Pharmaceutical preparation containing recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein and preparation thereof
A pharmaceutical preparation and protein technology, applied in the field of pharmaceutical preparations containing recombinant human serum albumin-human granulocyte colony-stimulating factor fusion protein and its preparation, can solve the problems that have not yet been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
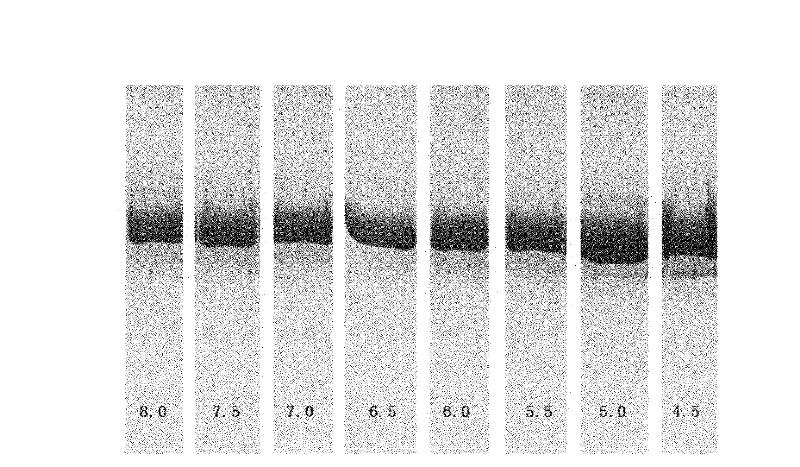
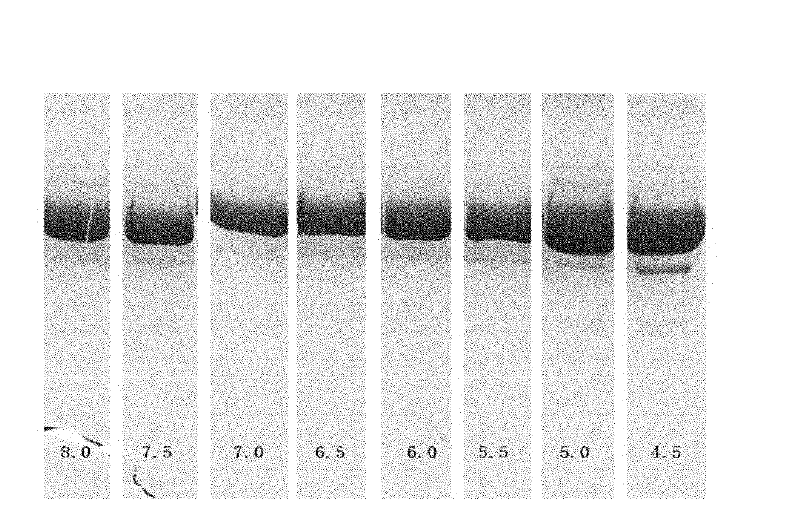
[0045] Example 1 The effect of pH value on the stability of rHSA / G-CSF formulation
[0046] rHSA / G-CSF refers to the description of Halpern W et al. (Pharm Res. 19: 1720-1729.2002), after the fusion of HSA and G-CSF gene, it was constructed in the expression plasmid pPIC9, and in Pichia pastoris GS115 (INVITROGEN company) expression obtained.
[0047] The present invention firstly studies the effect of pH on the stability of rHSA / G-CSF. In order to investigate the stability of the preparation under different pH conditions, the effects of different pH on the stability of the preparation were carried out according to the following conditions:
[0048] In test 1, the protein content of rHSA / G-CSF was 5.0 mg, and each group contained a certain concentration of acetate or phosphate buffer with different pH, pH 4.5 to pH 8.0, with an interval of 0.5, and a total of 8 points were set. Accelerated investigation at 37°C for four weeks, sampling analysis every two weeks, detection met...
Embodiment 2
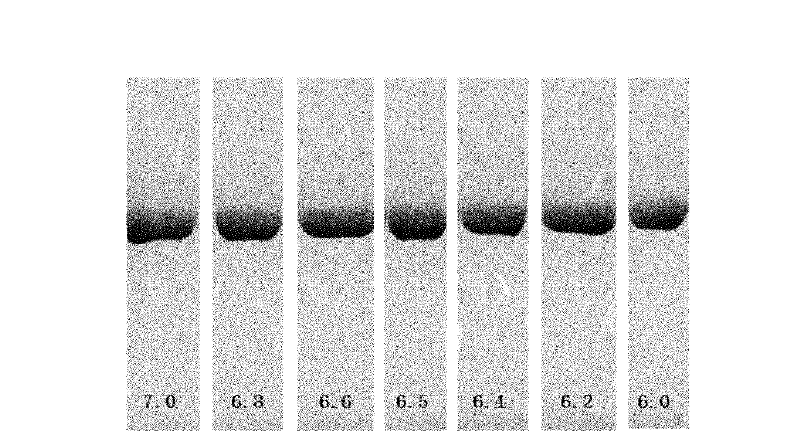
[0052] Example 2 Influence test of excipients on stability
[0053] The present invention screened some excipients and protective agents suitable for human application, such as mannitol, trehalose, PEG4000, glycerin, glycine, arginine, phenylalanine, methionine and histidine, and studied their effect on rHSA / G-CSF stability.
[0054] The excipients were prepared into a high-concentration stock solution, mixed with a high-concentration stock solution of pH 6.5 phosphate buffer, and then added to a high-concentration rHSA / G-CSF protein solution containing a pH 6.5 phosphate buffer, followed by 1 mol / L HCl Or adjust the pH value with 1 mol / L NaOH to obtain a certain volume of sample solution containing 10 mmol / L pH6.5 phosphate buffer and a protein concentration of 5.0 mg / ml. The sample solution was filtered and sterilized and then packed in sterile vials with a volume of 1.0 ml, half stoppered, and freeze-dried. The lyophilized preparations were visually inspected and analyze...
Embodiment 3
[0062] Weigh an appropriate amount of arginine, mannitol, disodium hydrogen phosphate and sodium dihydrogen phosphate, add water for injection and stir to dissolve, and then add an appropriate amount of high-concentration rHSA / G-CSF protein solution to obtain 5.0 rHSA / G-CSF protein per ml. mg, mannitol 30mg, arginine 10mg, phosphate 10umol solution, adjust the pH value to 6.5±0.1 with 1mol / L hydrochloric acid and 1mol / L sodium hydroxide.
[0063] The prepared solution was filtered with 0.22um PVDF or PES hydrophilic membrane.
[0064] The solution after sterilization and filtration is subpackaged and put into the freeze-drying box under aseptic conditions. The temperature of the freeze-drying machine is pre-frozen to below -10°C, and the half-stoppered sample is quickly put into the freeze-drying box. The average temperature of the product was kept below -35°C for 2 hours, and the cooling of the condenser was started. After the temperature of the condenser drops to -45°C, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com