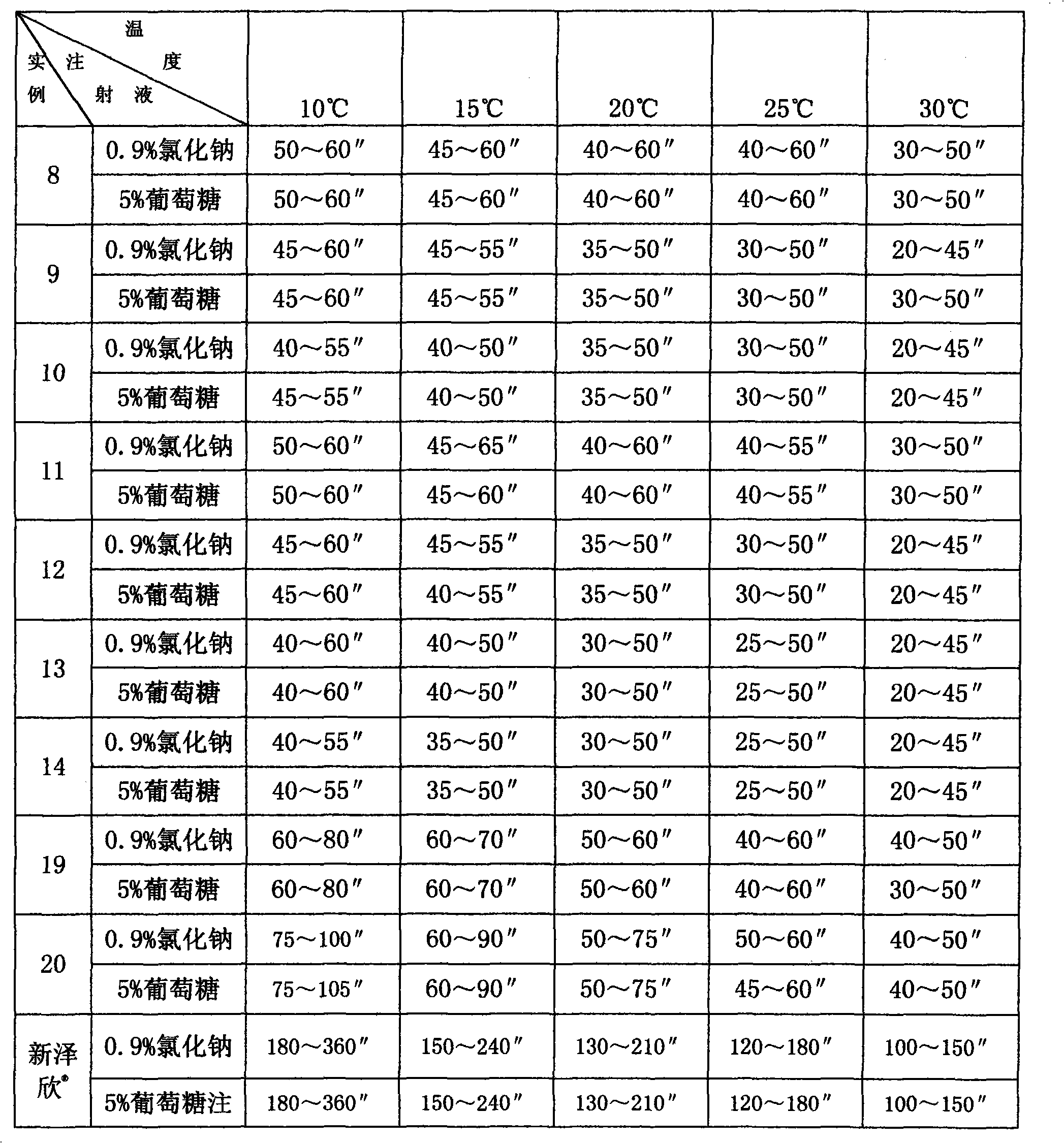
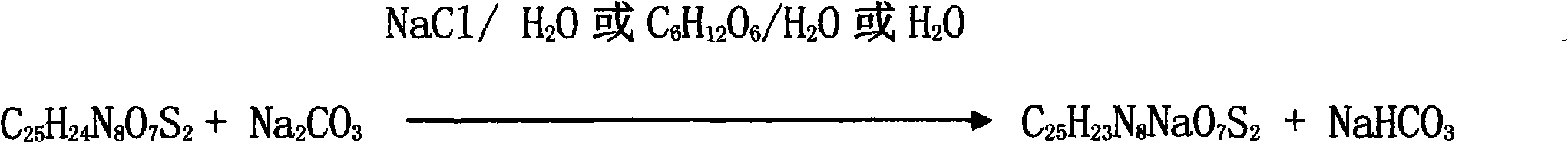Medicine composite of superfine sterile sodium carbonate and cephems
A technology of sodium carbonate and composition, applied in the field of medicine, can solve the problems of insoluble, inconsistent, no specific measurement method, etc., and achieve the effect of rapid dissolution and convenient clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] The preparation of example 1 ultrafine aseptic sodium carbonate
[0033] Weigh 1.5kg of anhydrous sodium carbonate, add 3.5 liters of water for injection and heat up to 40-45°C to dissolve to make a 30% sodium carbonate solution, filter aseptically through a 0.22 micron filter element to obtain a sterile solution of sodium carbonate, and use a clean and dry Sterile air or nitrogen, air inlet temperature 125±5℃, airflow pressure 0.4MPa, outlet temperature 80±2℃, solution flow rate 30ml / min, spray drying and cyclone separation to obtain 1.41kg of ultrafine non Bacterial sodium carbonate powder (average particle size 15μm).
example 2
[0034] The preparation of example 2 ultrafine aseptic sodium carbonate
[0035]Weigh 1.0kg of anhydrous sodium carbonate, add 5.67 liters of water for injection (30-35° C.) to dissolve, and make a 15% sodium carbonate solution, filter aseptically through a 0.22-micron filter element to obtain a sterile solution of sodium carbonate, and use a clean and dry Sterile air or nitrogen, air inlet temperature 150±5°C, air pressure 0.5MPa, outlet air temperature 85±2°C, solution flow rate 25ml / min, spray drying and cyclone separation to obtain 0.93kg of ultra-fine non-toxic particles with a particle size of 5-15μm Bacterial sodium carbonate powder (average particle size 10μm).
example 3
[0036] The preparation of example 3 ultrafine aseptic sodium carbonate
[0037] Weigh 0.5kg of anhydrous sodium carbonate, add 9.5 liters of water for injection (20-30° C.) to dissolve to make a 5% sodium carbonate solution, filter aseptically through a 0.22-micron filter element to obtain a sterile solution of sodium carbonate, and use a clean and dry Sterile air or nitrogen, air inlet temperature 175±5°C, airflow pressure 0.6MPa, outlet air temperature 88±2°C, solution flow rate 25ml / min spray drying cyclone separation and collection to obtain 0.45kg of ultra-fine powder with a particle size of 1-8μm Bacterial sodium carbonate powder (average particle size 4μm).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com