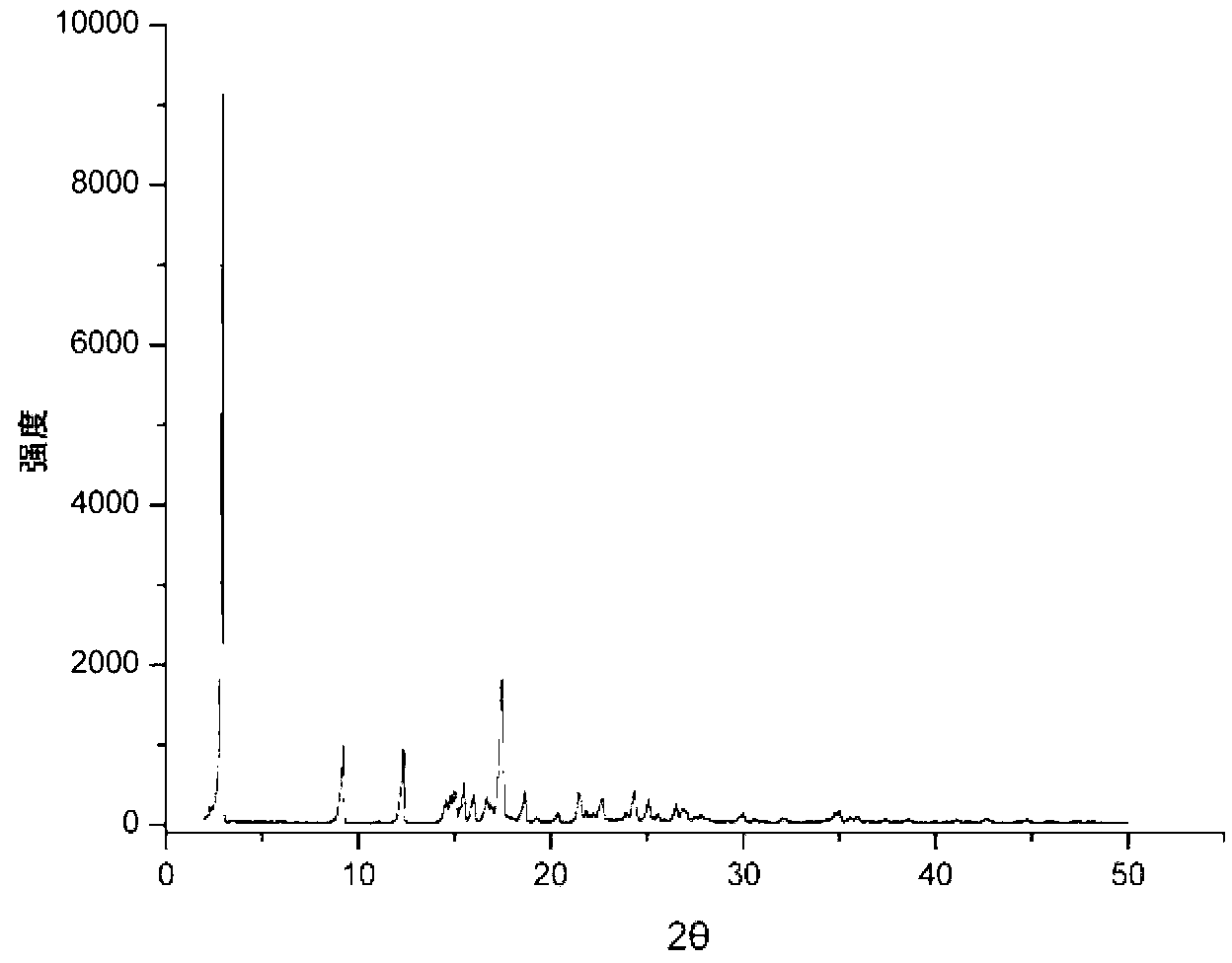
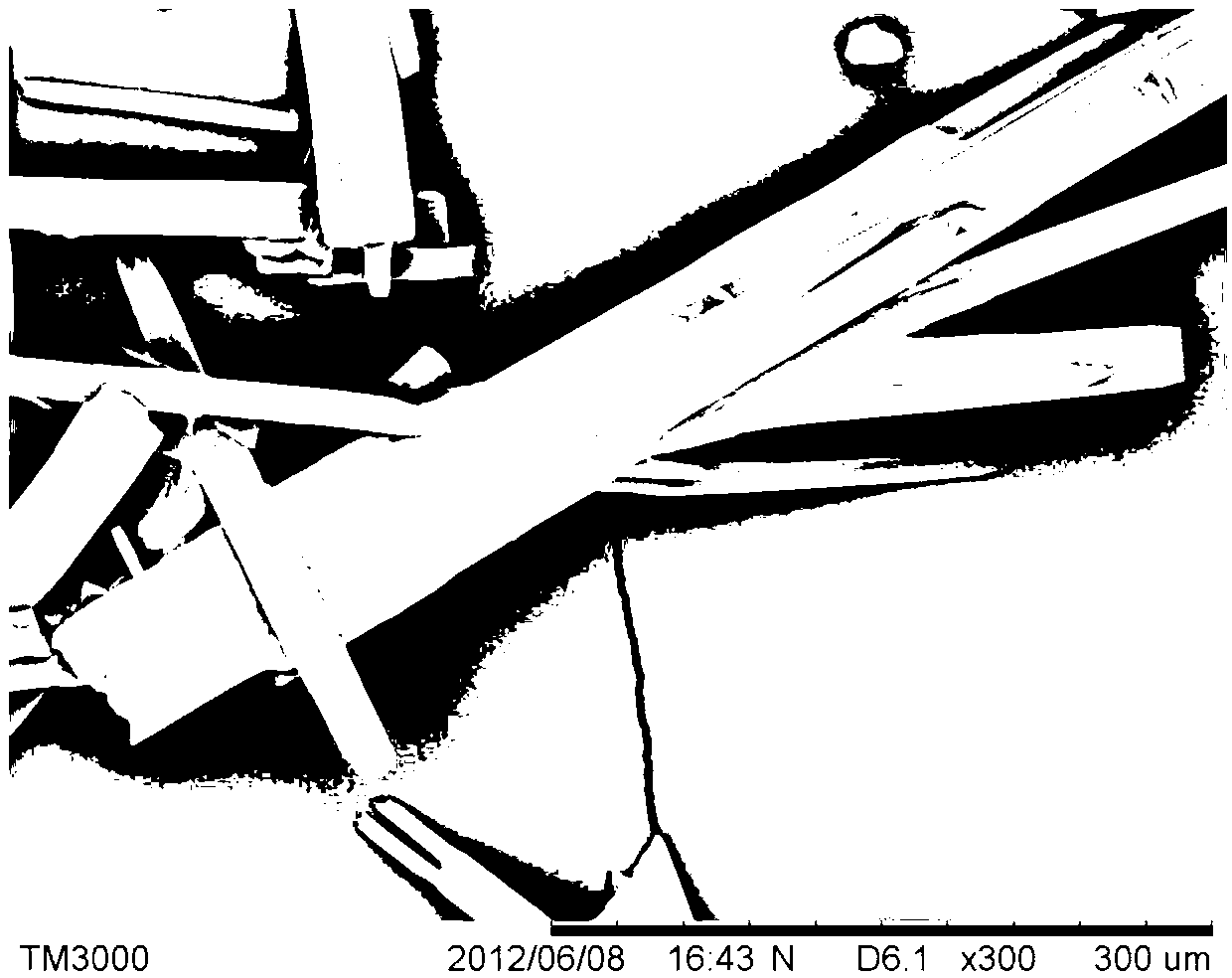
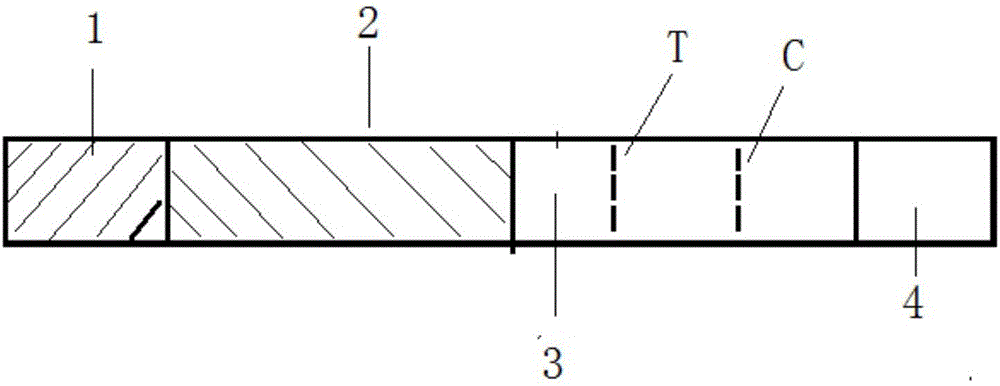
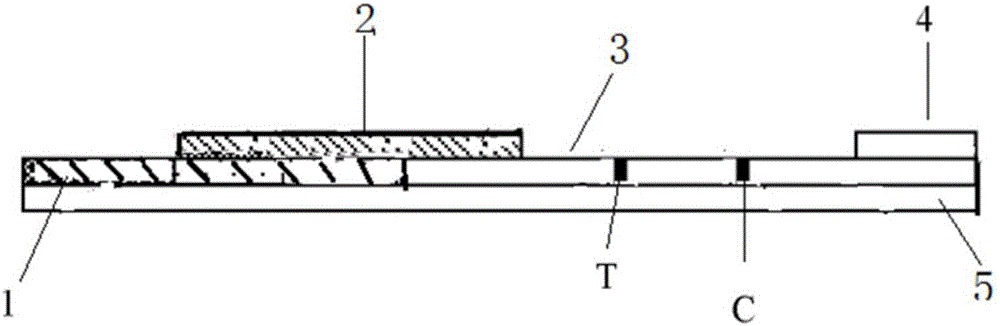
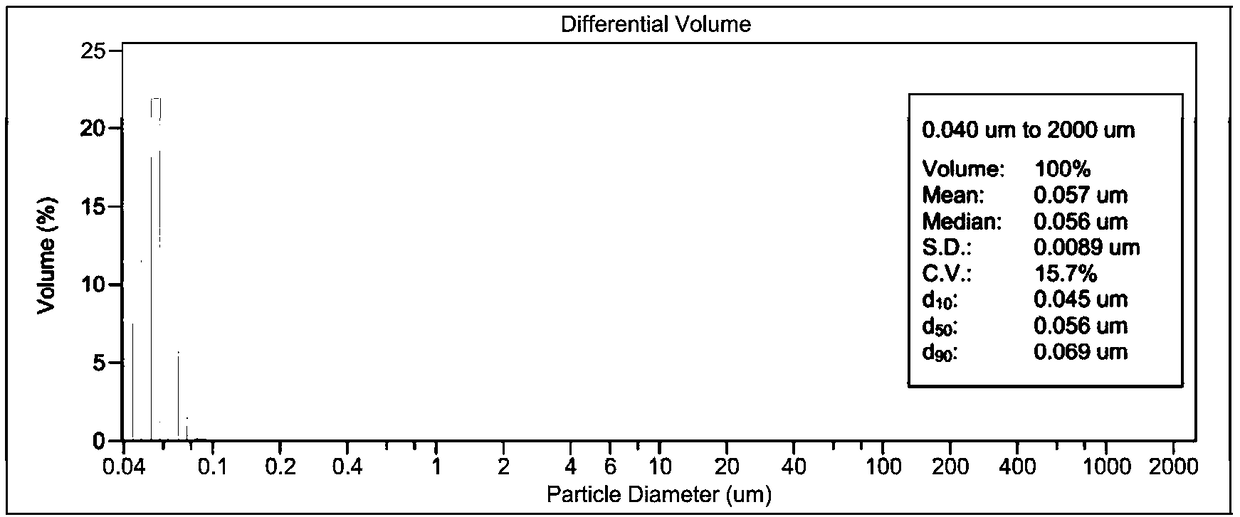
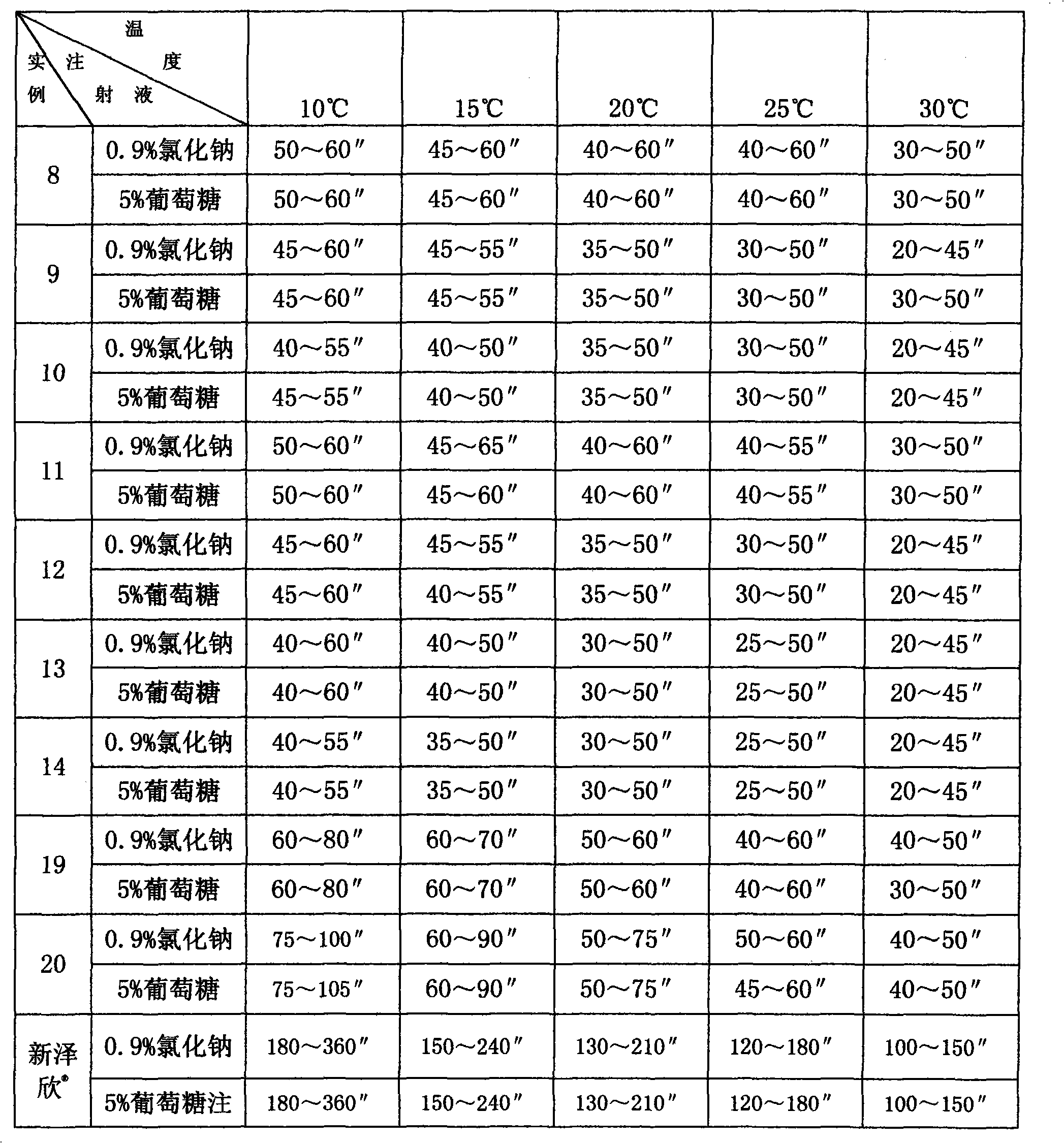
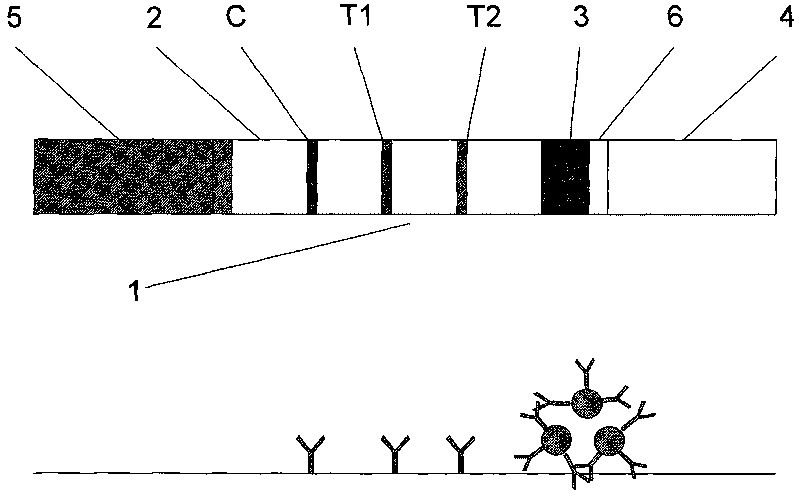
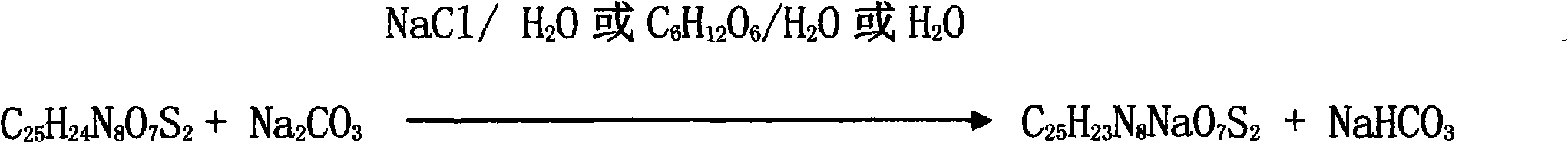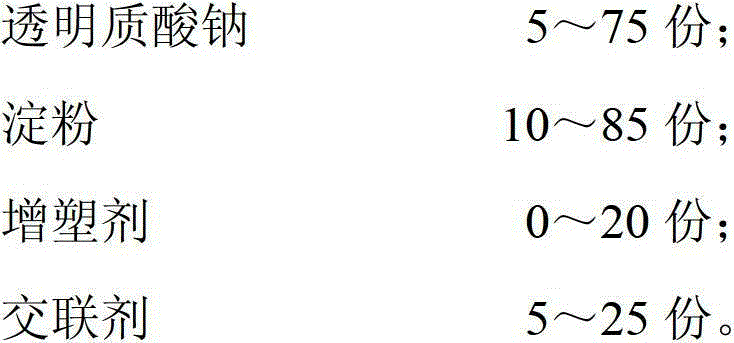Patents
Literature
139results about How to "Clinically convenient" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Double membrane tissue patching material and preparation method thereof
The invention discloses a double-layer membranous tissue repair material and a preparation method thereof, wherein, a cell-free membranous biological derivative material is used as a surface layer, and a fibroblast is compounded in the interior of a biological support material to form a substrate, and then the surface layer and the substrate are combined in a chimeric way to form the double-layer membranous tissue repair material; a compact surface layer structure can effectively reduce the loss of water, electrolytes and protein from surface of wound, avoid the invading and the reproduction of bacteria to the impaired surface of wound as well as prevent the infection of the surface of wound, thus being beneficial to epitheliosis and epithelial growth; the substrate can directly repair the surface of wound, promote the ingrowth of cells around the surface of wound and the angiogenesis, induce the differentiation from stem cells to skin cells and quicken wound healing; compared with the existing products, the tissue repair material has the advantages of being capable of promoting the regeneration of skin, improving the elasticity, the flexibility and the mechanical abrasion resistance of skin after the surface of wound is healed, reducing hyperplasia of scar tissues, controlling the contracture, having excellent biocompatibility, increasing the success rate of transplant and improving the quality of healing; the invention has wide material resources and simple production method; the double-layer membranous tissue repair material prepared is applicable to the clinical treatment of skin defect caused by inflammation, ulcer, thermal burns, iatrogenicity and the like.
Owner:SHAANXI RUISHENG BIOTECH
Time resolution immunochromatographic test strip for quantitatively detecting pepsinogen I as well as preparation method of time resolution immunochromatographic test strip
ActiveCN104422772AHigh sensitivitySmall difference between batchesDisease diagnosisPepsinPepsinogen I
The invention relates to the field of the clinic immunological detection, and in particular to a time resolution immunochromatographic test strip for quantitatively detecting pepsinogen I. The test strip comprises a plastic snap shell, a soleplate as well as a sample pad, a conjugate pad, a coating film and a piece of water absorption paper, which are sequentially adhered onto the soleplate in a staggering manner. The conjugate pad is coated with a pepsinogen I monoclonal antibody I marked by rare-earth ion microspheres; the coating film is coated with a detection band and a quality control band, a pepsinogen I monoclonal antibody II for recognizing different epitope is fixed on the detection band, and a rabbit anti-mouse IgG antibody is fixed on the quality control band. The invention also discloses a preparation method of the test strip. The two-antibody antigen sandwiched measuring technology and a time resolution immunochromatograhic technology are introduced to the detection of the pepsinogen I, and the single-person quantitative detection of the pepsinogen I is realized by combining a fluorescent detector; moreover, the sensitivity is high, the intra difference and inter difference are small, and great convenience is provided for the clinical use.
Owner:无锡市江原实业技贸有限公司
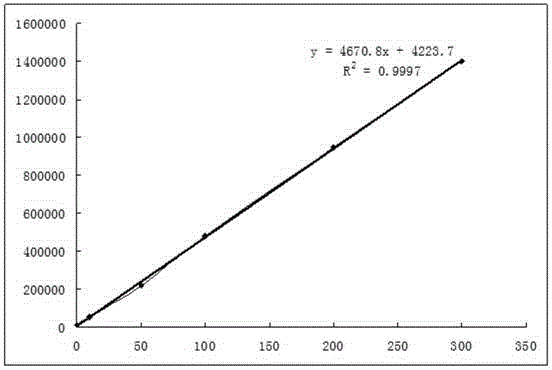
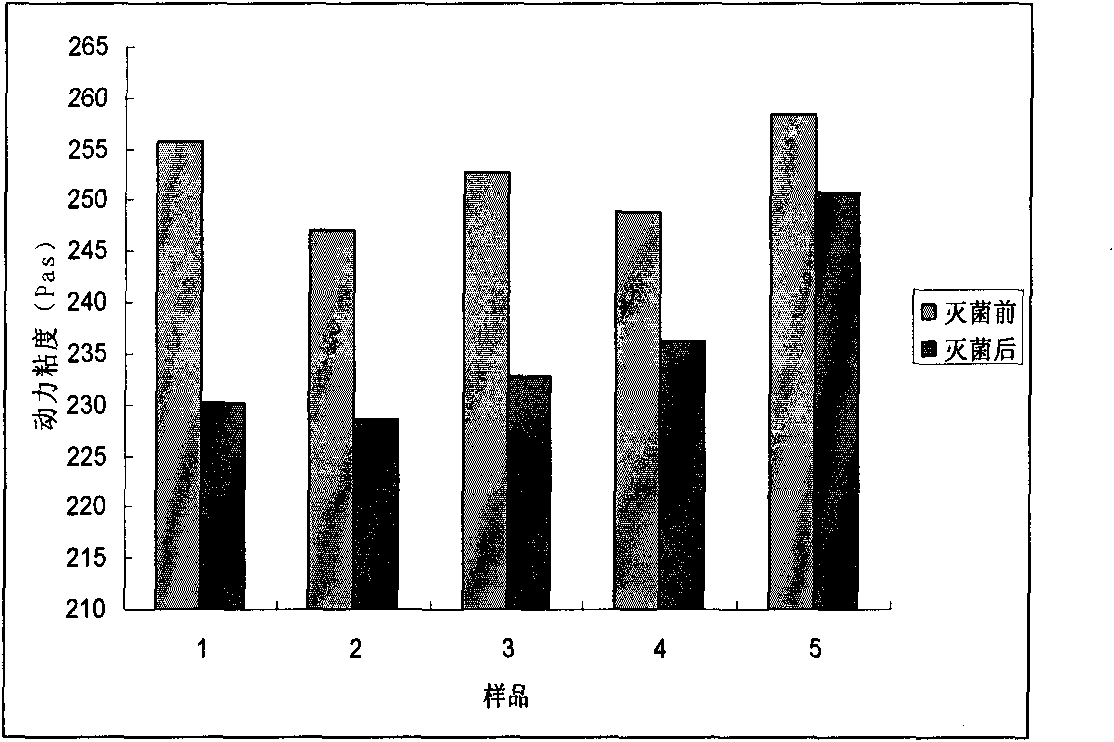
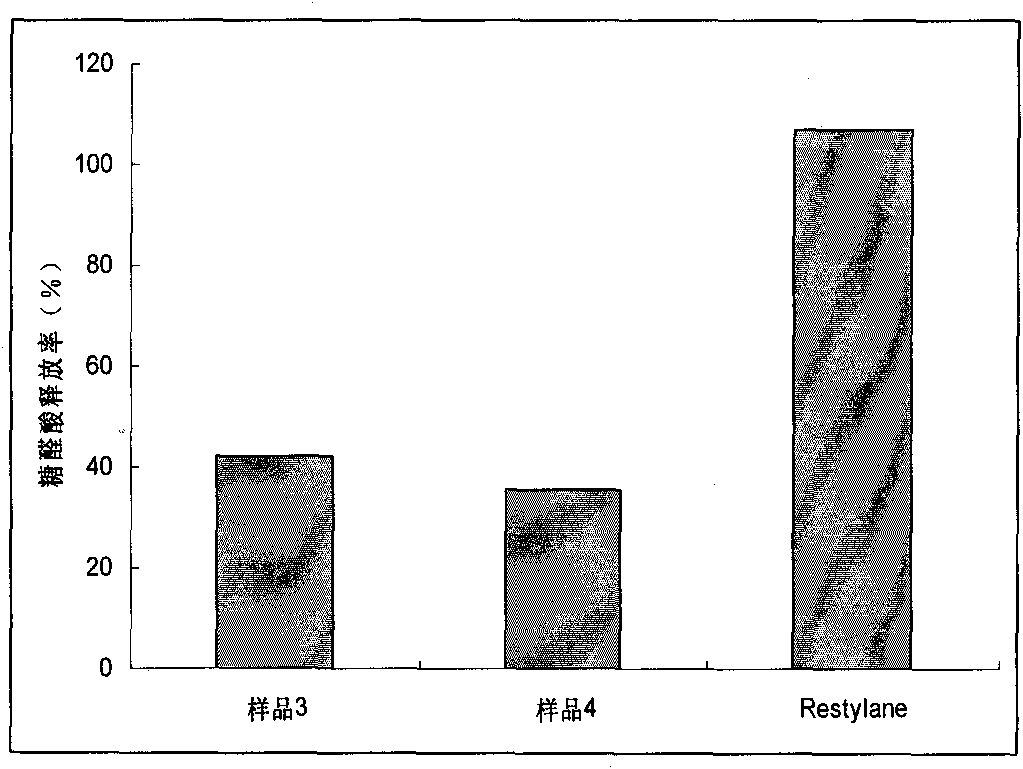
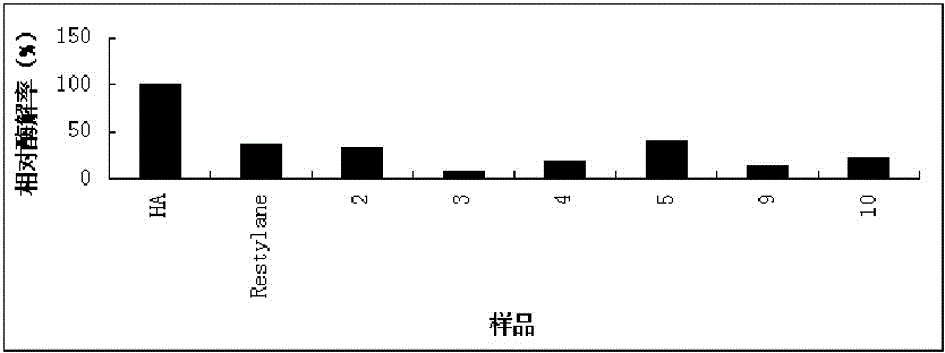
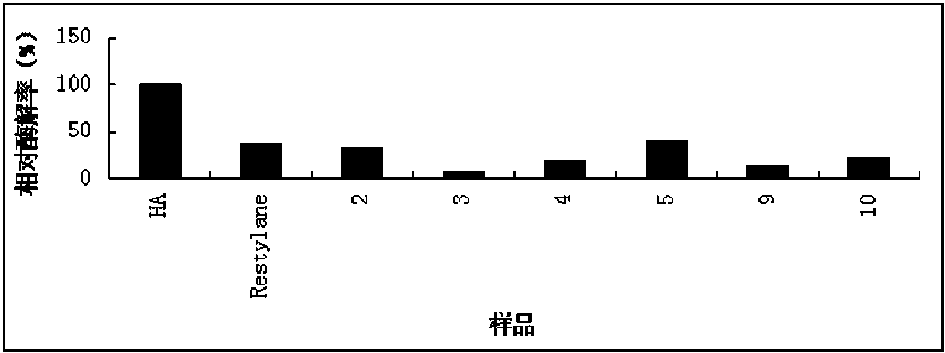
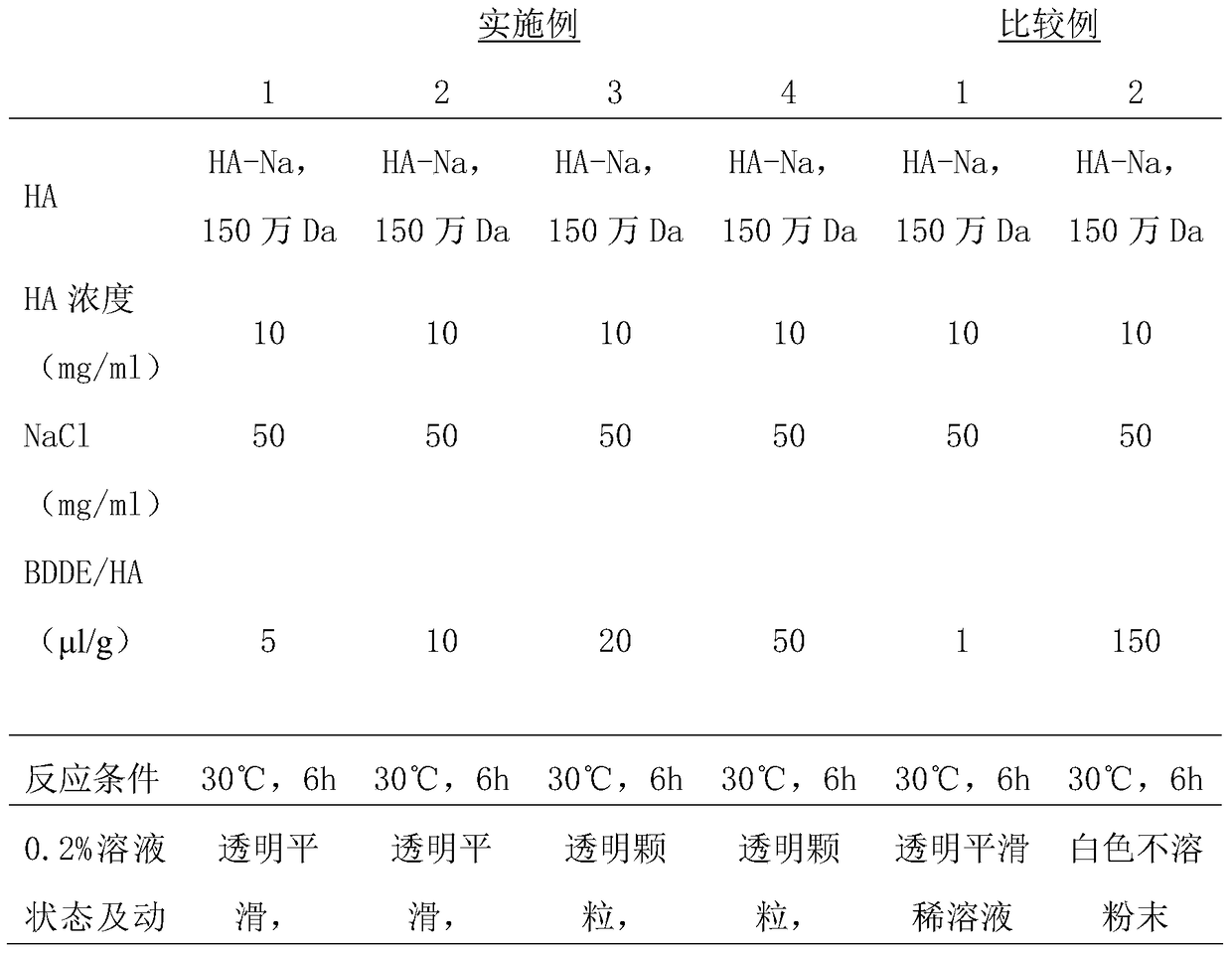
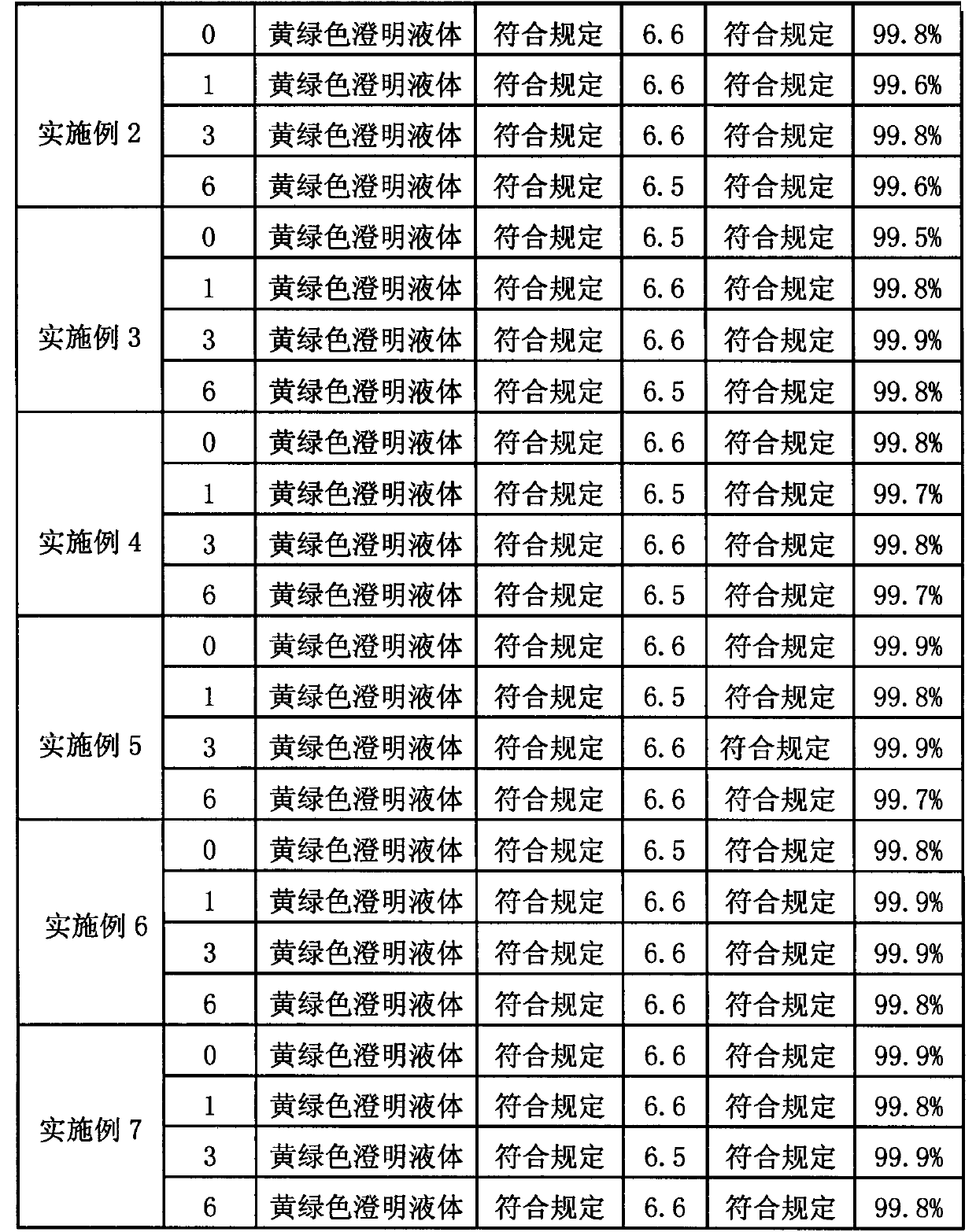
Preparation method for agranular crosslinking sodium hyaluronate with high-temperature-resistant and enzymatic-hydrolysis-resistant characteristics
InactiveCN102757572AGood gelLong retention time in the bodyProsthesisOrganic solventEnzymatic hydrolysis
The invention relates to a novel preparation method for agranular crosslinking sodium hyaluronate. Crosslinking reaction is performed in the low-concentration linear molecule state, and accordingly excess exposure of enzymatic-hydrolysis sites caused by crushing or extrusion of mechanical external forces of crosslinking sodium hyaluronate in traditional processes can be avoided. Redundant crosslinker can be eliminated effectively by white-powdered crosslinking sodium hyaluronate particles obtained by physical precipitation by using organic solvent, and accordingly accurate distribution of different concentrations of products can be guaranteed. The crosslinking sodium hyaluronate has the characteristics of high-temperature resistance, enzymatic-hydrolysis resistance and agranulation, and excellent injectability can be improved evidently. Kinetic viscosity of the agranular crosslinking sodium hyaluronate is reduced by 3-10% after sterilizing at 121 DEG C for 30 minutes, enzymatic hydrolysis is performed to 1000 U of sodium hyaluronate for 48 hours, and releasing amount of uronic acid is smaller than 40%.
Owner:SHANGHAI QISHENG BIOLOGICAL PREPARATION CO LTD
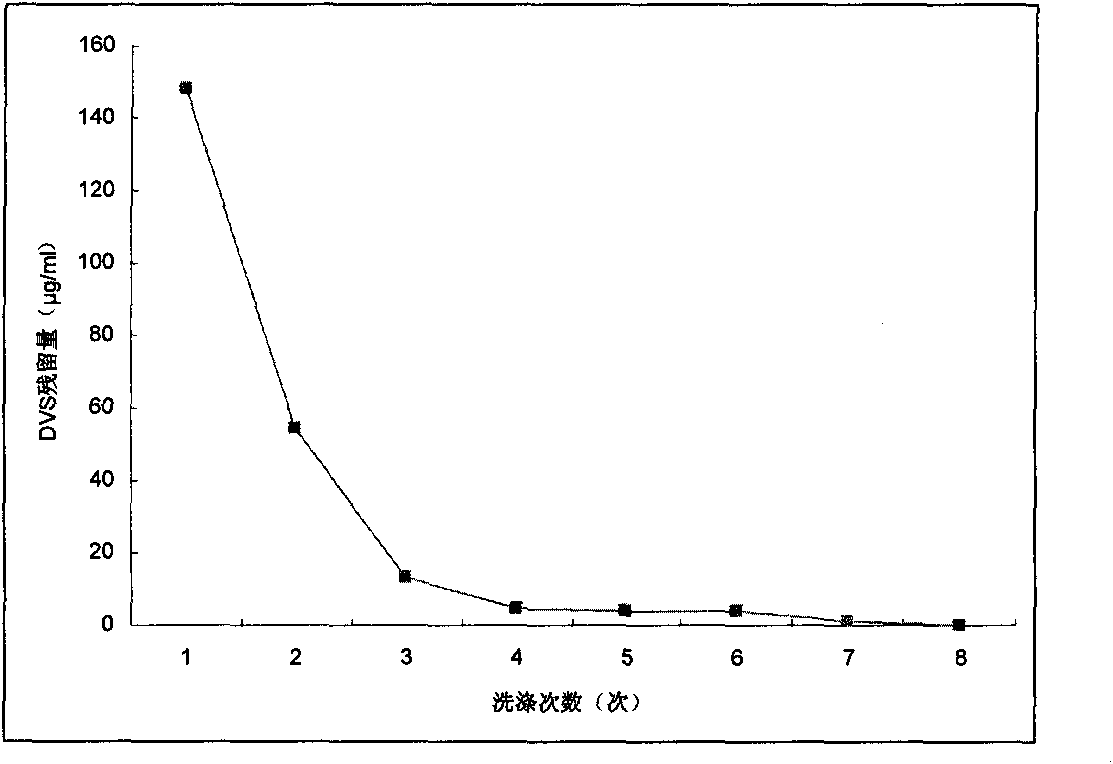
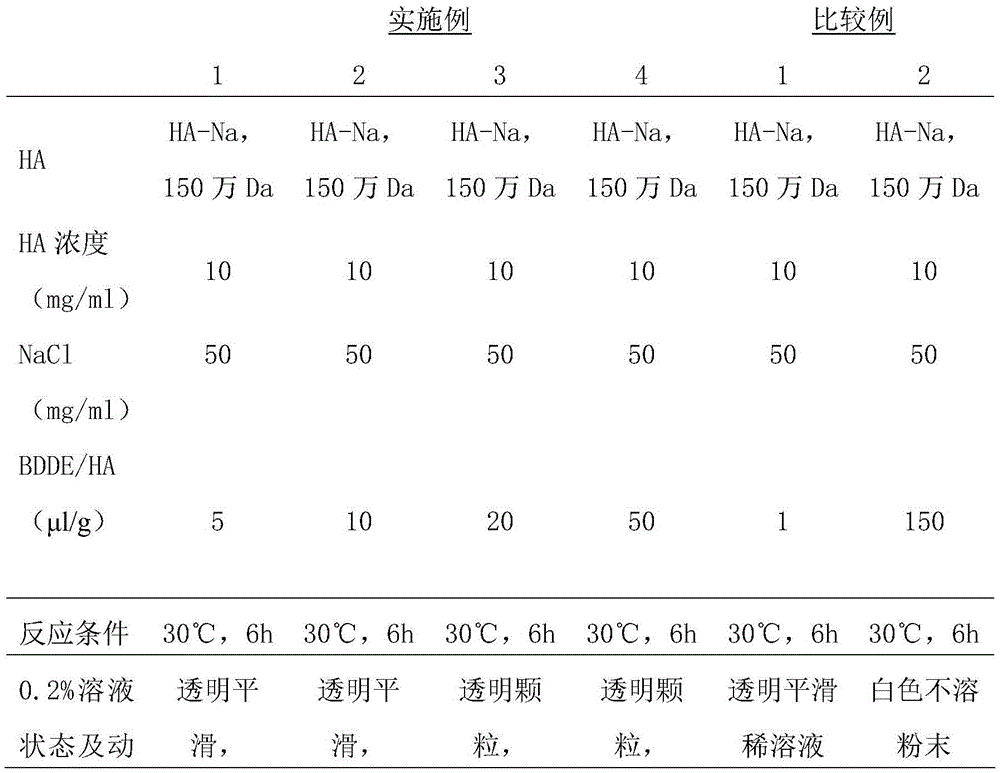
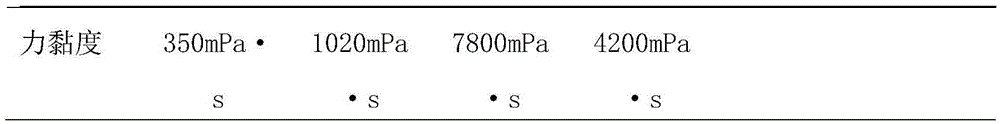
Crosslinked hyaluronic acid dry powder as well as preparation method and application thereof
ActiveCN105670011AImprove performanceClinically convenientCosmetic preparationsToilet preparationsMoisture retentionInorganic salts
The invention relates to the technical field of hyaluronic acid, in particular to crosslinked hyaluronic acid dry powder. Hyaluronic acid is dissolved in an inorganic salt water solution premixed with a crosslinking agent; an organic solvent is added, precipitates are separated out from the hyaluronic acid, a solid-liquid suspension system is formed, and a cross-linking reaction is performed; an organic solvent is added for washing the precipitates; vacuum drying is performed, and the crosslinked hyaluronic acid dry powder is obtained. The crosslinking reaction is performed in the solid-liquid suspension state, and the gelling property is better; crosslinked hyaluronic acid gels with different concentrations can be prepared from the obtained crosslinked hyaluronic acid dry powder, and use is facilitated. After the obtained crosslinked hyaluronic acid dry powder is dissolved in water, colorless transparent gel is formed, and the crosslinked hyaluronic acid dry powder has the effects that the viscoelasticity is excellent, the dry powder can perform the function of long-acting moisture retention after being applied to cosmetics, physical wrinkle filling is realized, active ingredients are released slowly, the cosmetics cosmetic is thickened, the skin feeling during use is improved and the like.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
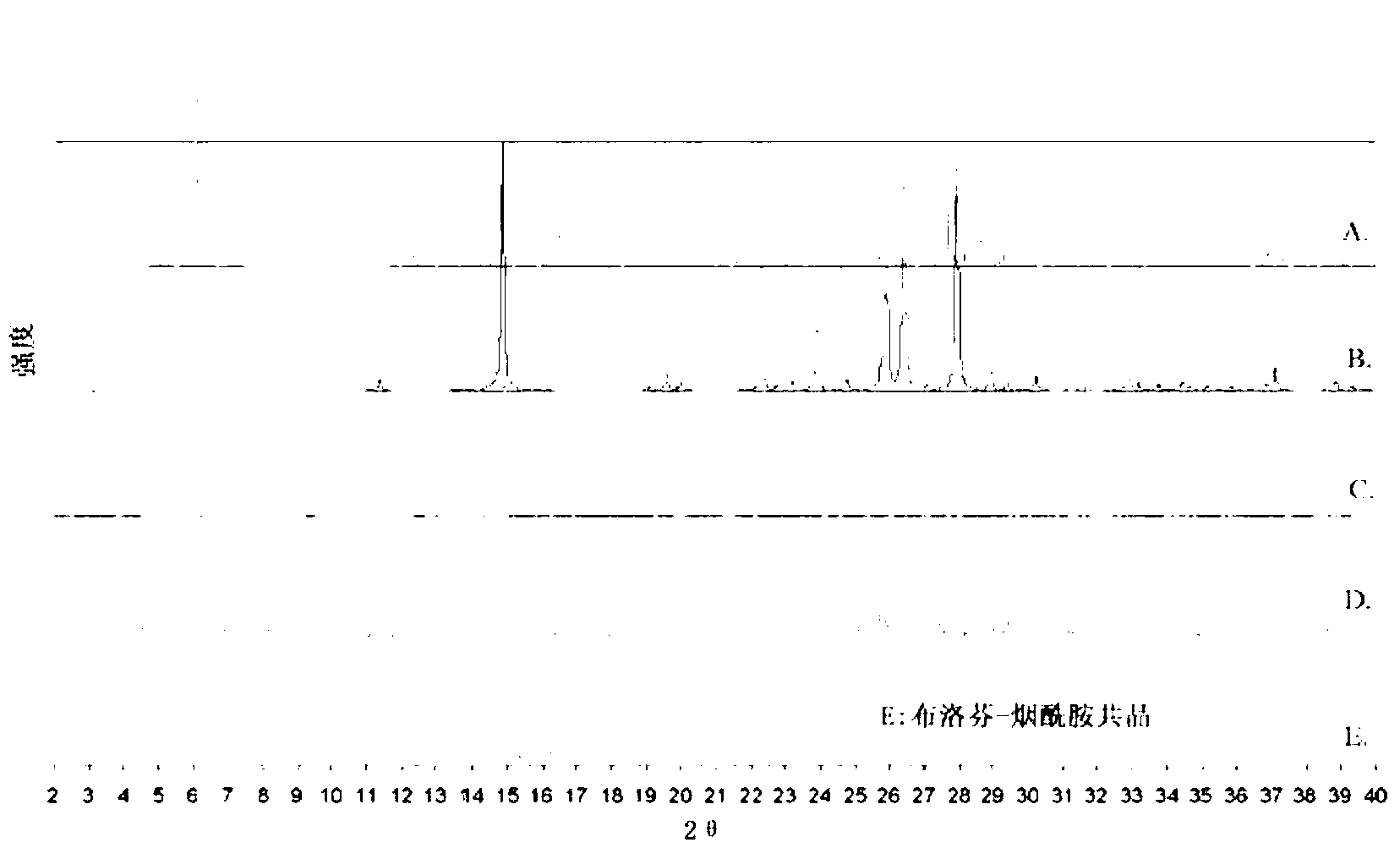
Preparation method of ibuprofen-nicotinamide eutectic crystals
The invention relates to a preparation method of ibuprofen-nicotinamide eutectic crystals. The preparation method comprises the following steps of: at the temperature of 70 to 60 DEG C, dissolving ibuprofen solids into a mixed solvent, wherein the concentration of the ibuprofen in the solution is 0.1 to 0.25g / ml, adding nicotinamide solids while stirring, wherein the mass ratio of ibuprofen to nicotinamide is (1.4:1) to (1.8:1), stirring, reacting fully until the solution is clear, reducing the temperature of the solution, wherein crystals start to separate at the temperature of 25 to 15 DEG C, then keeping on reducing the temperature to 0 to 5 DEG C, growing the crystals for 1 to 3 hours, filtering crystal pulp and drying, so as to obtain white ibuprofen-nicotinamide eutectic crystals. The dissolvability of the ibuprofen-nicotinamide eutectic crystals prepared by the preparation method is 158g / L in water, compared with the 100g / L dissolvability of the existing buprofen salts, the dissolvability is greatly improved, and the water solubility is increased accordingly; the preparation method is simple, the cost is low, and the quality is easy to control; the mass yield of the product is 56% to 70%.
Owner:TIANJIN UNIV
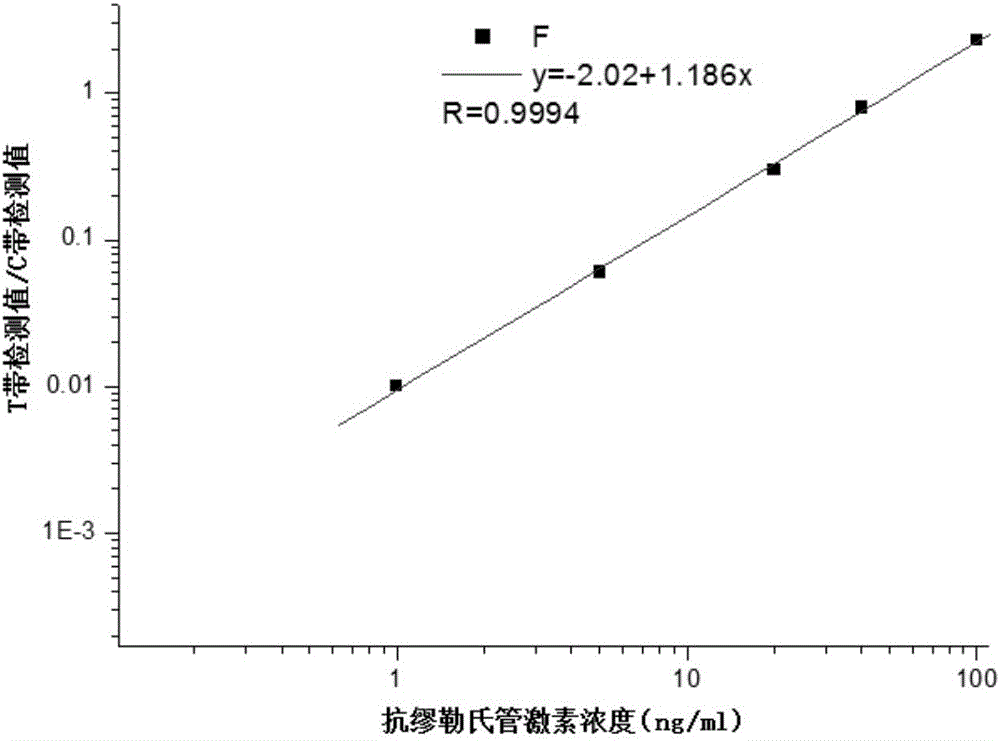
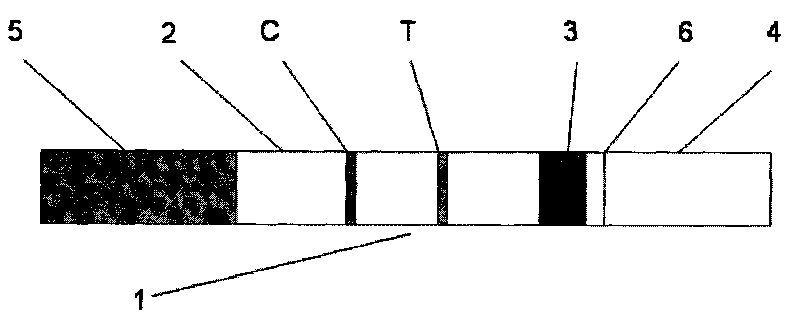
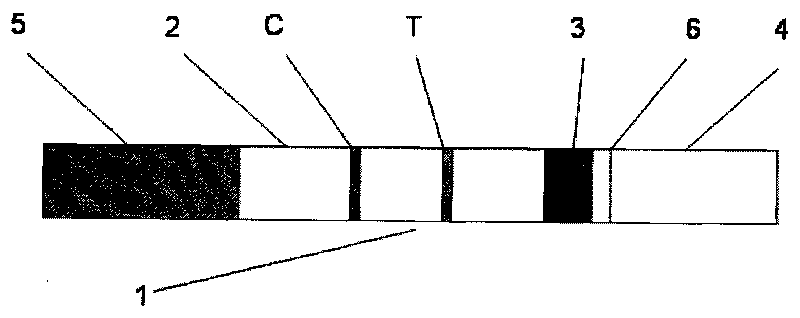
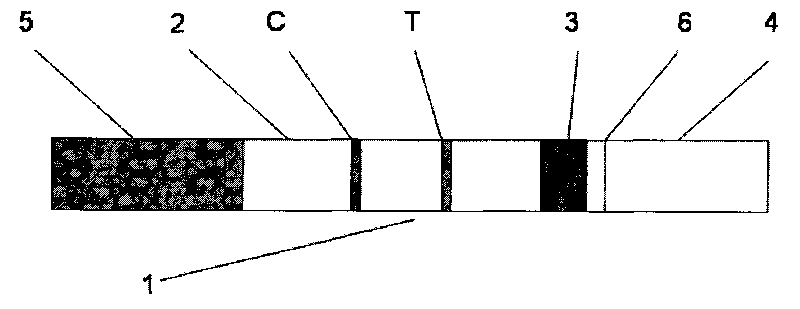
Test strip for quantitatively detecting anti-mullerian hormone, preparation method thereof and determination method for concentration of anti-mullerian hormone
InactiveCN105891490AShorten detection timeRealize quantitative detection of single servingMaterial analysisPhysical chemistryAnti-Müllerian hormone
The invention discloses a test strip for quantitatively detecting anti-mullerian hormone, a preparation method thereof and a determination method for the concentration of the anti-mullerian hormone, and belongs to the technical field of biology. The test strip comprises a base plate. A sample absorption pad, a labeled antibody pad, a coating film and a water absorption pad are sequentially attached to the base plate. The test strip has the advantages of being accurate in detection result, convenient to use and low in cost.
Owner:付国亮
Ornidazole injection and its preparation method
InactiveCN102335126AImprove solubilityReduce dosageAntibacterial agentsOrganic active ingredientsChemistryActivated carbon
The invention relates to a compound pharmaceutical preparation, i.e. an Ornidazole injection and its preparation method. The preparation contains Ornidazole, glacial acetic acid and injection water. The preparation method comprises the steps of: taking a proper amount of injection water (25DEG C-35DEG C), glacial acetic acid and Ornidazole, adding Ornidazole into water and stirring well, adding glacial acetic acid till complete dissolution, adjusting pH to 2.5-4.0, adding activated carbon which is 0.05% (g / ml) of the total volume of the solution, stirring the solution for 15min and conducting filtration, adding water to full capacity (with the temperature of the medical solution controlled between 25DEG C to 35DEG C), then carrying out filtration, embedding, disinfection, light inspection and packaging, thus obtaining the injection. The injection and its preparation method provided in the invention have the characteristics of simple prescription and process, high production efficiency, strong stability of the medical solution, etc.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Magnetic immuno-chromatographic test paper strip for quantitatively detecting tumor associated antigen 125 in blood and preparation method thereof
InactiveCN101762699ARealize single-serving wide-range quantitative detectionRealize a wide range of quantitative detectionMaterial analysisFluoresceinMedicine
The invention relates to a magnetic immuno-chromatographic test paper strip for quantitatively detecting tumor associated antigen 125 in blood and a preparation method thereof. A coating film, a magnet particle pad combined with tumor associated antigen 125 antibodies, a sample pad and a water absorbing pad are sequentially and alternately stuck on a base plate in a staggering way at intervals of 2mm, and then the upper layer is covered by a transparent plastic sealing film to form a test paper strip. A tumor associated antigen 125 antibody detection line and a quality control line are pre-coated on the coating film. The invention introduces the magnetic immuno-chromatographic technology and the fluorescein isothiocyanate system into the quantitative detection of tumor associated antigen 125, and has the advantages that the detection sensitivity is greatly improved, the accurate quantitative results can be given, the operation is simple and convenient and the diagnosis is rapid.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Magnetic immuno-chromatographic test paper strip for quantitatively detecting tumor associated antigen 15-3 in blood and preparation method thereof
InactiveCN101762697ARealize single-serving wide-range quantitative detectionClinically convenientMaterial analysisHematological testOncology
The invention relates to a magnetic immuno-chromatographic test paper strip for quantitatively detecting tumor associated antigen 15-3 in blood and a preparation method thereof. A coating film, a magnet particle pad combined with tumor associated antigen 15-3 antibodies, a sample pad and a water absorbing pad are sequentially and alternately stuck on a base plate in a staggering way at intervals of 2mm, and then the upper layer is covered by a transparent plastic sealing film to form a test paper strip. A tumor associated antigen 15-3 antibody detection line and a quality control line are pre-coated on the coating film. The invention introduces the magnetic immuno-chromatographic technology into the quantitative detection of tumor associated antigen 15-3, and has the advantages that the detection sensitivity is greatly improved, the accurate quantitative results can be given, the operation is simple and convenient and the diagnosis is rapid.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Pharmaceutical preparation containing recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein and preparation thereof
ActiveCN102670522AStable in natureClinically convenientPowder deliveryPeptide/protein ingredientsFreeze-dryingSubcutaneous injection
The invention discloses a pharmaceutical preparation containing recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein and preparation of the pharmaceutical preparation. According to the invention, the medical preparation containing the rHSA / G-CSF (recombinant human serum albumin-human granulocyte colony stimulating factor fusion protein) is a freeze-drying preparation, and each freeze-drying preparation comprises the following components: 1-30mg of rHSA / G-CSF protein, 10-80mg of pharmaceutically acceptable water-soluble excipient, 5-30mg of pharmaceutically acceptable protective agent and 5-50 mu mol of pH regulator. The pharmaceutical preparation containing the rHSA / G-CSF protein is suitable for administration in the ways of subcutaneous injection or intravenous injection and the like after being dissolved in water for injection, and the pharmaceutical preparation can be injected for the right dosage for treatment of neutrophilic granulocytopenia.
Owner:JIANGSU T MAB BIOPHARMA
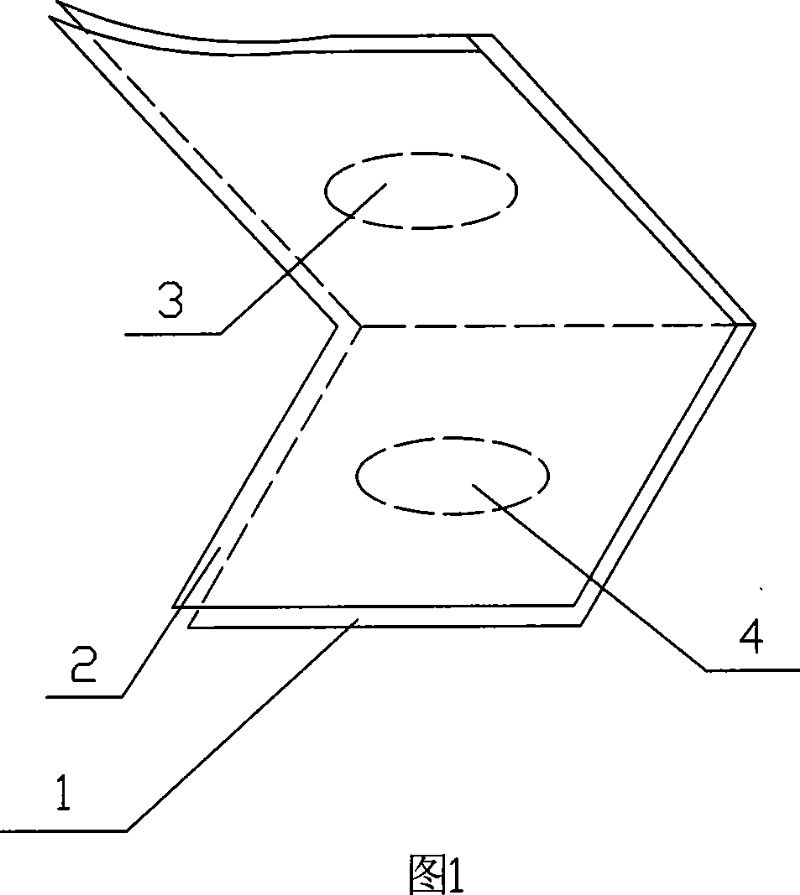
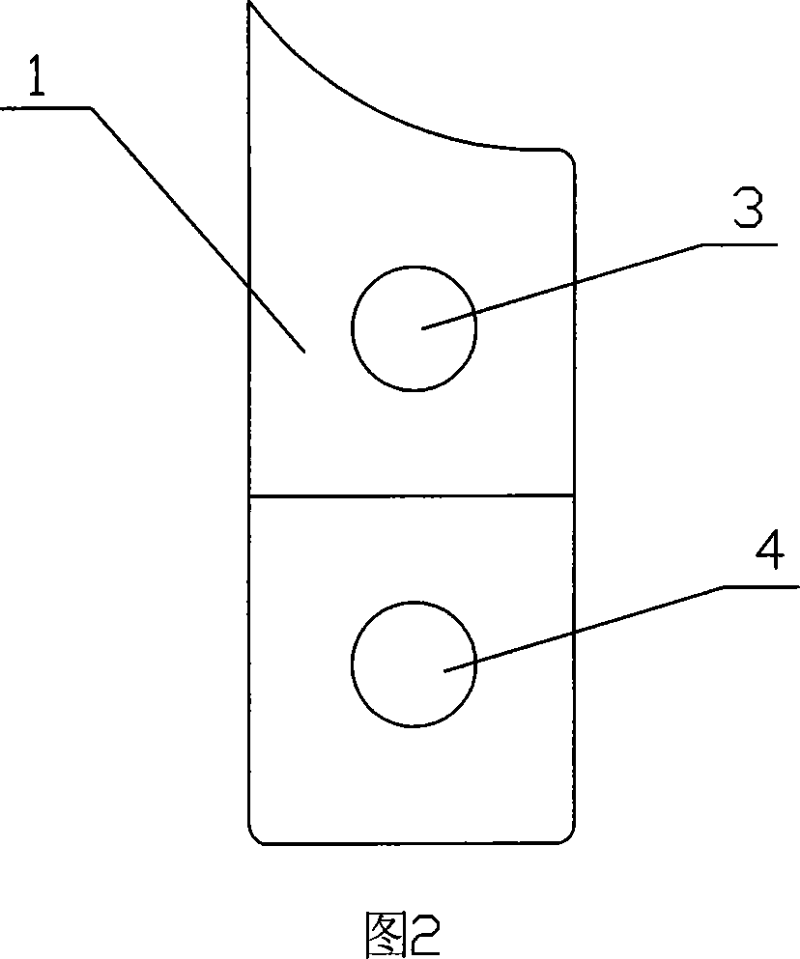
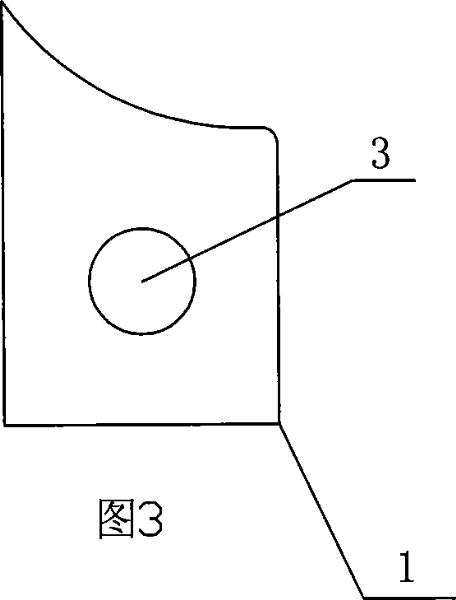
Amnion slice material and the preparing method and the application
The invention relates to an amnion sheet, relative preparation and application, used to plug lacrymal to treat xeroma, which resolves the problems of present lachrymal plug as complex preparation, high price and hard application. The inventive sheet comprises a support and an amnion, in same size and tight contact condition, wherein the support has two same holes, and the support can be folded along a long axis to engage two holes. And the preparation comprises that preparing support, preparing amnion and preparing amnion sheet. The invention can widen lacrymal plug treatment in xeroma with wide application. And the main component of amnion is kinds of collagens, which will degrade via humor / lacrimal collagenase in the lacrymal, while the amnion is soft to avoid hurt lacrymal wall and the held sharp will not bring bad feeling to user which can be taken out via clamping the sharp anytime.
Owner:何伟
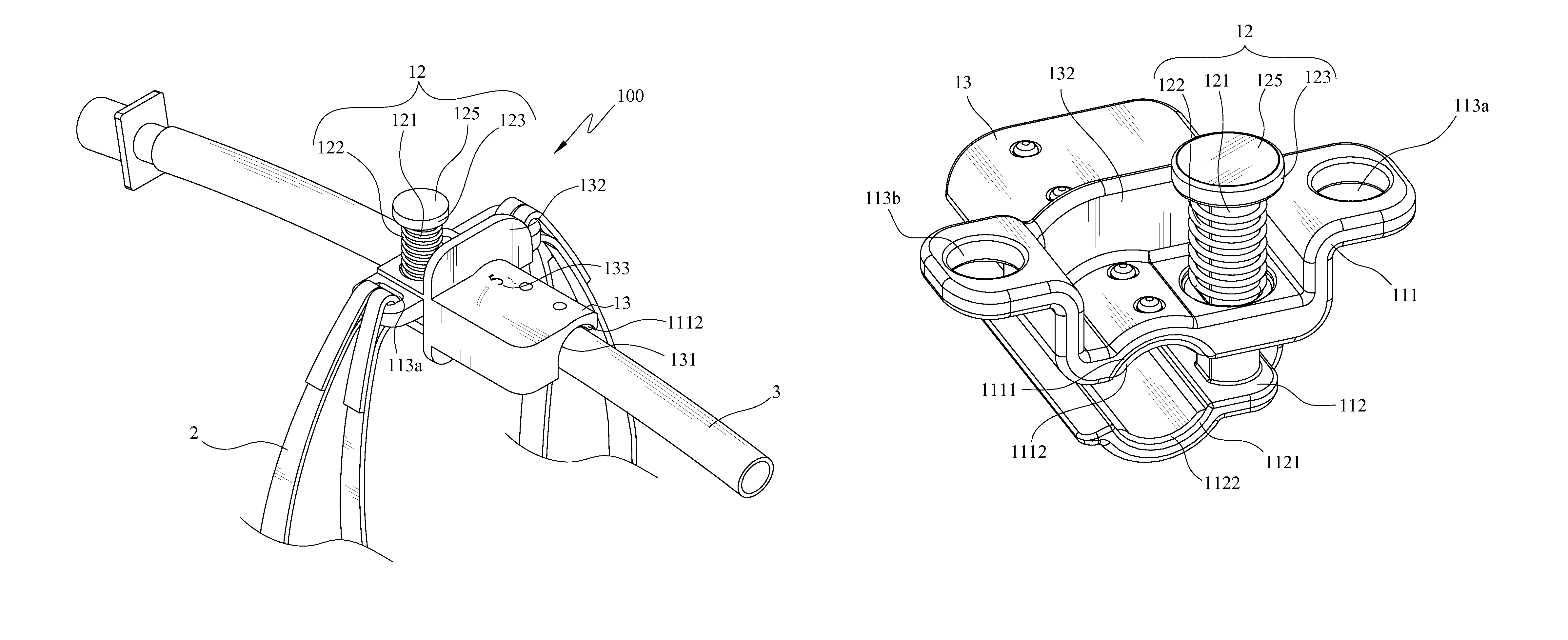
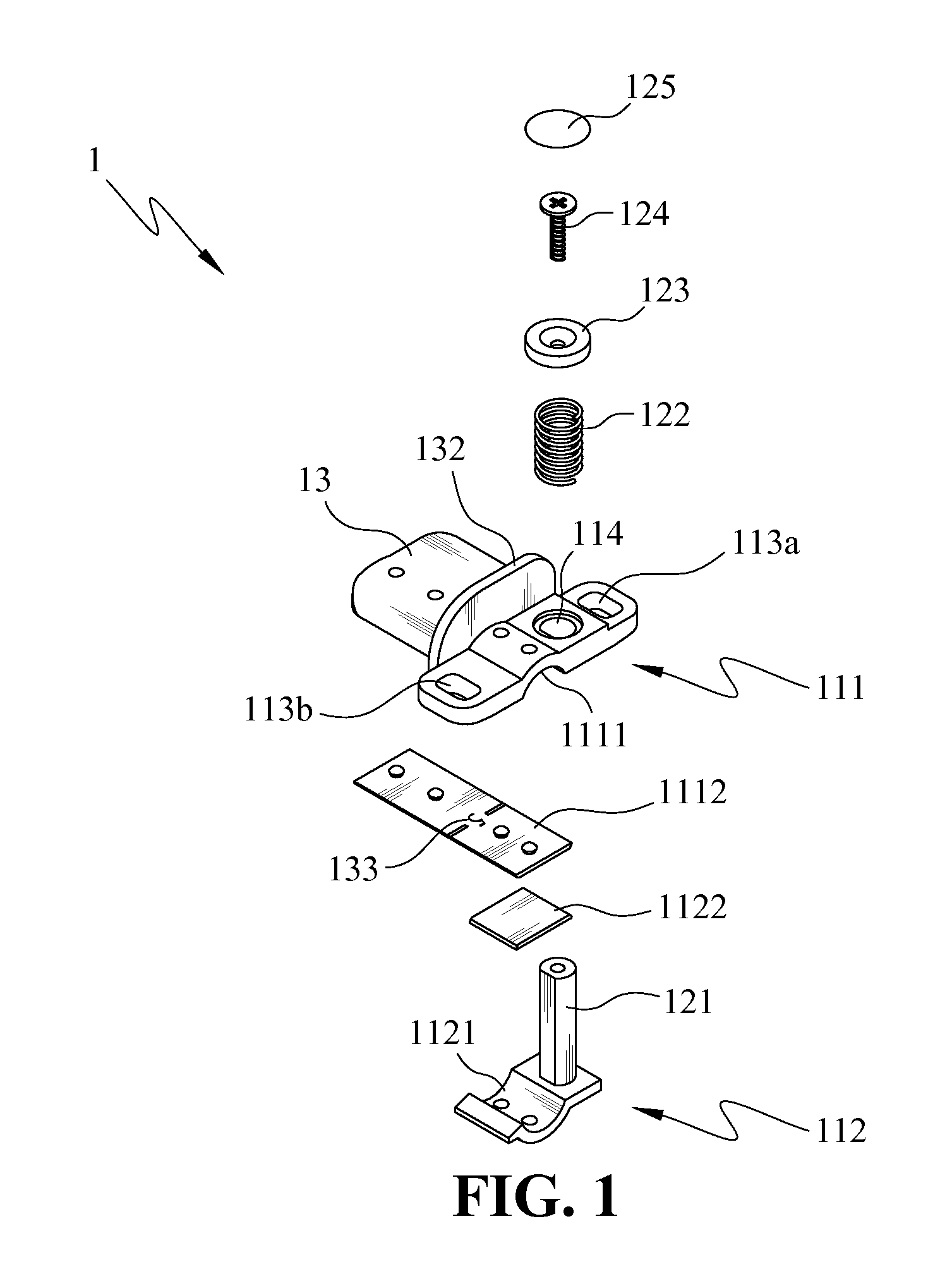
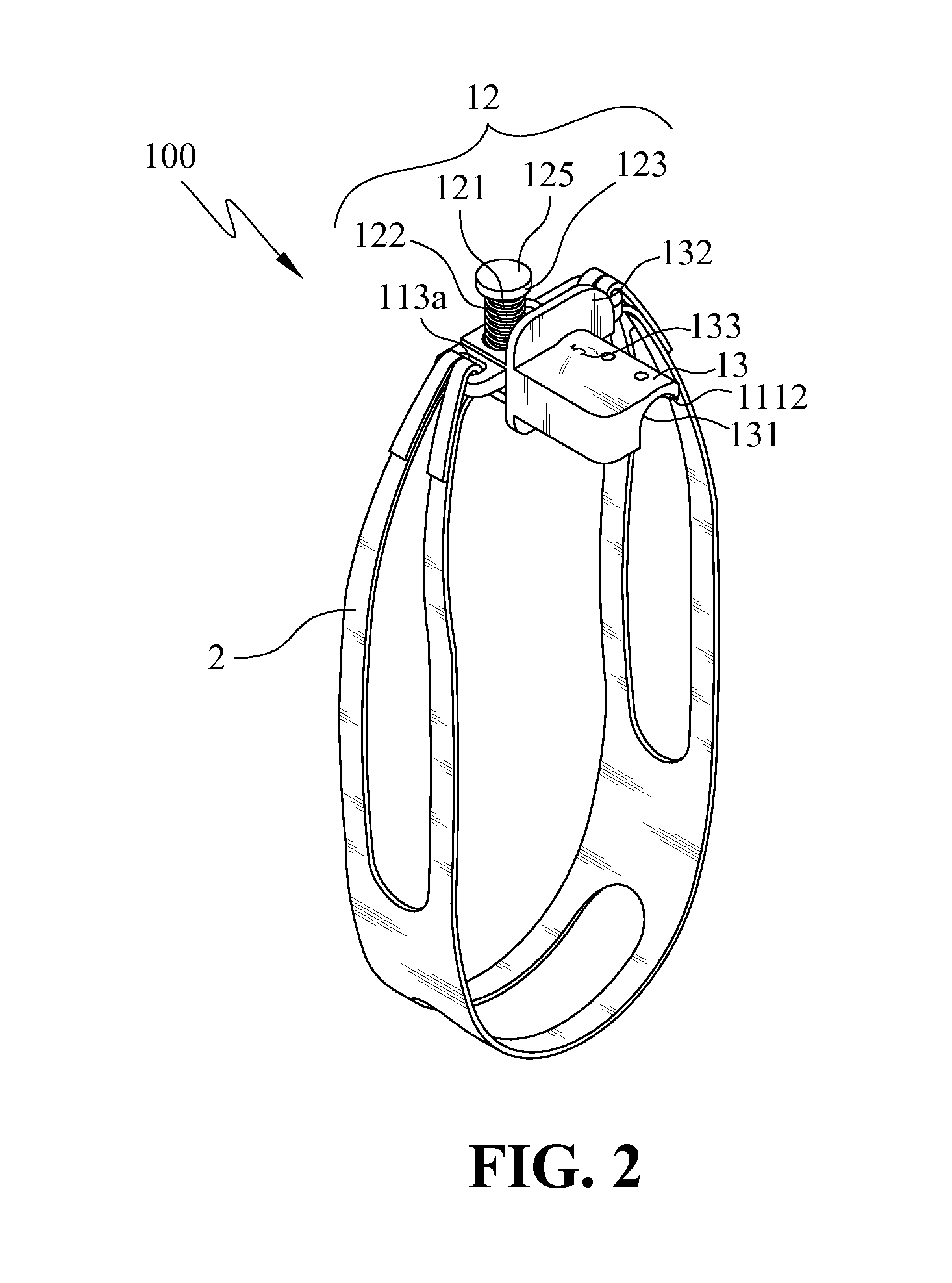
Endotracheal tube holder
InactiveUS8256427B2Fast and effectively securingReduce cavitiesTracheal tubesTeeth fillingEndotracheal tube holderEndotracheal tube insertion
Owner:MACKAY MEMORIAL HOSPITAL +1
Composition of taxone compound, and preparation method and application thereof
InactiveCN101485652AClinically convenientEasy to usePowder deliveryOrganic active ingredientsSolubilityChemical compound
The invention discloses a drug composition of taxanes, which comprises taxane compounds with effective dosage for treatment, a surfactant, a cosolvent and a stabilizer, wherein the cosolvent is selected from one or a mixture of more of polyethylene glycol, propylene glycol, glycerine or ethylene glycol; and the stabilizer is selected from hydrochloride, carbonate, phosphate and nitrate or a mixture of hydrochloride, carbonate, phosphate and nitrate. The composition can be an injection, a frozen dry powder needle or a kit for combined use. The composition can improve the solubility and stability of the taxane compounds. The invention also discloses a method for preparing the drug composition and application of the drug composition.
Owner:南通集智知识产权服务有限公司
Medical combination of teniposide, the preparing method and the function thereof
ActiveCN101062049AClinically convenientLess irritatingOrganic active ingredientsPowder deliverySolubilityFreeze-drying
The invention discloses a medicinal component of substitute nipa glycosides, which is characterized by the following: incorporating substitute nipa glycosides, Tuwen surface activator, assisting solvent and inorganic salt; choosing the assisting solvent as mixture of dimethyl ethanolamine and carbowax; choosing the inorganic salt from one or multiple common salt, potassium chloride, sulphate and sulphate; setting this component as injection or freeze dry powder needle; adding into Tuwen and the assisting dissolvent; adding into the inorganic salt at the same time; increasing solubility of substitute nipa glycosides. This invention also relates to the preparing method and usage of this medicinal component.
Owner:河北道恩药业有限公司
Carbataxel-elemene composite liposome and preparation method and application thereof
ActiveCN109260156ASmall particle sizeUniform particle size distributionOrganic active ingredientsPharmaceutical non-active ingredientsSide effectCholesterol
The invention discloses a capartasol-elemene composite liposome, preparation method and application thereof, and carbataxel-elemene composite liposome is prepared by dissolving carbataxel in absoluteethanol, adding polyethylene glycol derivative, cholesterol, phospholipid and elemene, and heating to 70-90 DEG C to melt to obtain organic phase; glycerol and water are mixed and heat preserve at 50-70 DEG C to obtain an aqueous phase; adding an organic phase into an aqueous phase, and homogenizing under high pressure after high-speed shearing to obtain the composite liposome; the mass ratio of carbataxel to elemene is 1:10-150. The obtained composite liposomes have small particle size and uniform particle size distribution, and can be used for the preparation and treatment of taxane-resistant tumors, which significantly improves the anti-drug resistance effect and curative effect to a variety of tumors, reduces the toxic and side effects of drugs, greatly alleviates the pain of tumor patients, and is very convenient for clinical use.
Owner:HANGZHOU NORMAL UNIVERSITY
Medicine composite of superfine sterile sodium carbonate and cephems
ActiveCN101927000ADissolve fastClinically convenientAntibacterial agentsOrganic active ingredientsSodium carbonateChemistry
The invention relates to medicine composite of superfine sterile sodium carbonate and cephems, which is applied to human medicine for injection and is formed by sterile cephems compound and superfine sterile sodium carbonate with grain size of below 20 Mum. The sterile cephems compound is sterile cefpiramide or sterile ceftazidime, the content of the superfine sterile sodium carbonate in the composite of superfine sterile sodium carbonate and sterile cefpiramide is 13.0-18.0%, and the content of the superfine sterile sodium carbonate in the composite of superfine sterile sodium carbonate and sterile ceftazidime is 8.0-12.0%. The invention has the advantages that when the prepared cefpiramide or ceftazidime powder for injection is clinically used, the prepared cefpiramide or ceftazidime powder for injection can be quickly solved by common transfused liquid medicine, such as 0.9% sodium chloride injection or 5% glucose injection and the like, by a nurse through normal operation even at the low temperature of 10 DEG C so as to be convenient for clinical use.
Owner:GUANGZHOU BAIYUSN TIANXIN PHARMA
Magnetic immuno-chromatographic test paper strip for quantitatively detecting PSA and fPSA in blood and preparation method thereof
InactiveCN101762691AFacilitate clinical judgmentSimple and efficient operationMaterial analysisMagnetEngineering
The invention relates to a magnetic immuno-chromatographic test paper strip for quantitatively detecting PSA and fPSA in blood and a preparation method thereof. A coating film, a magnet particle pad combined with PSA antibodies and rabbit IgG, a sample pad and a water absorbing pad are sequentially and alternately stuck on a base plate in a staggering way at intervals of 2mm, and then the upper layer is covered by a transparent plastic sealing film to form a test paper strip. A total PSA+fPSA antibody detection line and a goat anti-rabbit quality control line are pre-coated on the coating film. The invention introduces the magnetic immuno-chromatographic technology into the quantitative detection of PSA in blood, creatively places total PSA and free PSA on the same test paper strip for simultaneous detection, and has the advantages that the detection sensitivity is improved, the accurate quantitative results of the total PSA and the free PSA can be given, the ratio between the total PSA and the free PSA is clear, and great convenience is provided for clinicians to rapidly and accurately diagnose the illness of patients.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Traditional Chinese medicine powder for treating silky coccidiosis
InactiveCN103565987AShort course of treatmentGood curative effectPowder deliveryAntiparasitic agentsCoccidiosisMedicine
The invention discloses traditional Chinese medicine powder for treating silky coccidiosis and belongs to the field of traditional Chinese medicines. The traditional Chinese medicine powder is prepared from medicinal antifeverile dichroa root, root of Chinese pulsatilla, radix bupleuri, plantain seed, hairyvein agrimonia herb, radix astragali, garden burnet, radix sophorae flavescentis, licorice root, coptis root, cortex phellodendri, root of large-flowered skullcap and Chinese angelica according to a weight part ratio. A preparation method of the traditional Chinese medicine powder comprises careful selection on raw materials, sunning, crushing, sieving, weighing, mixing, traditional Chinese medicine powder division according to a dosage, packaging, and quality inspection. Each bag of the finished product comprises 200g of the traditional Chinese medicine powder. The traditional Chinese medicine powder treating silky coccidiosis has the advantages of short treatment course, high curative effect and low treatment cost.
Owner:吴昊
Traditional Chinese medicinal powder formulation for treating postpartum mastitis of Boer goat
InactiveCN102462827AShort course of treatmentGood curative effectUnknown materialsSexual disorderMyrrhPollen
The invention relates to a traditional Chinese medicinal powder formulation for treating postpartum mastitis of Boer goats, belonging to the field of traditional Chinese medicine. The traditional Chinese medicinal powder formulation is made from the following medicinal raw materials in parts by weight: medicinal honeysuckle, forsythia fruit, dandehon herb, Chinese violet, seed of snakegourd, burdock fruit, pollen, scutellaria root, bupleurum, angelica, frankincense, myrrh, red peony root, cowherb seed, pangolin scales, green tangerine peel, liquorice, white peony root, head stephania root, airpotato yam, nutagrass flatsedge rhizome, curcuma root, loofah, peach kernel, safflower, rehmannia dride rhizome and chuanxiong rhizome. The raw materials are carefully chosen, dried by air, ground, sifted, weighed, mixed, divided by a dose, packaged, checked by quality, and made into packages, wherein each package contains 200g of finished products. The traditional Chinese medicinal powder formulation has the advantages of short course of treatment, high curative effect and low Cost of treatment in the treatment of the postpartum mastitis of the Boer goats.
Owner:吴秀友
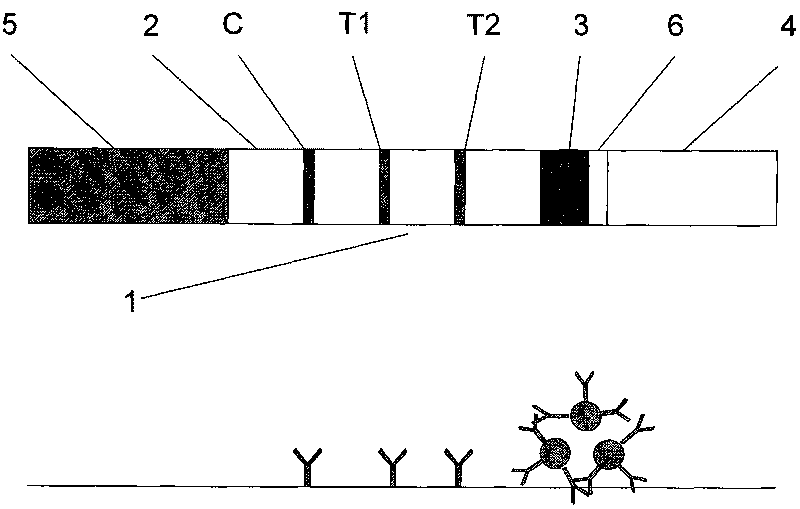
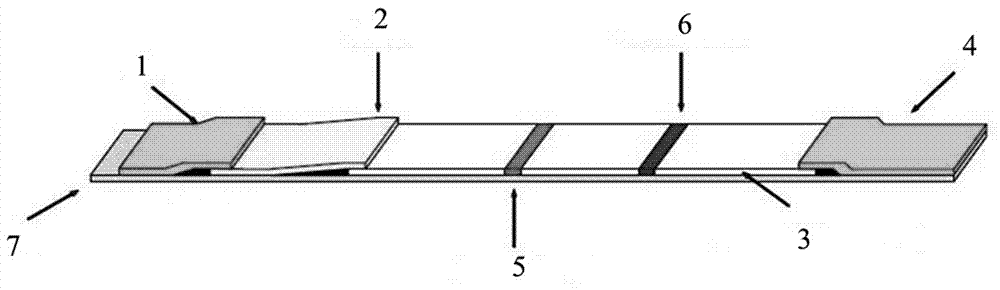
Test strip for detecting pepsinogen I and pepsinogen II as well as detection method and application of test strip
The invention relates to a time resolution fluorescence immunochromatographic test strip and test card for simultaneously and quantitatively detecting contents of pepsinogen I and pepsinogen II as well as an application of the test strip. According to the test strip disclosed by the invention, a nitrocellulose membrane is provided with a first detection zone, a second detection zone and a quality control zone which are sprayed at intervals, the first detection zone is coated with a pepsinogen I monoclonal antibody I, the second detection zone is coated with a pepsinogen II monoclonal antibody II, the quality control zone is coated with a rabbit anti-mouse polyclonal antibody, and a bonding pad is coated with a macromolecular nano microspherical coating with the surface decorated by the pepsinogen I monoclonal antibody I and the pepsinogen I monoclonal antibody and containing rare-earth ions; the test strip can simultaneously detect the contents of the pepsinogen I and the pepsinogen II, the problems that a pepsinogen test box cannot simultaneously detect the pepsinogen I and the pepsinogen II in the prior art can be solved, the high accuracy and the small error in the detection result of the pepsinogen I and the pepsinogen II in same sample liquid can be guaranteed, and the great convenience can be provided for the clinical use.
Owner:无锡市江原实业技贸有限公司
Multifunctional nursing bed
InactiveCN110251328AFacilitate body recoveryReduce work intensityElectrotherapyPneumatic massageMedical staffEngineering
The invention relates to a multifunctional nursing bed which comprises a bed body, a center control system, a massage mattress, an incubator and a toilet bowl. The center control system is arranged on the bed body and provided with a main controller, the main controller is connected with a temperature and humidity sensor and a temperature control device, the massage mattress is connected with the bed body and an overturning bed plate, the incubator is provided with a cover connected with the bed body, the cover is connected with a driving device and provided with a spraying pipe, the spraying pipe is connected with a water pumping device, and the toilet bowl is connected with the massage mattress. According to the multifunctional nursing bed, cleaning, excretion, treatment and the like of disabled patients are achieved, the working intensity of the medical staff is relieved, and excellent treatment environments are provided for patients.
Owner:GUANGDONG UNIV OF TECH
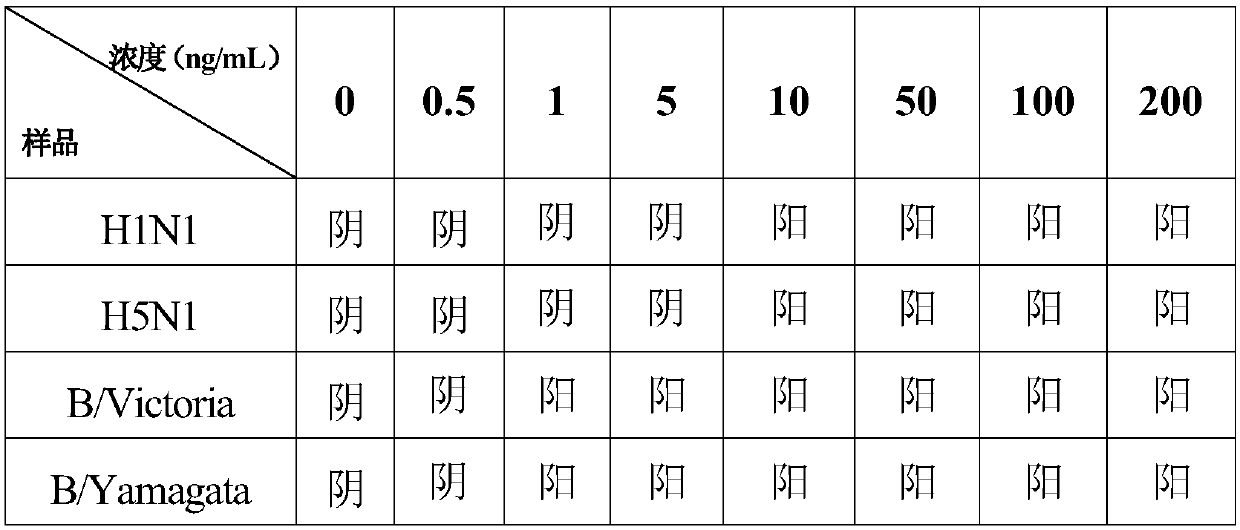
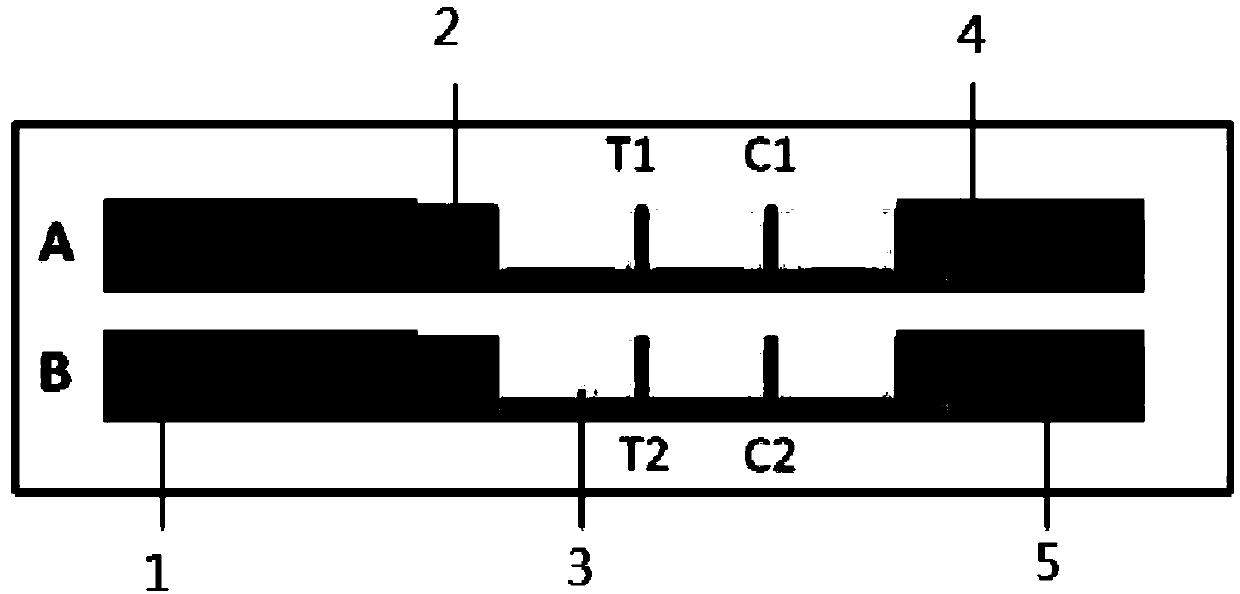
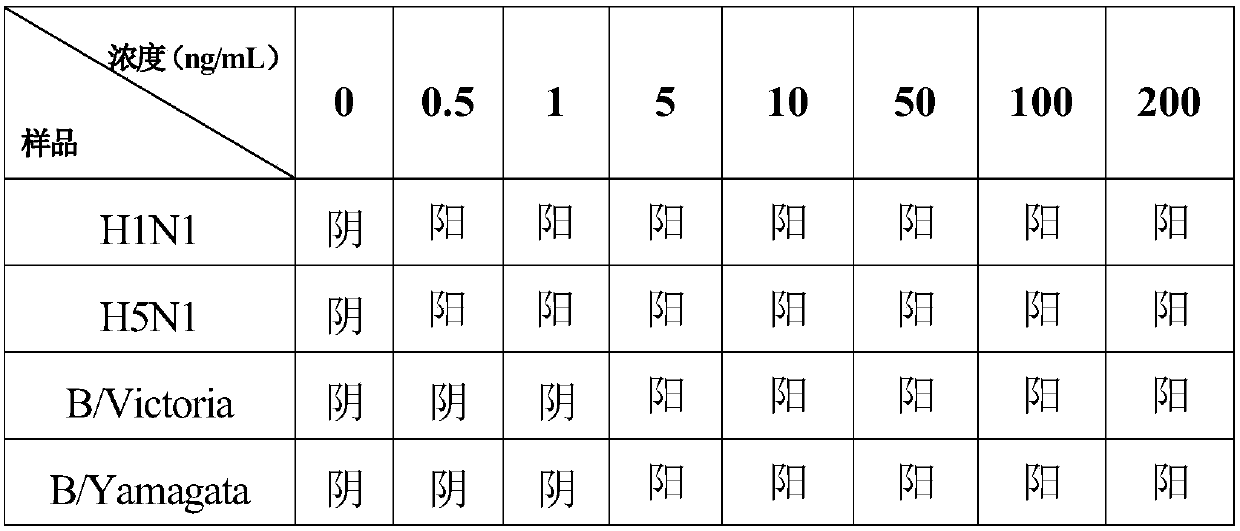
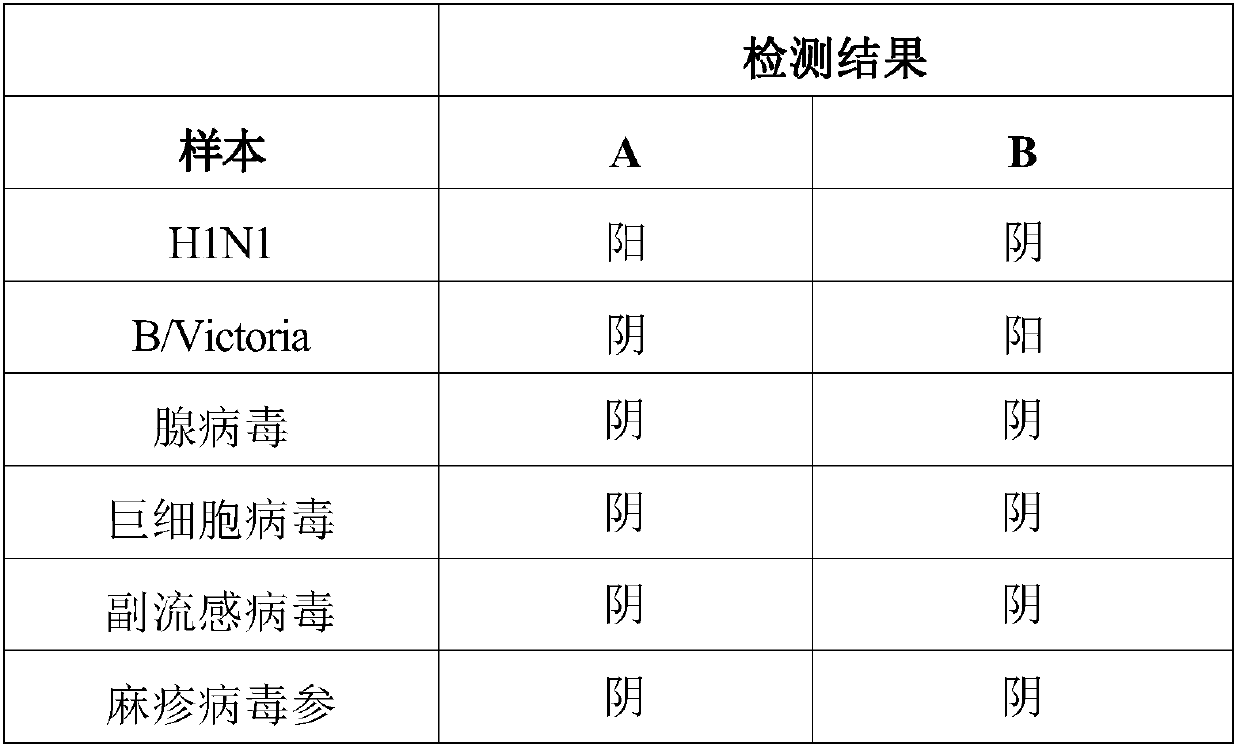
Test strip for simultaneously detecting influenza A and B viruses and preparation method
InactiveCN109580946AShorten detection timeImprove sensitivity and stabilityFluorescence/phosphorescenceImmunochromatographic testBiology
The invention discloses a time-resolved fluorescent immunochromatographic test strip for simultaneously detecting influenza A and B viruses and a preparation method. The test strip comprises a bottomplate, and a sample pad, a bonding pad, a reaction film and an absorbent pad which are sequentially adhered to the bottom plate. The binding pad is coated with influenza A and B viruses monoclonal detection antibodies marked with time-resolved fluorescent microspheres. The reaction film comprises a first detection zone, a second detection zone, and a control zone that are disposed in parallel andspaced apart from each other. The first detection zone is close to the bonding pad. The control area is close to the absorbent pad. The second detection zone is located in the middle of the first detection zone and the control zone. The first detection zone, the second detection zone, and the control zone are respectively coated with influenza A and B viruses monoclonal capture antibodies and goatanti mouse IgG antibodies that recognize a single antigenic epitope. The time-resolved fluorescent immunochromatographic test strip for simultaneously detecting influenza A and B viruses has high sensitivity, simple operation, low cost and short detection time, which can effectively meet the needs of clinical rapid inspection.
Owner:SHENZHEN YILIFANG BIOTECH CO LTD
A kind of cross-linked hyaluronic acid dry powder and its preparation method and application
ActiveCN105670011BImprove performanceClinically convenientCosmetic preparationsToilet preparationsWrinkle skinBULK ACTIVE INGREDIENT
The invention relates to the technical field of hyaluronic acid, in particular to crosslinked hyaluronic acid dry powder. Hyaluronic acid is dissolved in an inorganic salt water solution premixed with a crosslinking agent; an organic solvent is added, precipitates are separated out from the hyaluronic acid, a solid-liquid suspension system is formed, and a cross-linking reaction is performed; an organic solvent is added for washing the precipitates; vacuum drying is performed, and the crosslinked hyaluronic acid dry powder is obtained. The crosslinking reaction is performed in the solid-liquid suspension state, and the gelling property is better; crosslinked hyaluronic acid gels with different concentrations can be prepared from the obtained crosslinked hyaluronic acid dry powder, and use is facilitated. After the obtained crosslinked hyaluronic acid dry powder is dissolved in water, colorless transparent gel is formed, and the crosslinked hyaluronic acid dry powder has the effects that the viscoelasticity is excellent, the dry powder can perform the function of long-acting moisture retention after being applied to cosmetics, physical wrinkle filling is realized, active ingredients are released slowly, the cosmetics cosmetic is thickened, the skin feeling during use is improved and the like.
Owner:BLOOMAGE BIOTECHNOLOGY CORP LTD
Medical orthopedic cryotherapy ice packing machine
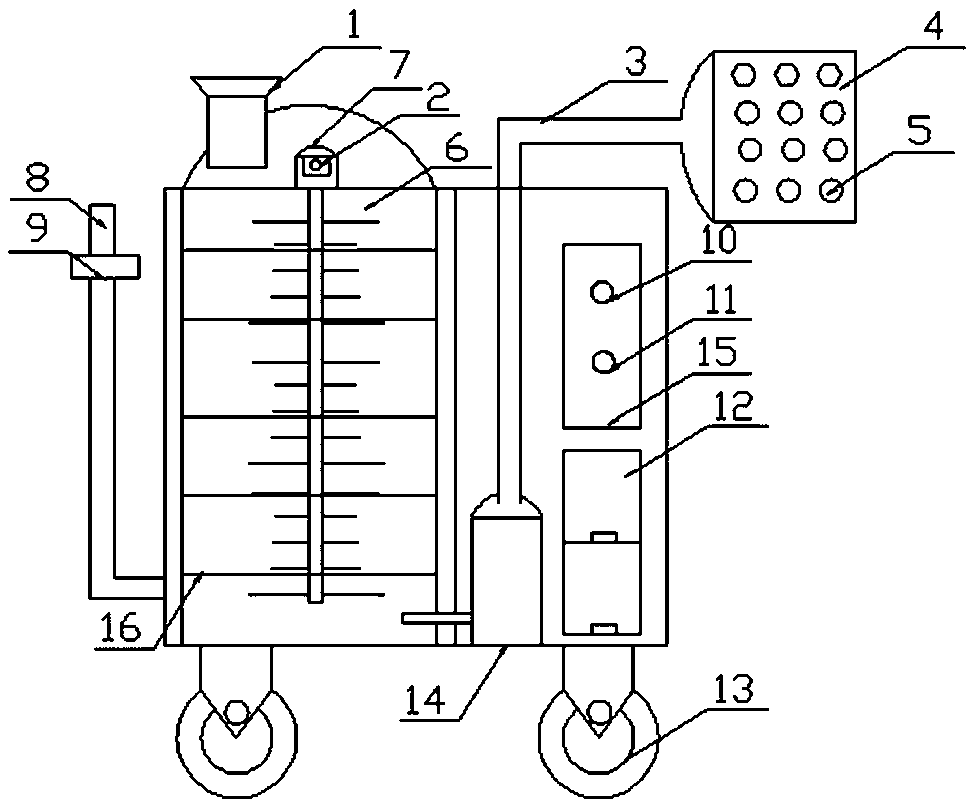
InactiveCN108836614ASimple structureStrong temperature controlRoller massageFirst-aid kitsExcessive BleedingEmergency Supply
The invention discloses a medical orthopedic cryotherapy ice packing machine which comprises a water adding funnel, a temperature display, a circulation tube, an ice pad, a massage ball, a water tank,a thermometer, a liquid level gauge, a positioning clip, a running switch, a temperature adjustment button, a first-aid box, a roller, a micro water pump, a controller and a cooling tube, wherein thetemperature display, the micro water pump and the cooling tube are fixedly mounted at the bottom in a water tank body; the water adding funnel is arranged at an upper end of the water tank; the thermometer is arranged in the water tank; a top end of the thermometer is arranged outside the water tank; the temperature display is arranged on the thermometer; the liquid level gauge is connected to the bottom of a left side of the water tank; the positioning clip is arranged at the upper end of the liquid level gauge; the controller is arranged on an outer housing of a right side of the water tank; the cooling tube is controlled to control the cooling by the controller conveniently and rapidly. Drugs, tourniquets and other emergency supplies are placed inside the first-aid box so that first aid can be performed when bleeding due to injury to prevent excessive bleeding to cause risks.
Owner:赵晓晨
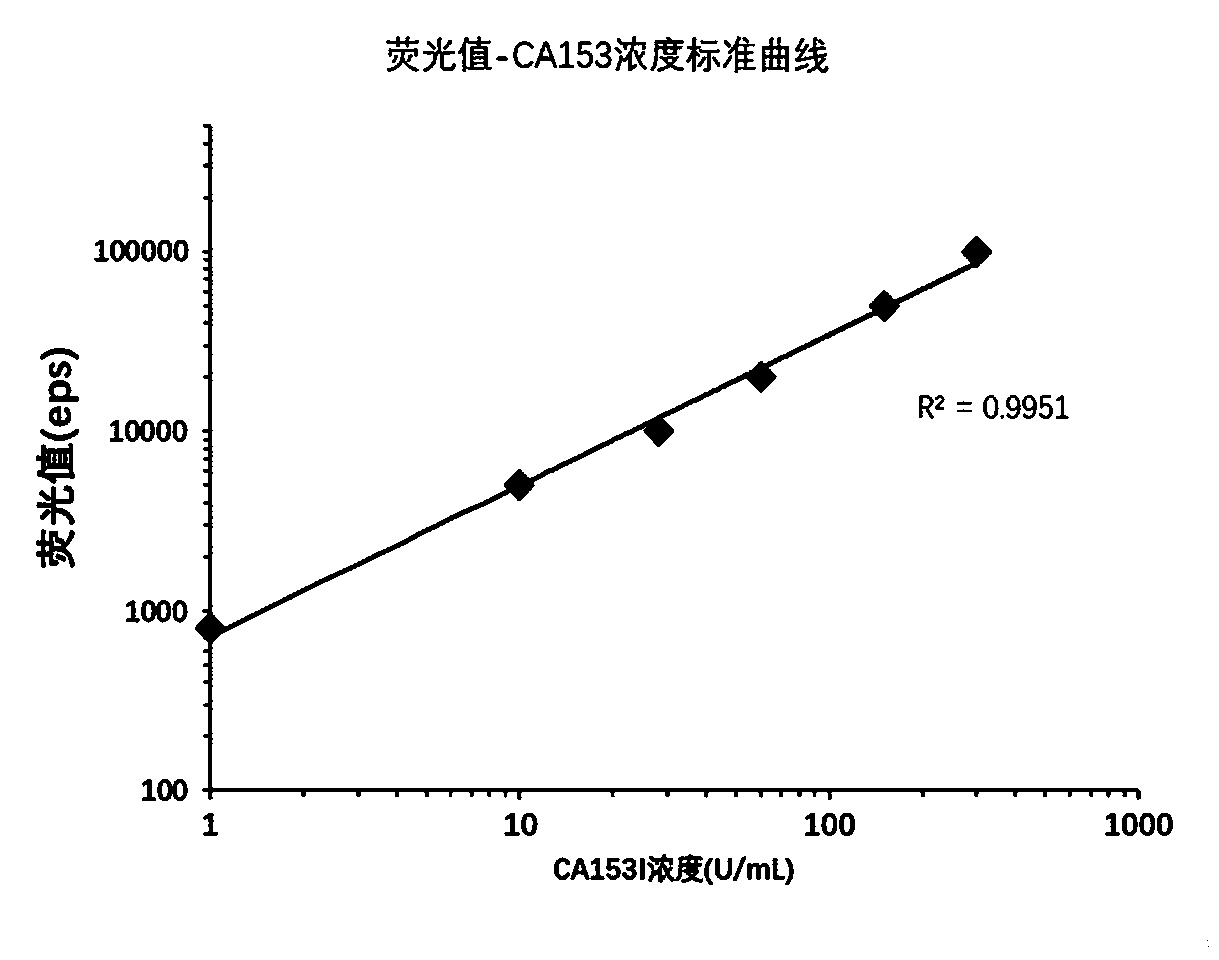
Time-resolved fluorescent immunochromatographic test strip for quantitatively detecting CA153 in blood and preparation method
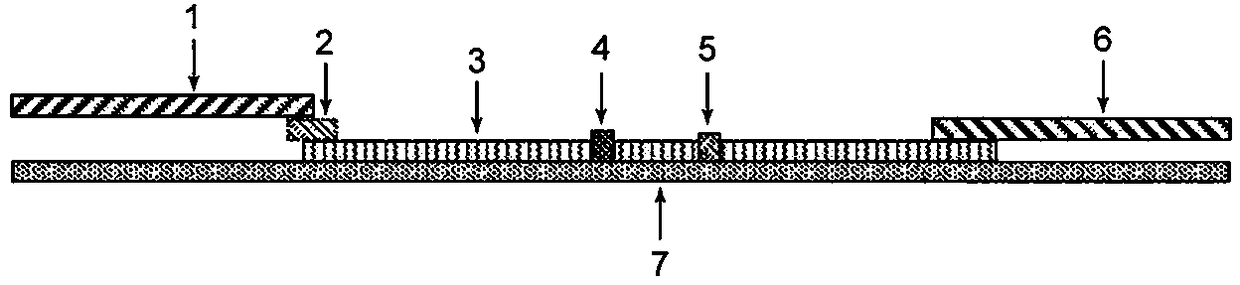
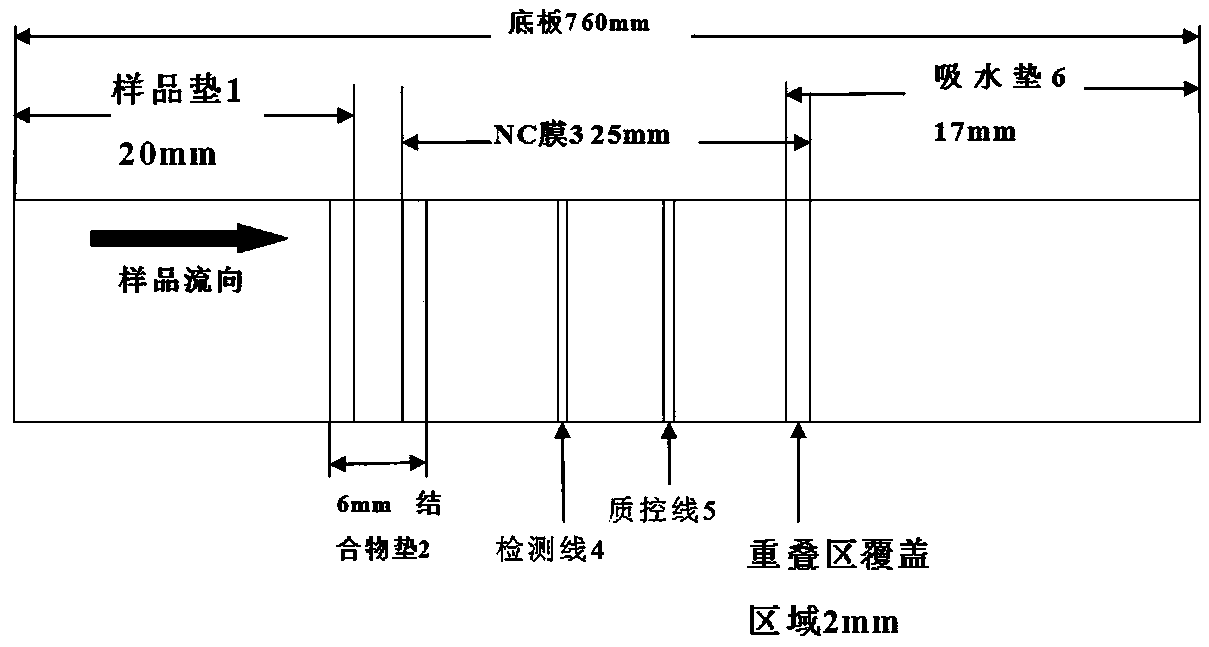
InactiveCN108535485ARealize quantitative detectionClinically convenientMaterial analysisQuality controlAbsorbent Pads
The invention relates to a time-resolved fluorescent immunochromatographic test strip for quantitatively detecting CA153 in blood and a preparation method thereof. The test strip is a test card formedby successively bonding an NC film, a CA153 antibody and mouse anti-rabbit IgG marked fluorescent microsphere conjugate pad, a sample pad and an absorbent pad on a white PVC substrate staggeredly at2 mm and then using upper and lower matched plastic card holder to fix the test strip for assembling, and the NC film is pre-coated with a CA153 antibody detection line and a rabbit IgG quality control line. According to the test strip, a time-resolved fluorescence immunochromatography technology is introduced into the quantitative detection of the CA153 in the blood, the detection time is greatlysaved, the stability and sensitivity of detection are improved, the operation is simple and convenient, and the test strip can be used for on-site screening; the test strip has the advantages of lowcost and high cost performance.
Owner:SHENZHEN MICRO BIOLOGICAL TECH CO LTD
Test strip for detecting influenza viruses and preparation method thereof
InactiveCN109580933AShorten detection timeImprove stabilityFluorescence/phosphorescenceEpitopeImmunofluorescence
The invention discloses a time resolution immunochromatographic test strip for detecting influenza A / B viruses and a preparation method thereof. The test strip comprises a bottom board and a sample pad, a combination pad, a reaction membrane and a water absorption pas, which are pasted on the bottom board in sequence, wherein the combination pad is coated with an influenza A / B virus monoclonal detection antibody marked by a time resolution fluorescent microsphere; the reaction membrane comprises a detection area and a quality control area parallel to and spaced from the detection area; the detection area is close to the combination pad; the quality control area is close to the water absorption pad; and the detection area and the quality control area are respectively coated with an influenza A / B viruses monoclonal capture antibody and a goat anti mouse IgG antibody for recognizing single epitopes. The time resolution immunochromatographic test strip for detecting influenza A / B viruses is high in sensitivity, simple to operate, low in cost and short in detection time, and is capable of effectively satisfying the demand of rapid clinical examination.
Owner:SHENZHEN YILIFANG BIOTECH CO LTD
Ornidazole injection and preparation method thereof
ActiveCN103006554BLow impurity contentGuaranteed stabilityAntibacterial agentsOrganic active ingredientsAnhydrous ethanolSide effect
The invention relates to the technical field of medicines, and particularly discloses an ornidazole injection and a preparation method thereof. The ornidazole injection comprises 500 grams of ornidazole and the balance of solvents added till the volume of total liquid medicine is 2000-3000 milliliters. The ornidazole injection and the preparation method thereof which are disclosed by the invention have the advantages of simple process, low cost, safety and reliability. Compared with the traditional ornidazole injection, the ornidazole injection increases the using amount of anhydrous ethanol without adding propanediol, plays cosolvent and stabilizer roles through the anhydrous ethanol, ensures the stability of the injection by reducing the impurity content of the injection, is convenience for clinical application, prevents the toxic and side effects of the propanediol on human bodies because the ethanol is safer than the propanediol and reduces the medical risk.
Owner:JINAN RUIFENG PHARMA +3
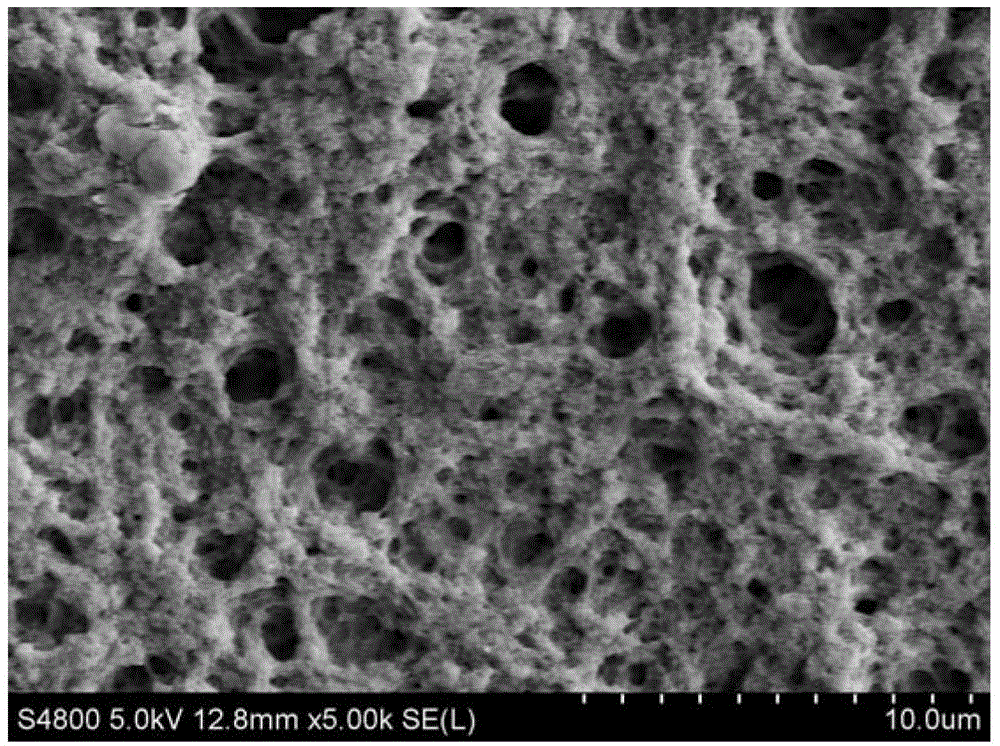
A kind of composite polysaccharide hemostatic material and its preparation method and application
ActiveCN103041448BHigh liquid absorptionRapid hemostasisOrganic active ingredientsSurgical drugsCross-linkSurgical operation
The invention relates to the field of a clinical surgery, and in particular relates to a compound polysaccharide hemostatic material. The compound polysaccharide hemostatic material comprises the following components in parts by weight: 5 to 75 parts of sodium hyaluronate, 10 to 85 parts of starch, 0 to 20 parts of plasticizer and 5 to 25 parts of cross-linking agent. According to the invention, sodium hyaluronate and starch are compositely cross-linked to obtain a porous support material, which is high in material liquid absorbing rate, fast in hemostasis and low amount of bleeding, so that a hemostatic effect is good. Meanwhile, sodium hyaluronate has the effects of endowing good anti-adhesion effect on the material, promotes wound healing and defected tissue repairing. The compound polysaccharide hemostatic material can be crushed in a granule or powder shape or ground in a soft sponge film shape, thereby being applied to the hemostasis of a traditional surgical operation, a minimally invasive surgery and trauma and wound tissue bleeding, being served as a filling and repairing and anti-adhesion material of defected tissues, and being served as a tissue engineer scaffold material, a medicine carrying support material and a wound dressing.
Owner:SUZHOU BOCHUANG TONGKANG PHARM TECH CO LTD
Immunochromatography reagent strip for AMH fluorescent quantitative detection and preparation method of immunochromatography reagent strip
An immunochromatography reagent strip for AMH fluorescent quantitative detection and a preparation method of the immunochromatography reagent strip belong to the technical field of immunodiagnosis. Aiming at defects in the prior art, an adopted technical scheme comprises that the immunochromatography reagent strip comprises a sample pad, a label pad, a coating membrane, and absorbent paper, which overlap a PVC base plate in sequence, wherein the label pad is sprayed with avidin; and a quality control line is coated with a rabbit anti-avidin antibody for specific recognition of avidin. The strip has advantages that independent reaction systems are adopted in detection and quality control lines and don't affect each other, and a T / C value manner is adopted for labeling, so that the accuracy of test results is ensured.
Owner:SHENZHEN YHLO BIOTECH
Chinese medicine for treating acute pharyngitis
InactiveCN1879759AEffective treatmentSimple recipeRespiratory disorderPlant ingredientsAcute PharyngitisTreatment effect
The invention discloses a Chinese medicinal preparation for treating acute pharyngitis, which is prepared from Philippine violet herb 1-10 weight parts, Raphanus sativus L 1-8 parts through conventional procedures. The medicament can be prepared into any of the conventional dosage forms.
Owner:张永顺
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com