF conjugate
ActiveUS8207112B2Prevents acid hydrolysisImprove solution stabilityPeptide/protein ingredientsPharmaceutical delivery mechanismSuccinic acidG-csf therapy
Owner:RATIOPHARM GMBH
External Agent for Treatment of Skin Ulcer
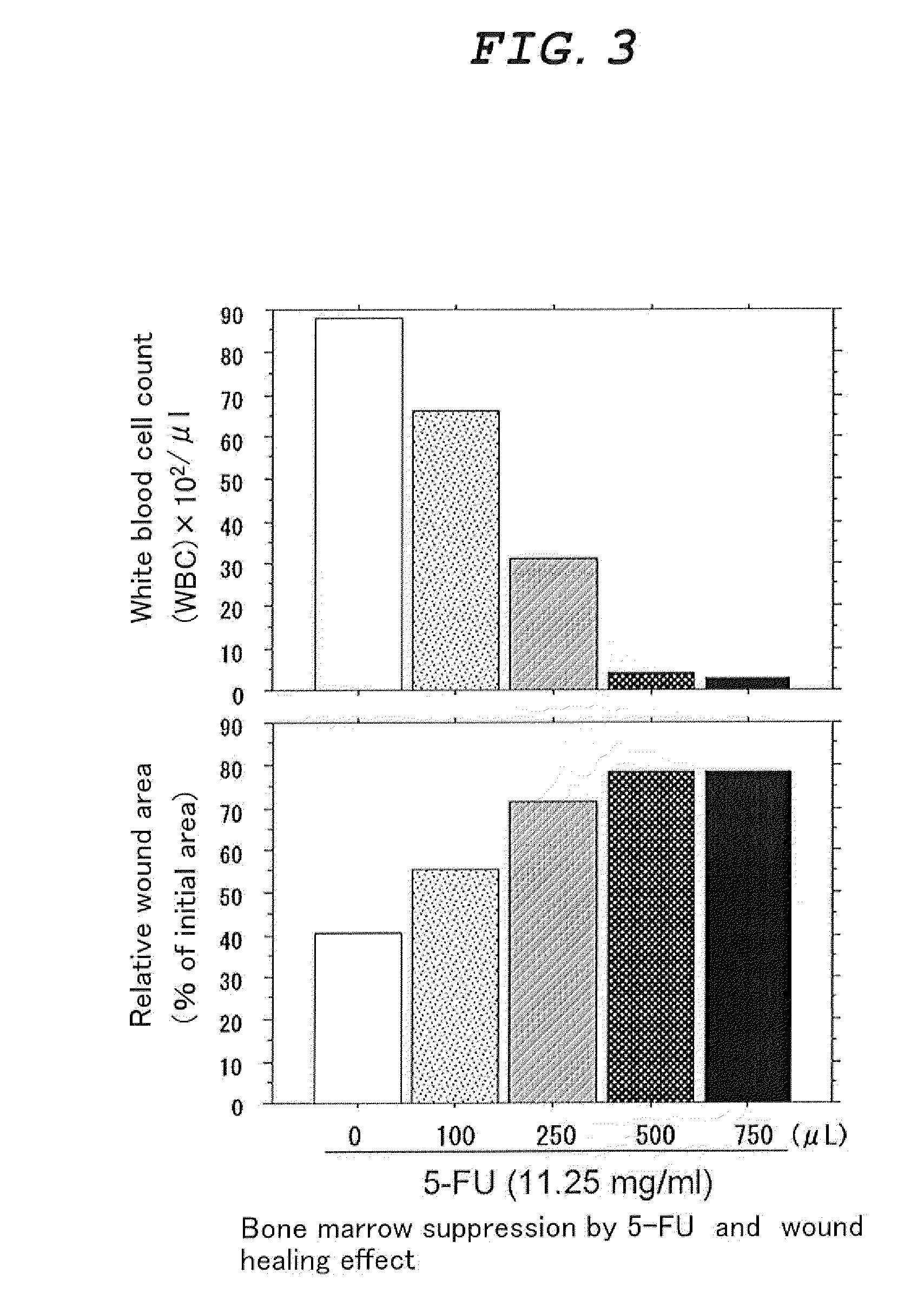
InactiveUS20090285785A1Good curative effectBioreactor/fermenter combinationsBiological substance pretreatmentsStromal cellG-csf therapy
[Problems] To provide a novel external agent for treatment of skin ulcer which has an excellent healing effect on intractable skin ulcer such as bedsore, diabetic skin ulcer and ischemic skin ulcer.[Means for Solving Problems] The agent is characterized in that it comprises a composition containing at least one selected from the group consisting of granulocyte colony stimulating factor (G-CSF), stromal cell-derived factor-1 (SDF-1) and CD41-positive cells, and a hydrophilic high molecular substance.
Owner:CELLGENTECH
G-CSF therapy as an adjunct to reperfusion therapy in the treatment of acute myocardial infarction
InactiveUS7220407B2Minimize damageImprove patient outcomesPeptide/protein ingredientsDead animal preservationReperfusion therapyG-csf therapy
The present invention relates to methods of using Granulocyte Colony Stimulating Factor (G-CSF) polypeptide in conjunction with reperfusion therapy in the treatment of acute myocardial infarction or other ischemic events. This treatment can be used alone or in combination with other well-known methods of treatment.
Owner:NORTHWESTERN UNIV
Recombinant human granulocyte colony stimulating factor and one-step purifying process for chemical modifications thereof
InactiveCN1663962AHigh purityCarrier-bound/immobilised peptidesCytokines/lymphokines/interferonsG-csf therapyHigh activity
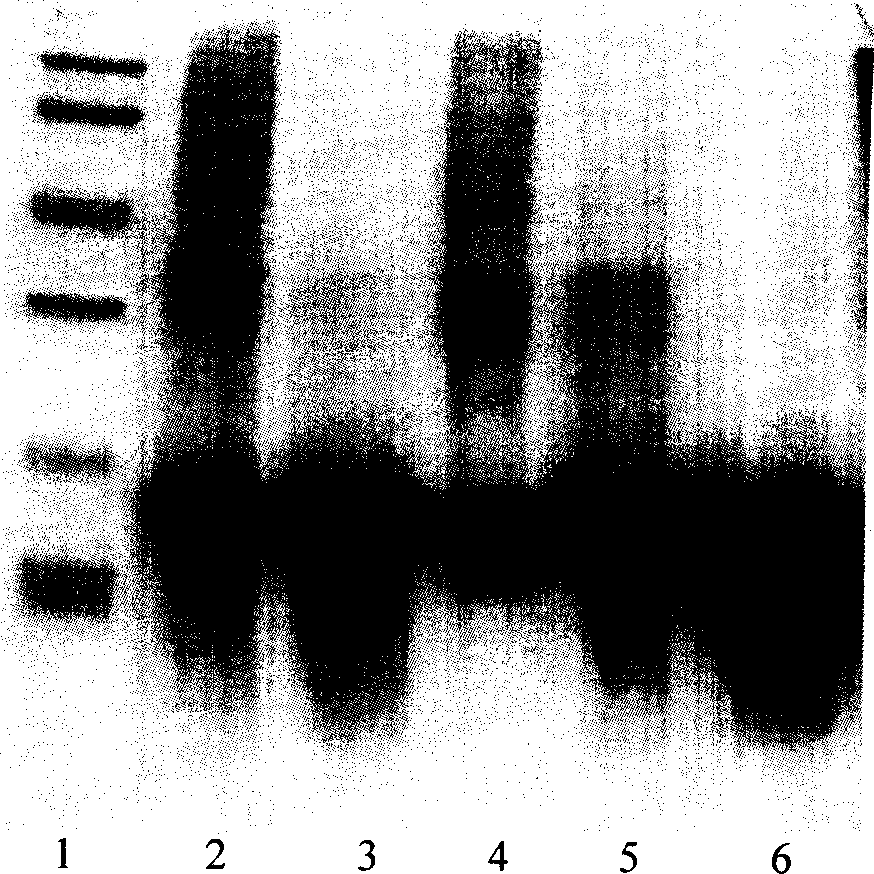
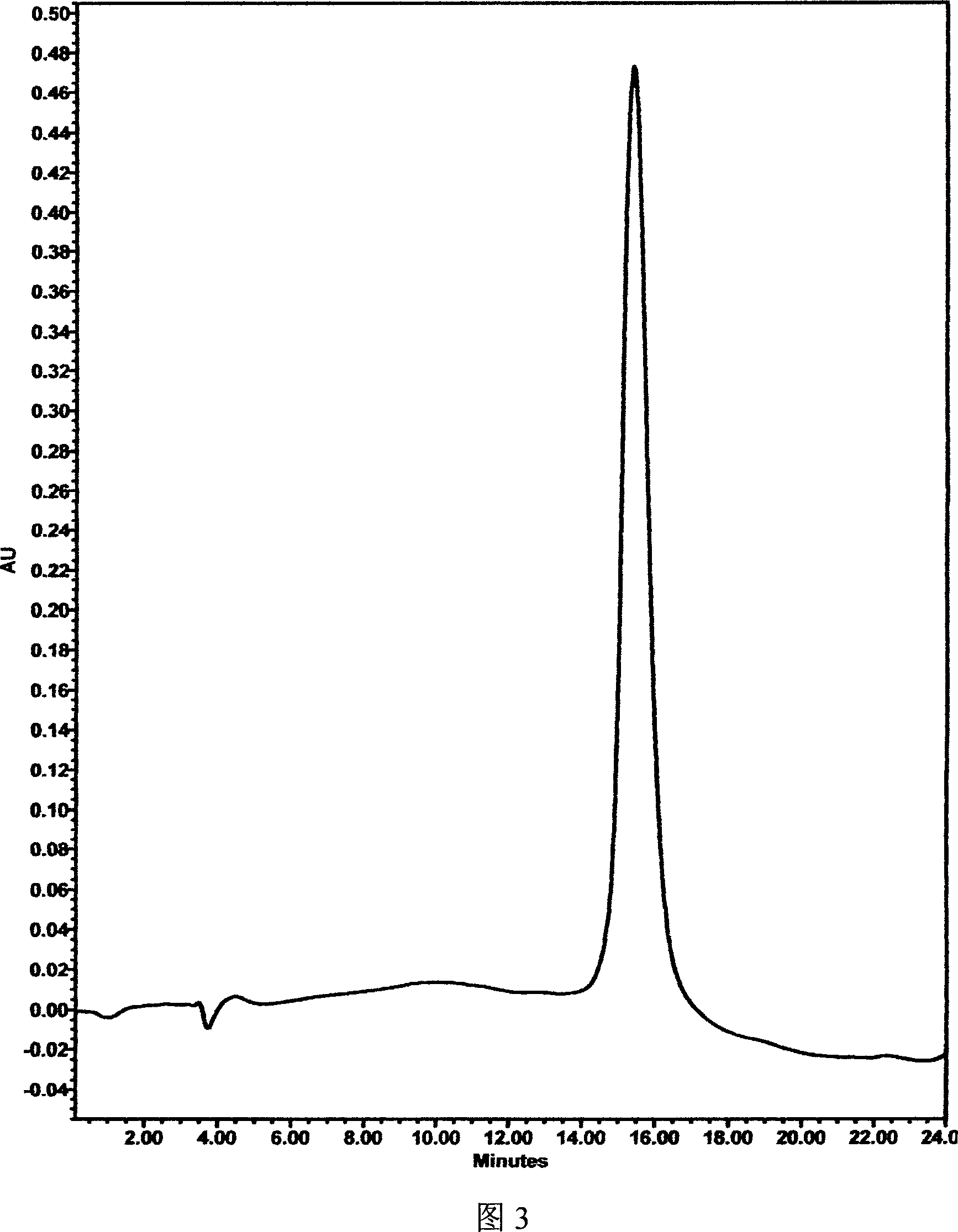
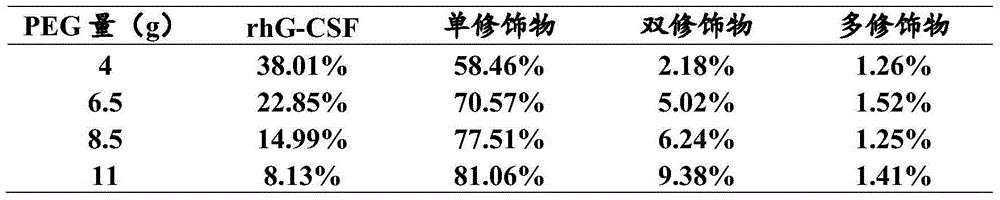
The invention relates to a recombinant human granulocyte colony stimulating factor and one-step purifying process for chemical modifications, which employs the method of cation exchange chromatography, wherein one-step chromatography is utilized for obtaining high activity, high purity and high recovery ratio rhG-CSF and polyethylene glycol chemically modified rhG-CSF. The process is especially suitable for industrial production.
Owner:CHONGQING FAGEN BIOMEDICAL
Materials and methods for providing nutrition to neonates
InactiveUS6956023B1Promote and facilitate nutritional intakeReducing and preventing villous atrophyOrganic active ingredientsPeptide/protein ingredientsSodium acetateNutrition
The subject invention provides methods for reducing or preventing villous atrophy and feeding intolerance in infants, particularly low birth weight and / or preterm infants, by enterally administering granulocyte-colony stimulating factor (G-CSF), erythropoietin (Epo), or both G-CSF and Epo. The subject invention also provides compositions that comprise G-CSF and / or Epo that may be administered to infants in need thereof. In one embodiment, the composition of the subject invention comprises recombinant G-CSF, recombinant Epo, and one or more electrolyte additives. In a specific embodiment, the electrolyte additive is selected from the group consisting of sodium chloride, sodium acetate, and potassium chloride.
Owner:FLORIDA UNIV OF A FLORIDA
Methods and compositions for diagnosis and prognosis of renal injury and renal failure
ActiveUS8778615B2Easy to adaptMicrobiological testing/measurementAntibody ingredientsInterleukin 10Soluble P-Selectin
The present invention relates to methods and compositions for monitoring, diagnosis, prognosis, and determination of treatment regimens in subjects suffering from or suspected of having a renal injury. In particular, the invention relates to using assays that detect one or more markers selected from the group consisting of Cytoplasmic aspartate aminotransferase, soluble Tumor necrosis factor receptor superfamily member 5, soluble CD40 Ligand, soluble C-X-C Motif chemokine 16, S100-A12, Eotaxin, soluble E-selectin, Fibronectin, Granulocyte colony-stimulating factor, Granulocyte-macrophage colony-stimulating factor, Heparin-binding growth factor 2, soluble Hepatocyte growth factor receptor, Interleukin-1 receptor antagonist, Interleukin-1 beta, Interleukin-10, Interleukin-15, Interleukin-3, Myeloperoxidase, Nidogen-1, soluble Oxidized low-density lipoprotein receptor 1, Pappalysin-1, soluble P-selectin glycoprotein ligand 1, Antileukoproteinase, soluble Kit ligand, Tissue inhibitor of metalloproteinase 1, Tissue inhibitor of metalloproteinase 2, soluble Tumor necrosis factor, soluble Vascular cell adhesion molecule 1, and Vascular endothelial growth factor A as diagnostic and prognostic biomarkers in renal injuries.
Owner:ASTUTE MEDICAL
Methods for mobilizing hematopoietic facilitating cells and hematopoietic stem cells into the peripheral blood
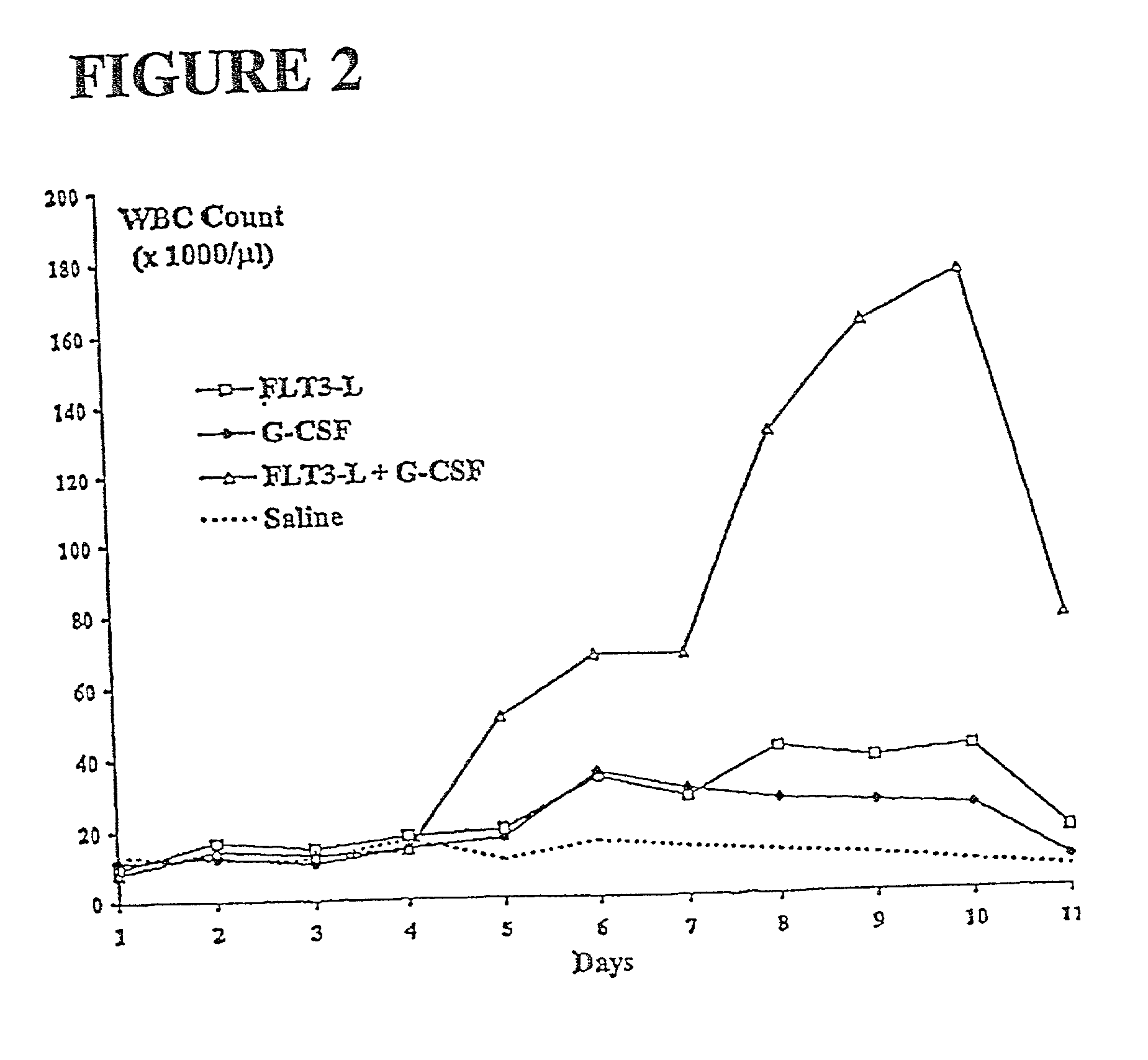
The present invention relates to methods for mobilizing hematopoietic facilitating cells (FC) and hematopoietic stem cells (HSC) into a subject's peripheral blood (PB). In particular, the invention relates to the activation of both FLT3 and granulocyte-colony stimulating factor (G-CSF) receptor to increase the numbers of FC and HSC in the PB of a donor. The donor's blood contains both mobilized FC and HSC, and can be processed and used to repopulate the destroyed lymphohematopoietic system of a recipient. Therefore, PB containing FC and HSC mobilized by the method of the invention is useful as a source of donor cells in bone marrow transplantation for the treatment of a variety of disorders, including cancer, anemia, autoimmunity and immunodeficiency. Alternatively, the donor's hematopoietic tissue, such as bone marrow, can be treated ex vivo to enrich selectively for FC and HSC populations by activating appropriate cell surface receptors.
Owner:ILDSTAD SUZANNE T +1
Preparation method of recombination human granular cell colony stimulating factor
A process for preparing the recombinant human granulocyte colony stimulating factor includes such steps as fermenting culture of DH5 alpha-PBV220-hGCSF strain, separating cytoryctes, washing, modifying renaturation, ultrafilter, the second renaturation, and chromatography. Its advantages are high purity and high activity.
Owner:深圳未名新鹏生物医药有限公司
Production method for recombinant human granulocyte colony-stimulating factor
ActiveCN103233053ALarge amount of processingReduce difficultyMicroorganism based processesPeptide preparation methodsEscherichia coliYeast
The invention discloses production method for a recombinant human granulocyte colony-stimulating factor. According to the invention, an engineered strain of an escherichia coli expressed recombinant human granulocyte colony-stimulating factor is cultured to obtain an inclusion body, the engineered strain of the escherichia coli expressed recombinant human granulocyte colony-stimulating factor is pKG931 / HB101, a culture method comprises multiplication culture and culture in a fermentation cylinder, an antibiotic-free medium containing yeast and peptone is employed in both culture, a high pressure homogenizer is used for breaking of bacteria, and denaturation, renaturation and chromatography are carried out so as to obtain a high purity G-CSF stock solution. The production method provided by the invention has the advantages of a short production period, high production efficiency, a great production scale, especial suitability for industrial production and reduction in production cost.
Owner:BEIJING FOUR RINGS BIOPHARM
Use of SCF and G-CSF in the treatment of cerebral ischemia and neurological disorders
InactiveUS20060153799A1Affect body weightAbility to focus on taskNervous disorderPeptide/protein ingredientsDiseaseNervous system
The present invention relates to the use of stem cell factor (SCF) polypeptide, alone and in combination with granulocyte colony stimulating factor (G-CSF) polypeptide, in the prevention or treatment of injury to the brain after cerebral ischemia or neurological disorder. More particularly, the invention provides methods of improving neurological function and outcome after stroke by the administration of SCF polypeptide, alone and in combination with G-CSF polypeptide. This treatment can be used alone or in combination with other well-known methods of treatment of cerebral ischemia and neurological disorders in a mammal.
Owner:NORTHWESTERN UNIV
Non-animal-source serum-free culture medium for umbilical cord blood stem cells
ActiveCN102827810AAvoid instabilityClear natureBlood/immune system cellsCell phenotypeLipid formation
The invention relates to the field of biology, and discloses a non-animal-source serum-free culture medium which essentially comprises an IMDM (Iscove Modified Dulbecco Medium), L-glutamine, sodium bicarbonate, recombinant human insulin, recombinant human transferrin, recombinant human albumin, 2-mercaptoethanol, phytohaemagglutinin (PHA), lipid, amino acid, vitamins, trace elements, interleukin-3(IL-3), stem cell factor, (SCF), Fit3-L, IL-6 and granulocyte colony-stimulating factor (G-CSF). The non-animal source serum-free culture medium is clear in chemical components, free from animal sources and serum and safe and ideal in cell cultivation, avoids the doped animal components and unstability of batches, and the results of cultured umbilical cord blood stem cells show that the total cellular score, the cell phenotype and the secretory cell factors are normal, so that the non-animal-source serum-free culture medium has good industrial application prospect.
Owner:内蒙古干细胞医学工程技术研究中心
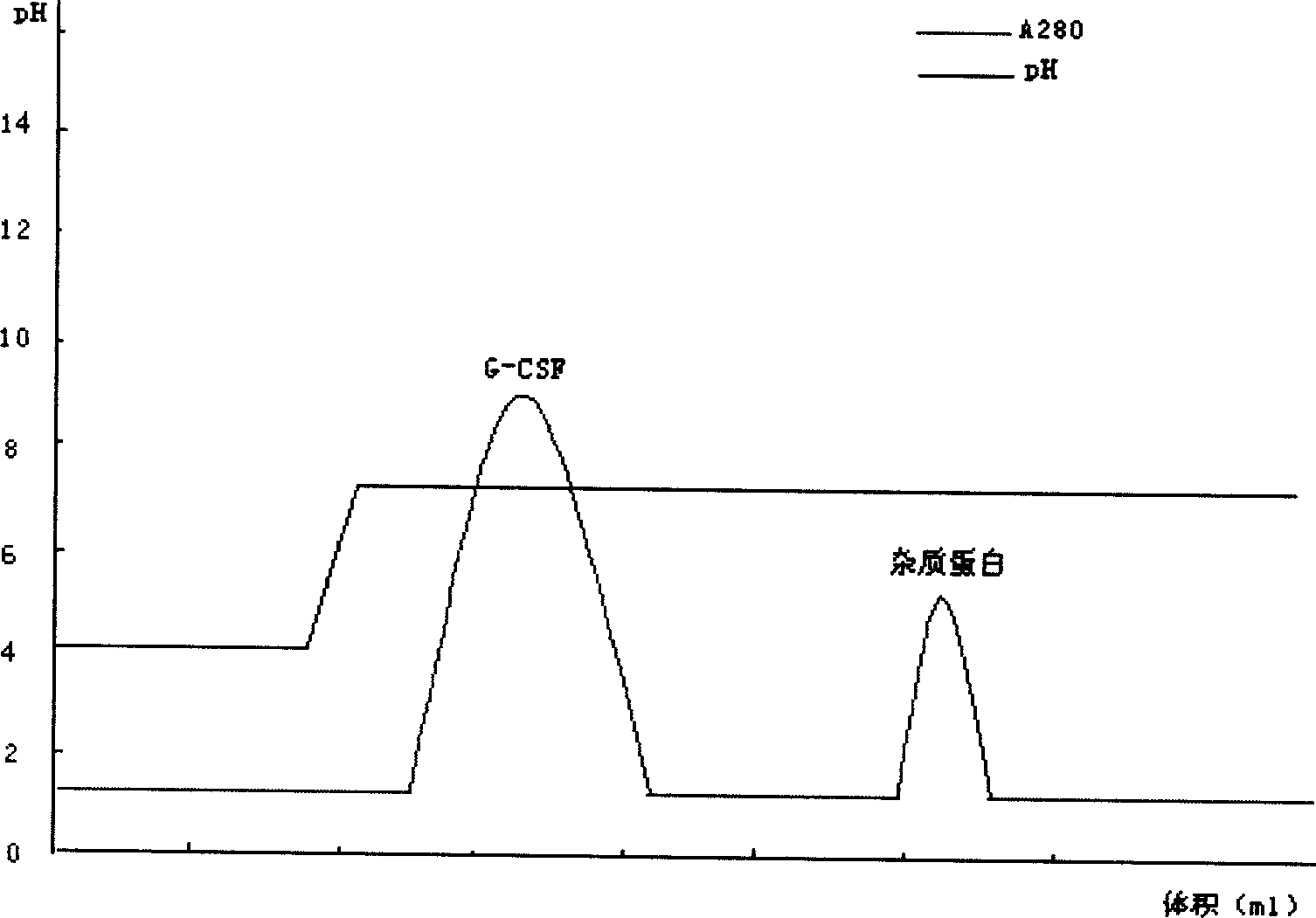
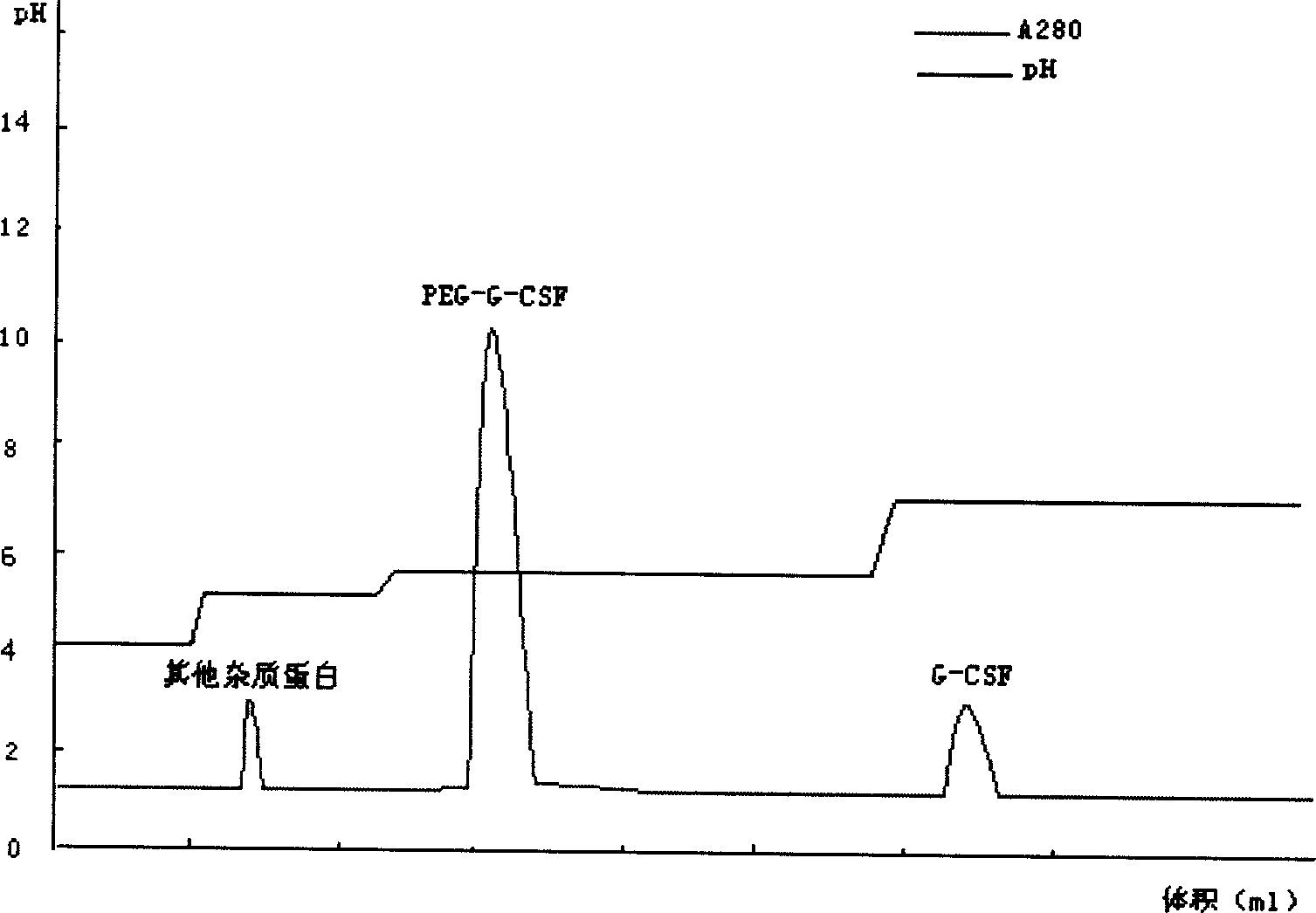
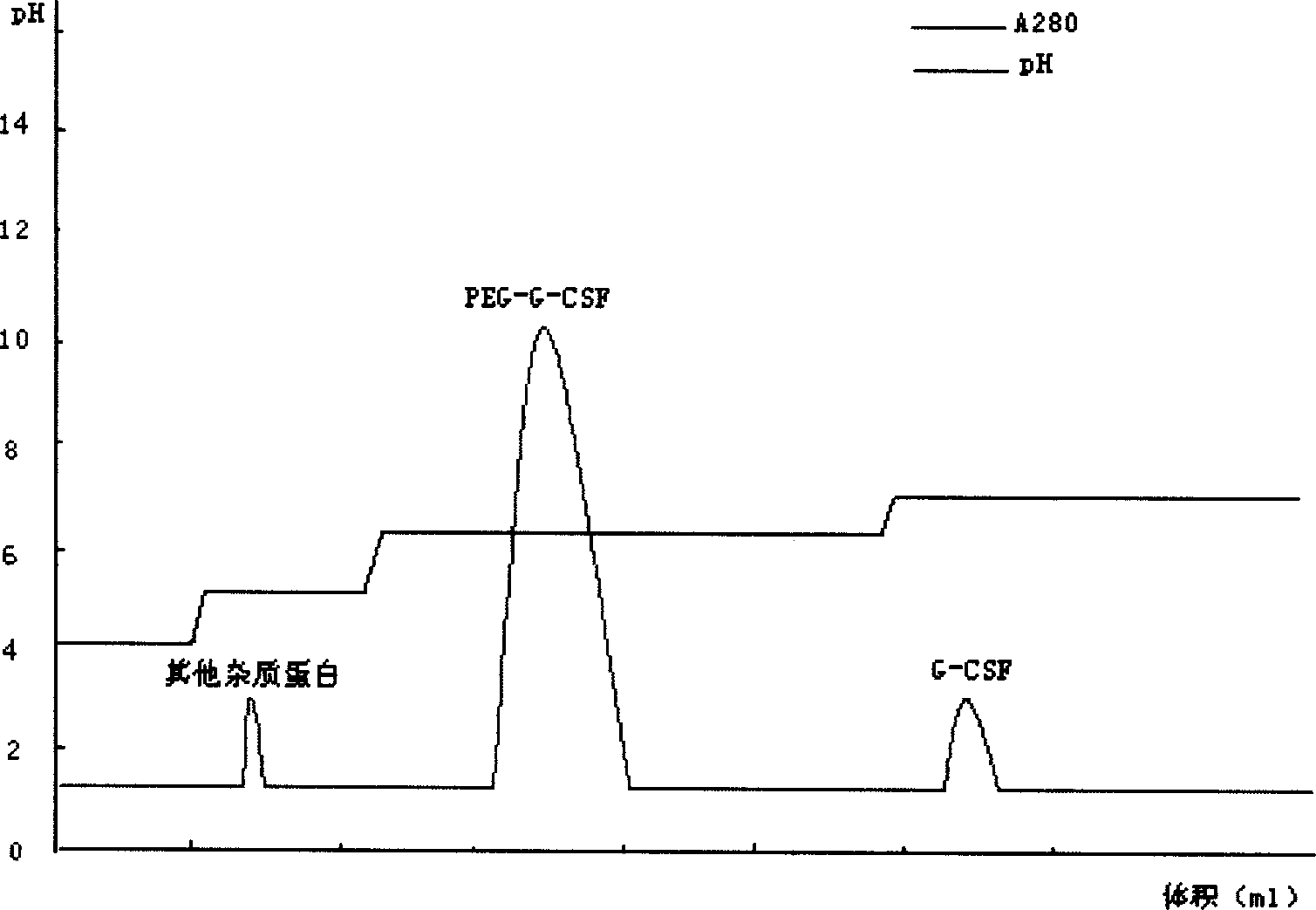
Purification method of pegylated recombinant human granulocyte colony stimulating factor
ActiveCN102850450AIncrease lossReduce lossesPeptide preparation methodsDepsipeptidesPurification methodsEndotoxin removal
The present invention provides a purification method of pegylated recombinant human granulocyte colony stimulating factor. The inventor performed extensive researches of a variety of chromatographic methods, and found that high-purity pegylated recombinant human granulocyte colony stimulating factors can be obtained by using desalting chromatography, ion exchange tandem chromatography and desalting chromatography to perform sequential combined purification. The purification process of the present invention makes full use of the characteristics of the target protein in the surface charge and hydrophobicity, firstly uses an anion exchange chromatography column to effectively remove endotoxin and to increase controllability of the process; and makes full use of strong hydrophobicity of the target protein in the second-stage chromatography, realizes tandem chromatography, and combines removal of endotoxin and protein purification into a step. The process can not only effectively remove endotoxin, but also simplify operation steps, save production time, and improve production efficiency. The process is very suitable for large-scale industrial production.
Owner:QILU PHARMA
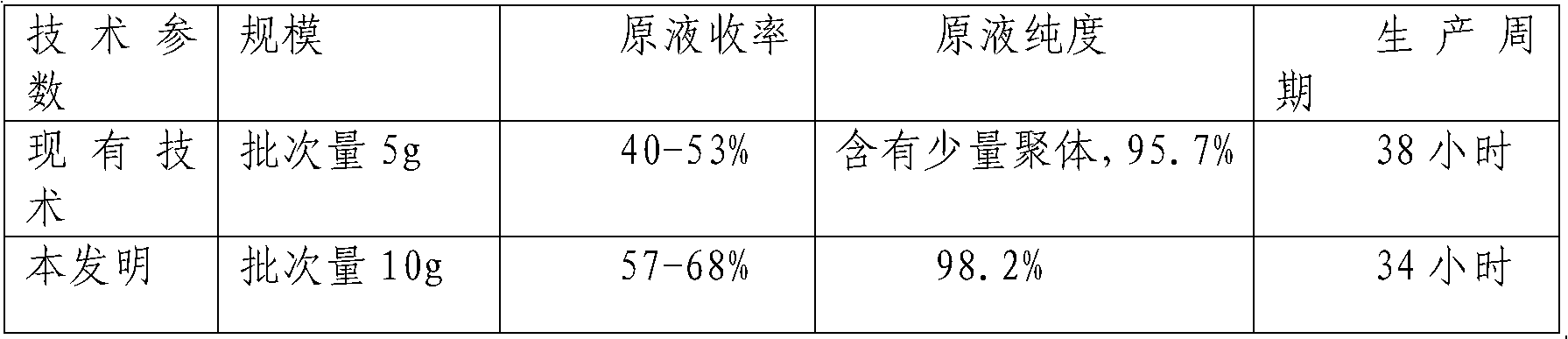
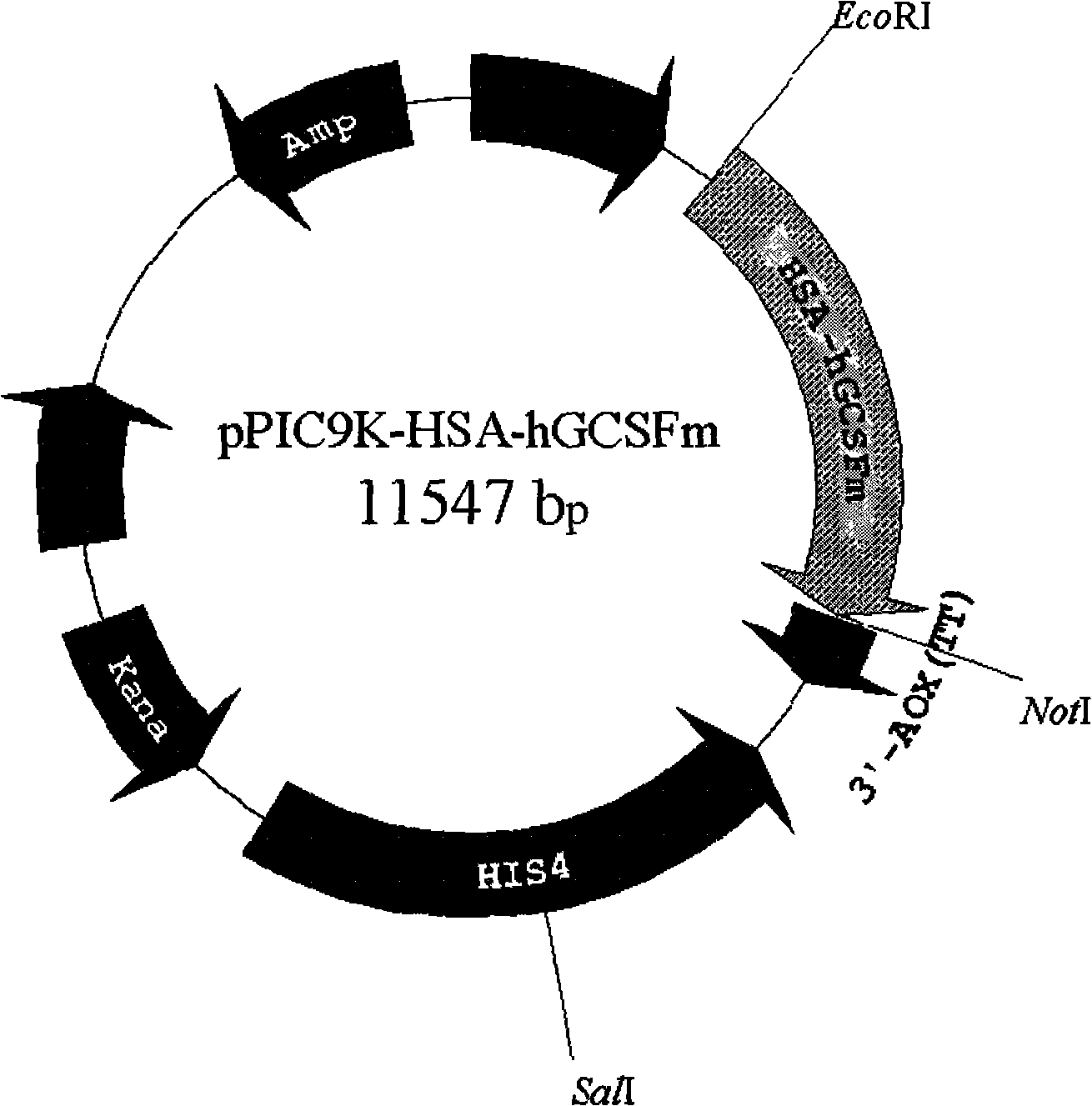
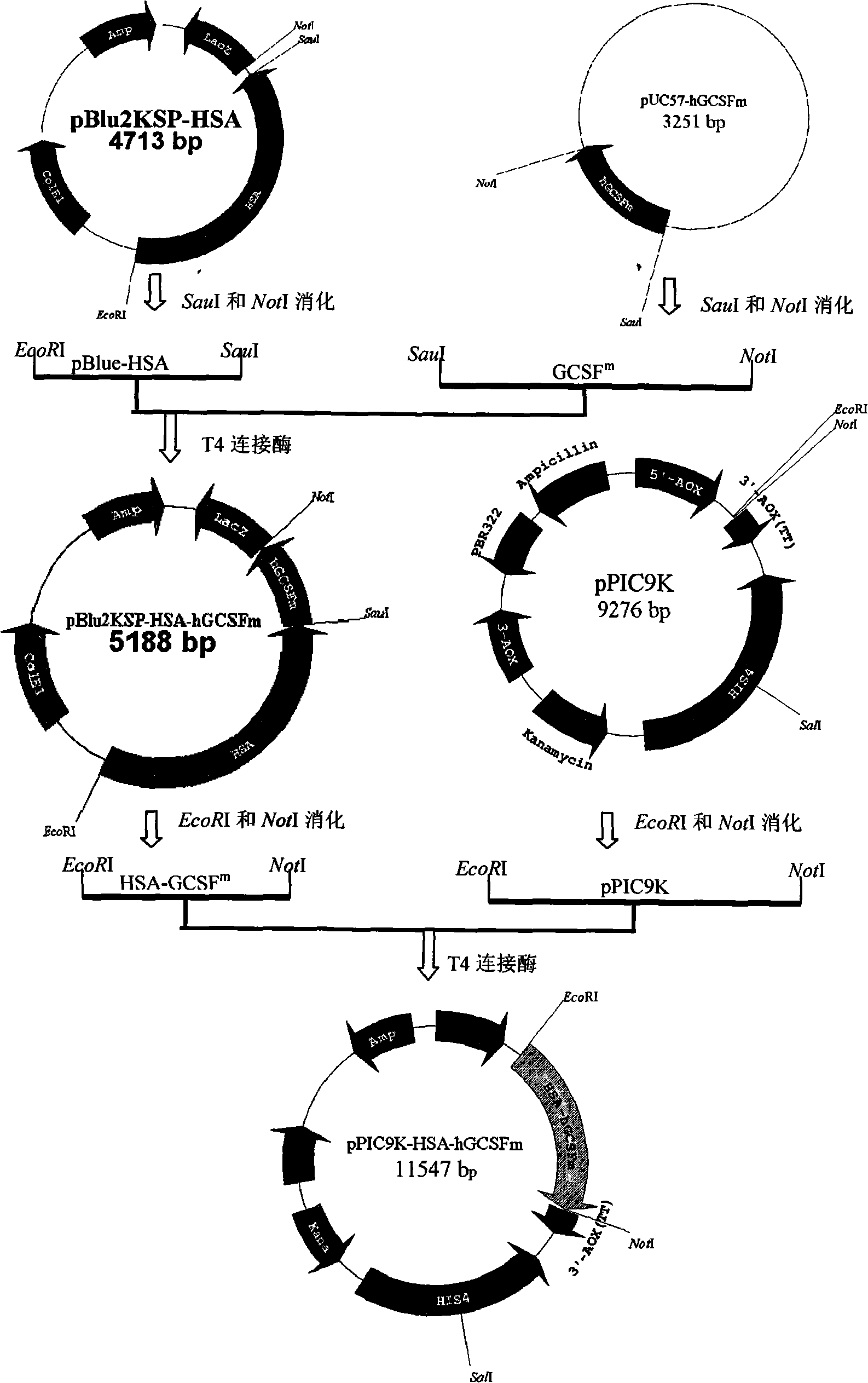
Fused protein of human serum albumin and human granulocyte colony stimulating factor mutant, and preparation thereof
InactiveCN101280019AEasy to operateAvoid degradationHybrid peptidesVector-based foreign material introductionHuman bodyHalf-life
The invention relates to a preparation method of a fused protein of human serum albumin and mutant human granulocyte colony stimulating factor, and the product thereof, belonging to the technical field of long-effetive recombinant fused protein drugs. Human serum albumin(HSA) cDNA and mutant human granulocyte colony stimulating factor(hGCSF) cDNA are directly connected, without adding any connecting peptide therevetween, obtaining HAS- hGCSFm cDNA which is then integrated with a cell of the host to express. The fused protein of the invention comprises a first region with at least 85% of the sequence congenetic with human serum albumin and a second region with at least 85% of the sequence congenetic with the mutant human granulocyte colony stimulating factor, and the two regions are directly connected without any connecting peptide therebetween. Under the premise of not being changed in characteristics, the fused protein is capable of the substitution, deletion or addition of specific amino acid residues. The host cell can be bacteria, barm, insect cell, zooblast, plant cell, etc. The fused protein maintains the physiological characteristics of the mutant human granulocyte colony stimulating factor and prolonges the half life of the mutant human granulocyte colony stimulating factor within human body; therefore the fused protein is of good application prospect in pharmacy.
Owner:JIANGNAN UNIV
Method for producing recombinant human granulocyte colony stimulating factor
ActiveCN1814779ASimple production methodProduction method is stablePeptide preparation methodsCytokines/lymphokines/interferonsInclusion bodiesIon exchange
This invention relates to a method for producing recombined human granular leukocyte colony stimulating factors including: fermenting, breaking bactrriums, extracting occlusion bodies, chromatographing with molecular sieves, renaturating, exchanging anions, exchanging cations to get the raw fluid, in which, BL21 is selected as the host bacterium and the engineering bacterium type is got in high expression volume, quick reproduction and stable passage, several purification steps greatly reduce the residural toxin in the bacterium and the specific activity is increased greatly, a dialysis method is applied for the renaturation to reduce the concentration of the denaturalization agent steadily so the renaturation rate is at high level.
Owner:山东泉港药业有限公司
Dipurification process of recombinant humangranulocyte colony stimulating factor
ActiveCN101045742AHigh annealing quality yieldPeptide preparation methodsRecombinant DNA-technologyInclusion bodiesGlycerol
This invention relates to a preparation method of recombinant human granulocyte colony stimulating factor (rhG - CSF). It includes cracking liquid of inclusion bodies, composition of renaturation liquid, cracking and renaturation method.The renaturation liquid composed by guanidine hydrochloride ( Guanidine - HCl),urea, trihydroxymethyl aminomethane hydrochloride ( Tris - HCl), cysteine, cystine and glycerol.
Owner:NCPC NEW DRUG RES & DEV +1
Preparation method and pharmaceutical composition of PEGylated recombinant human granulocyte colony-stimulating factor
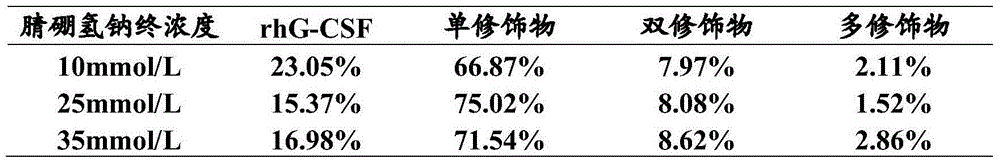
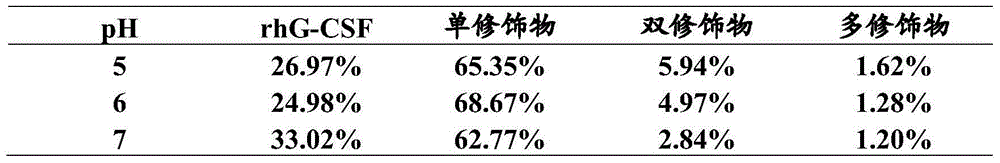
InactiveCN105273076AHigh selectivityStrong acidPeptide/protein ingredientsDepsipeptidesFactor iiG-csf therapy
The invention provides a preparation method of PEGylated recombinant human granulocyte colony-stimulating factor. The preparation method comprises following steps: (a), an alkali conditioning agent is used for adjusting pH value of a mother liquor containing recombinant human granulocyte colony-stimulating factor to a first pH value; (b), methoxy-polyethylene glycol-propionaldehyde is added into the mother liquor, and an obtained solution is stirred for dissolving; (c), NaBH3CN is added, and an obtained mixture is stirred for reaction; and (d) an acid conditioning agent is used for adjusting pH value of an obtained reaction liquid to a second pH value so as to stop reaction and obtain the PEGylated recombinant human granulocyte colony-stimulating factor. The preparation method is simple; buffer system of a reaction liquid is the same to buffer system of the mother liquor of the recombinant human granulocyte colony-stimulating factor, so that buffer solution replacing is avoided; reaction is stopped by adjusting pH value with hydrochloric acid; product purifying difficulty is reduced; and the preparation method is suitable for industrialized production. The kinds of added reagent are few; and safety of the finished products in clinical application is improved.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Preparation method and separation and purification method of polyethylene glycol single modified recombinant human granulocyte-colony stimulating factor
InactiveCN102485742AHigh purityReduce generationPeptide preparation methodsDepsipeptidesPurification methodsPolyethylene glycol
The invention belongs to the field of biomedicine, specifically relates to polyethylene glycol modification and separation and purification of protein and especially relates to preparation and purification of a polyethylene glycol single modified recombinant human granulocyte-colony stimulating factor (PEG-rhG-CSF). According to optimized modification on a reaction system and reaction conditions, single modified PEG-rhG-CSF larger than 70% and multiple modified PEG-rhG-CSF less than 2.3% are obtained; according to control on technological parameters in the separation and purification process, single modified PEG-rhG-CSF with high purity and high activity are obtained, and a yield of the PEG-rhG-CSF reaches higher than 85%. The purification method of the invention employs a one-step cation exchange chromatography to simplify steps and save cost, and is suitable for industrialized large-scale production.
Owner:SHANDONG NEWTIME PHARMA
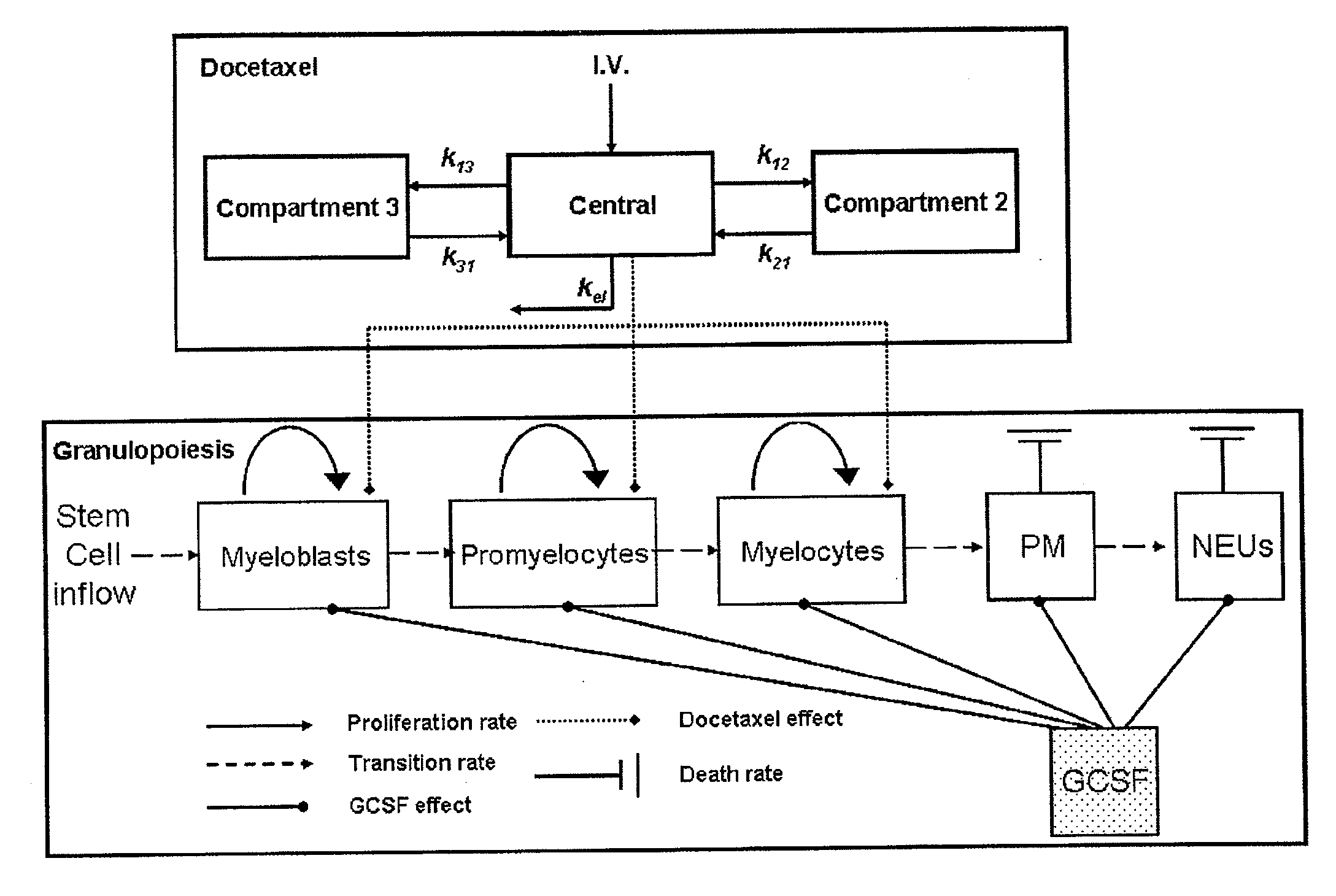
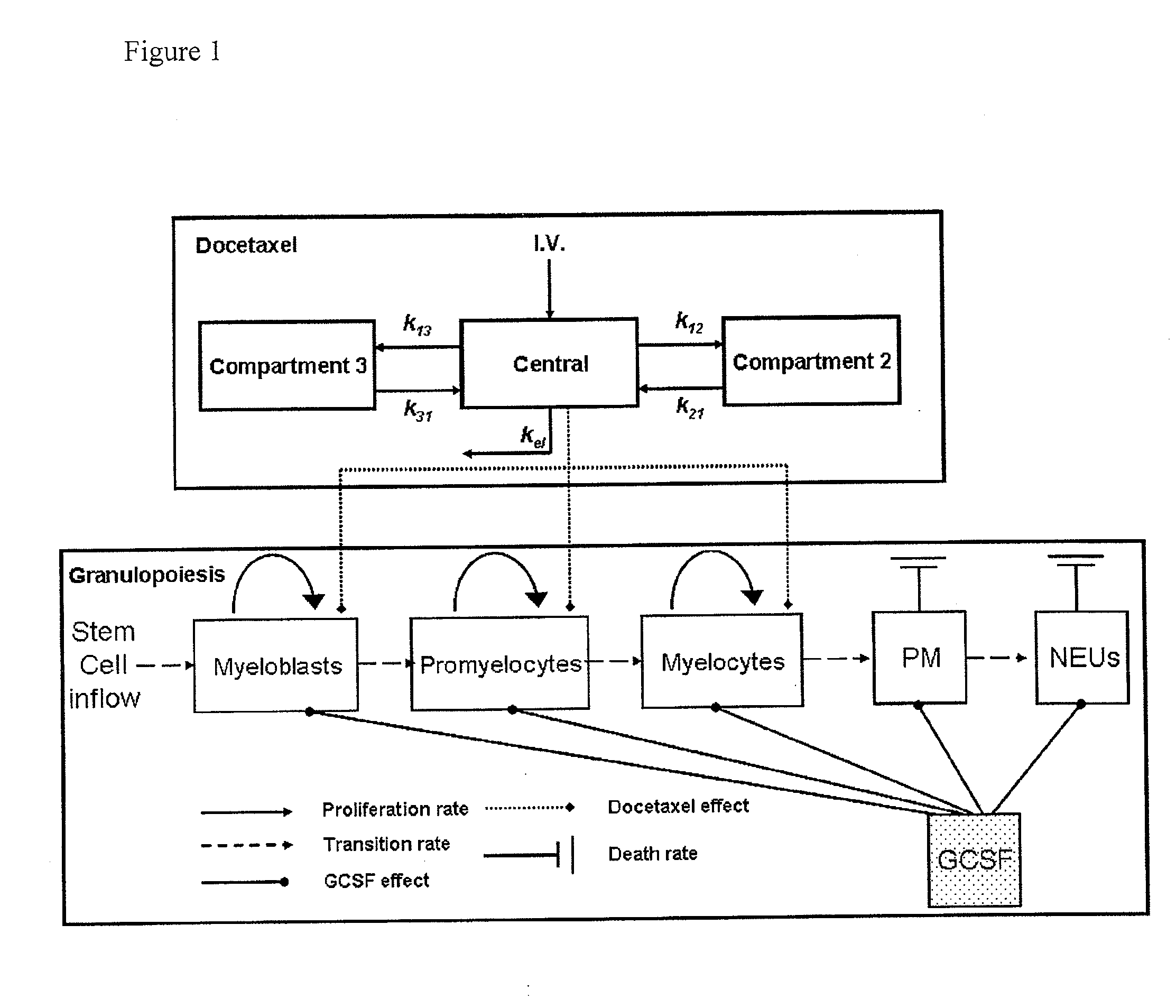
Cancer therapy by docetaxel and granulocyte colony-stimulating factor (g-csf)
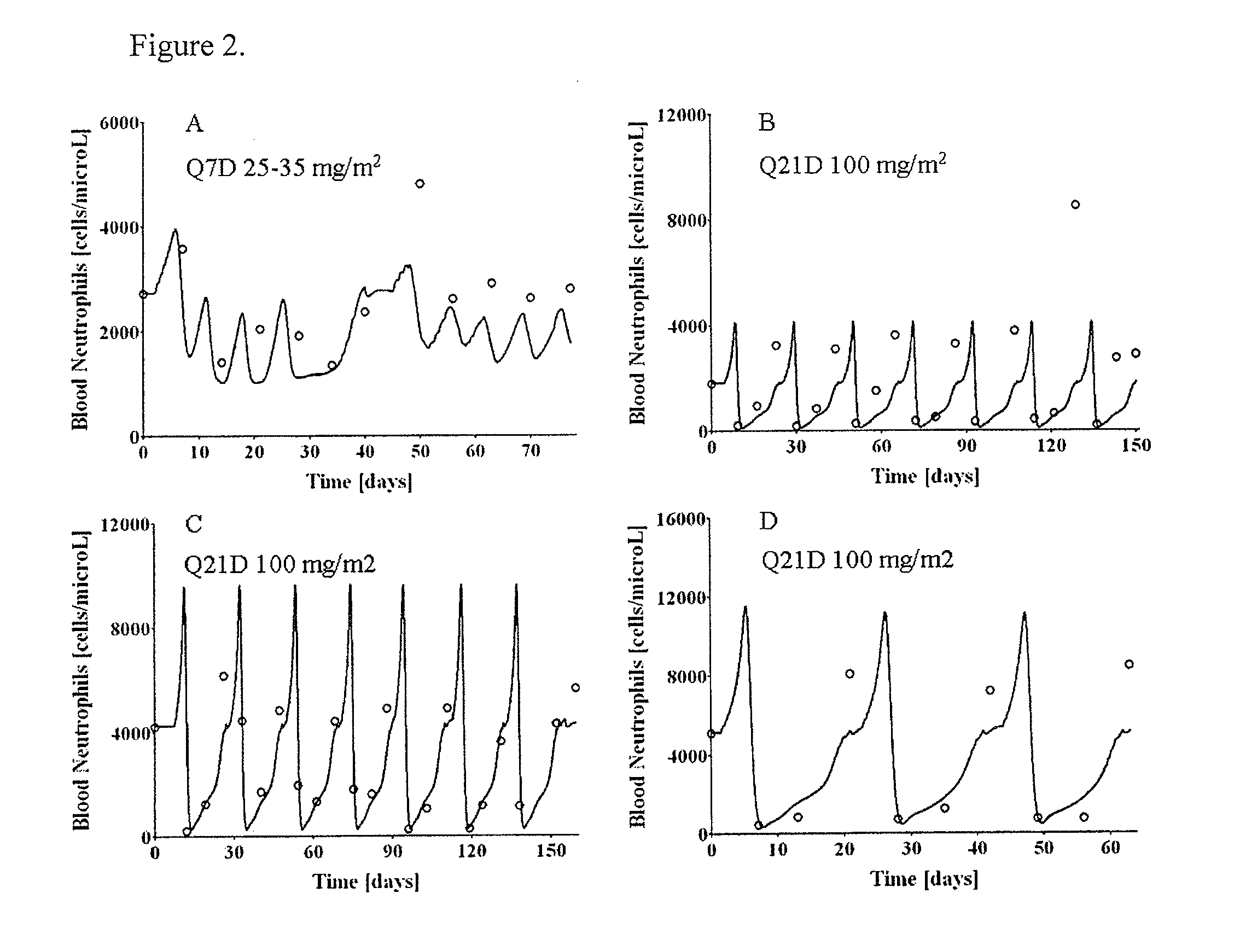
Neutropenia is the dose-limiting toxicity of the tri-weekly docetaxel (Taxotere®) schedule. Here, we evaluate in Metastatic Breast Cancer (MBC) patients (N=38) a computerized method for predicting docetaxel-induced neutropenia, and use the model to identify improved docetaxel and Granulocyte Colony Stimulating Factor (G-CSF) regimens. Pharmacokinetics / pharmacodynamics (PK / PD) models were created and simulated concomitantly with a mathematical granulopoiesis model. Individual baseline neutrophil counts and docetaxel schedules served as inputs. Our trial validated the model accuracy in predicting nadir timings (r=0.99), grade 3 / 4 neutropenia (86% success) and neutrophil profiles (r=0.62). Model was robust to CYP3A-induced variability, except for slightly less accurate grade 3 / 4 neutropenia predictions. Simulations confirm smaller toxicity of the weekly docetaxel regimen than the tri-weekly one, and suggest an optimal G-CSF support for alleviating neutropenia, 60 μg / day QD×3, 6-7 days post-docetaxel, administered tri- and bi-weekly, and 4 days post weekly docetaxel>33 mg / m2.
Owner:OPTIMATA
Pharmaceutical preparation containing recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein and preparation thereof
ActiveCN102670522AStable in natureClinically convenientPowder deliveryPeptide/protein ingredientsFreeze-dryingSubcutaneous injection
The invention discloses a pharmaceutical preparation containing recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein and preparation of the pharmaceutical preparation. According to the invention, the medical preparation containing the rHSA / G-CSF (recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein) is a freeze-drying preparation, and each freeze-drying preparation comprises the following components: 1-30mg of rHSA / G-CSF protein, 10-80mg of pharmaceutically acceptable water-soluble excipient, 5-30mg of pharmaceutically acceptable protective agent and 5-50 mu mol of pH regulator. The pharmaceutical preparation containing the rHSA / G-CSF protein is suitable for administration in the ways of subcutaneous injection or intravenous injection and the like after being dissolved in water for injection, and the pharmaceutical preparation can be injected for the right dosage for treatment of neutrophilic granulocytopenia.
Owner:JIANGSU T MAB BIOPHARMA
Escherichia coli strain secreting human granulocyte colony stimulating factor (G-CSF)
The present invention provides a recombinant plasmid vector comprising a kanamycin resistance gene, a promoter, an endoxylanase signal sequence, a nucleotide sequence coding for an oligopeptide consisting of 13 amino acids including 6 consecutive histidine residues, and a human granulocyte colony stimulating factor(hG-CSF) gene; an E. coli transformed with the said vector; and, a process for producing complete hG-CSF protein with high purity from the protein pool secreted by the said microorganism. In accordance with the invention, the hG-CSF protein can be prepared with high purity through rather simple process facilitating secretion of large amount of hG-CSF fusion protein into the periplasm, which does not require complicated processes such as solubilization and subsequent refolding required for isolation of the hG-CSF protein produced in cytoplasm as insoluble inclusion bodies by conventional techniques, thus, the hG-CSF protein can be widely used as an active ingredient in the development of supplementary agents for anticancer therapy.
Owner:KOREA ADVANCED INST OF SCI & TECH
Expression of soluble therapeutic proteins
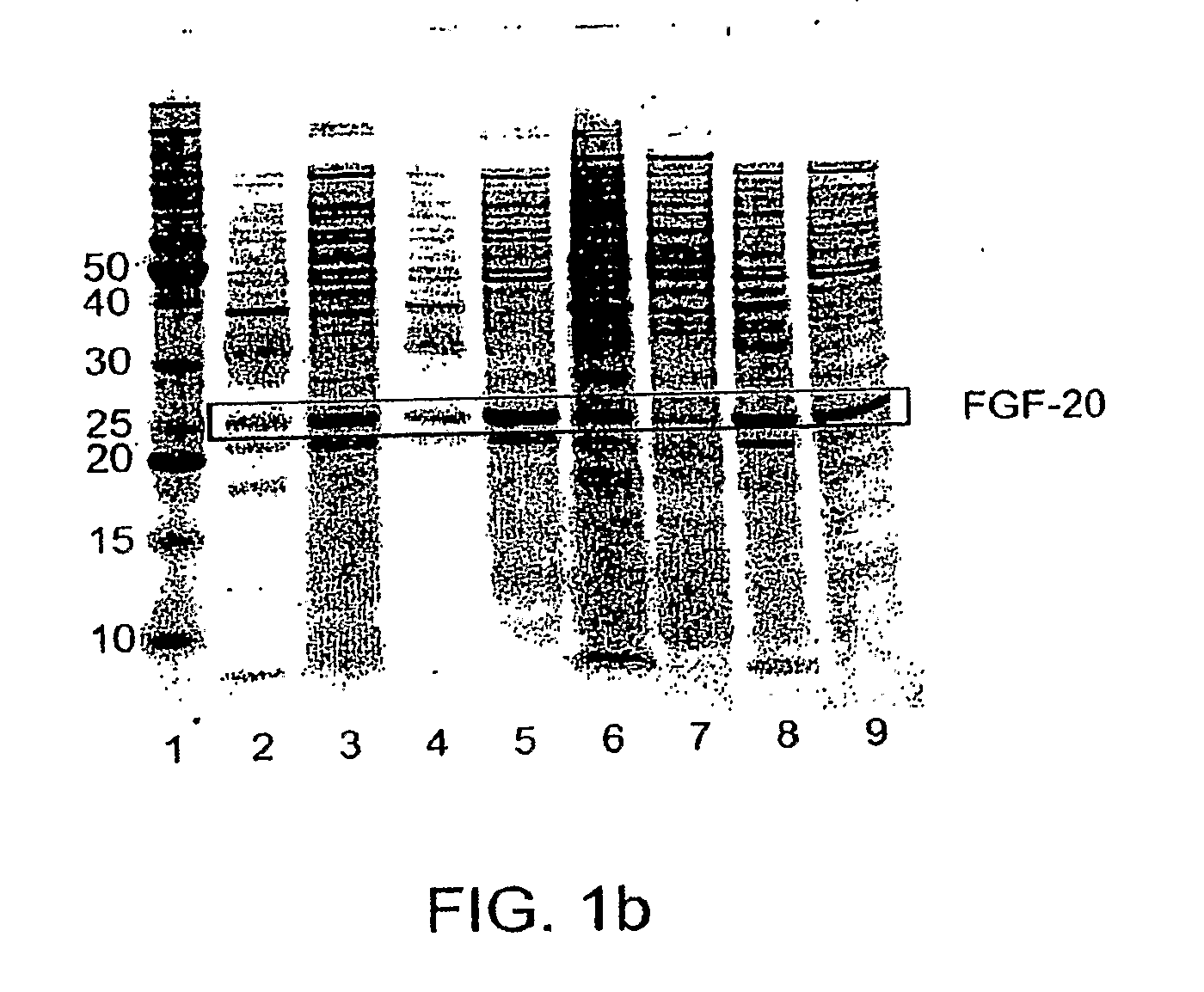
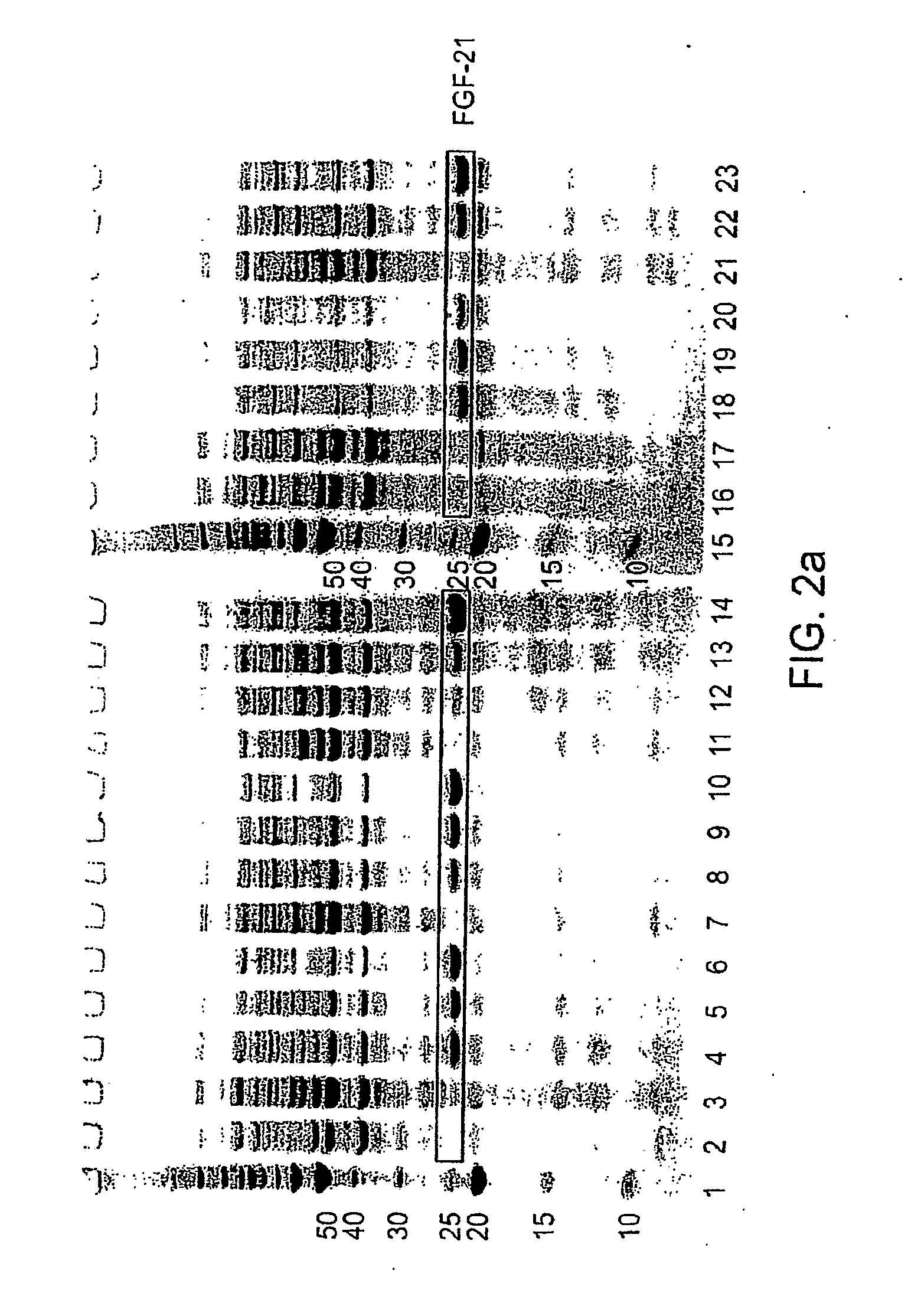
ActiveUS20090298121A1Improve solubilityFactor VIIPeptide/protein ingredientsMicroorganismNeurotrophin-3
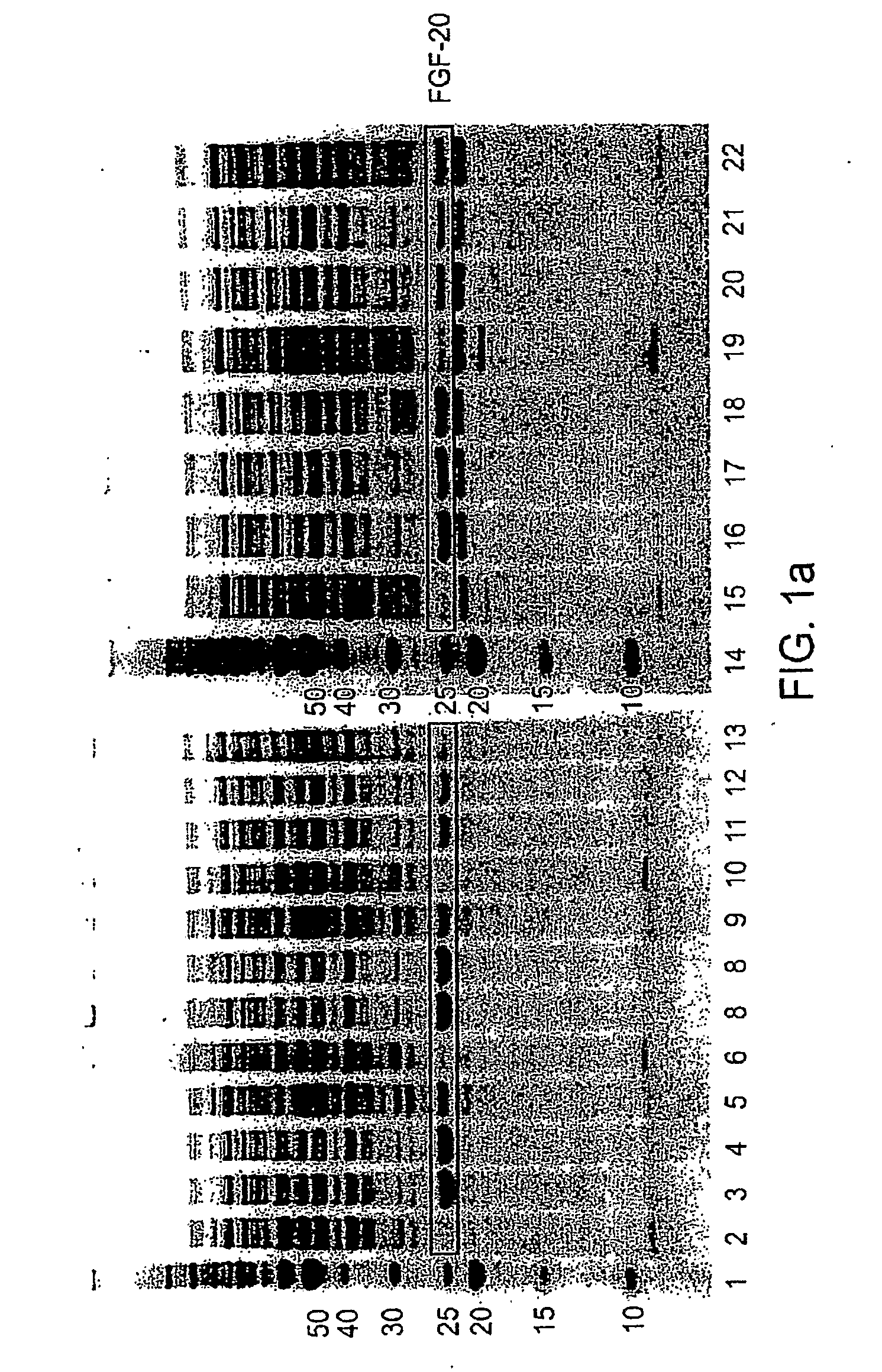
The present invention provides enhanced methods of producing soluble, active fibroblast growth factor-20 (FGF-20), FGF-21, neurotrophin-3 (NT-3), growth hormone (GH), granulocyte colony stimulating factor (G-CSF), or glucocerebrosidase proteins in microorganisms that have an oxidizing environment.
Owner:NOVO NORDISK AS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com