Immunochromatography reagent strip for AMH fluorescent quantitative detection and preparation method of immunochromatography reagent strip
A technology of fluorescent quantitative detection and immunochromatographic test paper, which is applied in the field of immunodiagnosis, can solve the problems of many influencing factors, high price, false positives, etc., and achieve the effect of ensuring accuracy, high sensitivity and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of AMH fluorescent immunochromatographic test strips:
[0032] (1) Preparation of fluorescent microsphere-labeled protein
[0033]Take 0.1 mL of 10% fluorescent microspheres, centrifuge at 15,000 rpm for 15 minutes for the first time, adjust the concentration of the precipitate to 1% with 50 mM pH 6.5 phosphate buffer, and ultrasonically disperse; add carbon dioxide at a final concentration of 2 mg / mL imine (EDC), mix well, then add N-hydroxysuccinimide (NHS) with a final concentration of 5 mg / mL, mix well; incubate at room temperature for 20 minutes and then centrifuge at 15,000 rpm for a second time for 15 minutes. Dissolve in 50 mM pH 6.5 phosphate buffer. Ultrasonic disperse the reconstituted fluorescent microspheres, add 0.2 mg AMH monoclonal antibody and 0.1 mg avidin into two tubes respectively, mix well, rotate and mix at room temperature for 2 hours, centrifuge for the third time at 15,000 rpm for 15 minutes, and precipitate The material was redis...
Embodiment 2
[0042] Quantitative detection of anti-Müllerian hormone (AMH) concentration in blood samples by fluorescence immunochromatography
[0043] (1) Standard curve drawing
[0044] The AMH antigen was prepared with negative plasma to 25 ng / mL, 10 ng / mL, 5 ng / mL, 1.0 ng / mL, 0.5 ng / mL, 0.1 ng / mL, 0 ng / mL, using the same batch of reagents, Each concentration point was tested 6 times. Take the fluorescence intensity ratio of the detection line (T band) and the quality control line (C band) as the ordinate, and the AMH reference substance concentration as the abscissa, establish an equation and fit it into a standard curve, and write the standard curve information into In the ID chip.
[0045] (2) Detection of samples:
[0046] Take out the test strip from the kit, tear open the aluminum foil bag, lay the test strip flat, and equilibrate for 5 minutes. Take 100 μL of sample and add it to the sample well, and react at room temperature for 15 minutes in the dark. Insert the ID chip int...
Embodiment 3
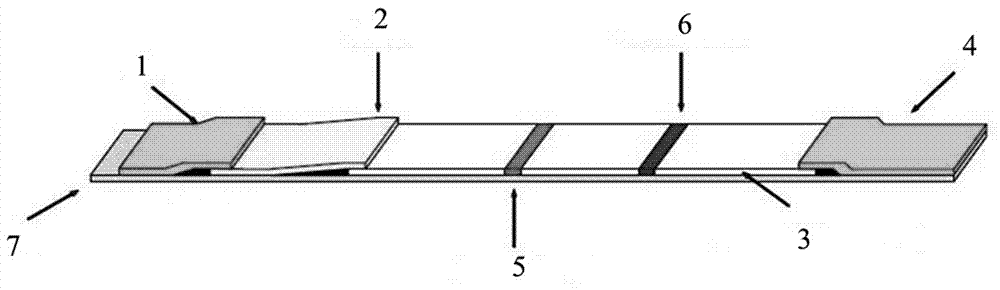
[0050] Please refer to figure 1 , an immunochromatographic test strip for fluorescent quantitative detection of AMH, the immunochromatographic test strip includes a PVC base plate 7, and the PCV base plate 7 is sequentially provided with a sample pad 1, a marker pad 2, and a coating film 3. Absorbent paper 4, the marking pad 2 is connected to the sample pad 1, the coating film 3 is connected to the marking pad 2, the absorbent paper 4 is connected to the coating film 3 are connected; the AMH monoclonal antibody labeled with fluorescent microspheres and avidin labeled with fluorescent microspheres are sprayed on the marker pad 2; a detection line 5 and a quality control line 6 are provided on the coating film 3, and the detection line 5 and the quality control line 6 are separated by 4-8 mm, and the detection line 5 is coated with another AMH monoclonal antibody at a different epitope from the AMH monoclonal antibody labeled with the fluorescent microspheres, and the quality co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com