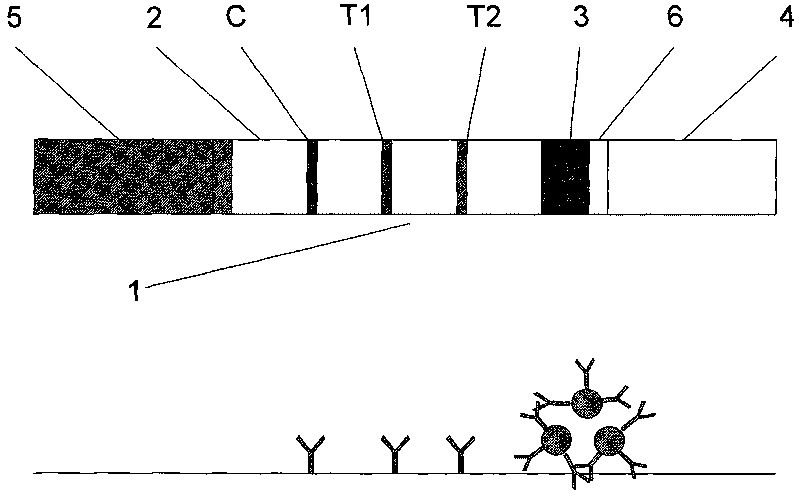
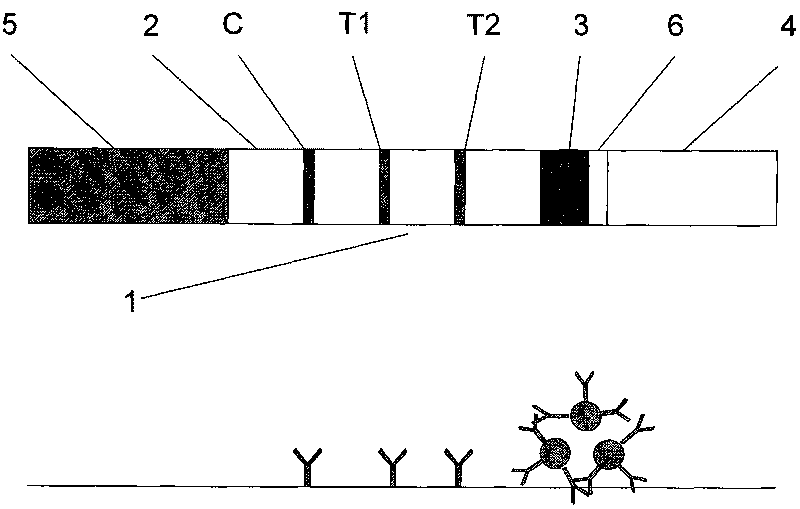
Magnetic immuno-chromatographic test paper strip for quantitatively detecting PSA and fPSA in blood and preparation method thereof
A magnetic immunochromatography, quantitative detection technology, applied in the field of medical testing, can solve the problems of difficult to use in large areas, operator hazards, time-consuming, etc., to achieve the effect of convenient clinical judgment and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation method of magnetic immunochromatography test strip and test paper box for quantitative detection of PSA and fPSA in blood
[0030] The preparation method of the present embodiment comprises the following steps:
[0031] A. Antibody preparation: commercialized total PSA+fPSA paired antibody (Hytest company, cat: 4P33) was selected, dialyzed overnight in 20 mM PBS, pH 7.24° C. for use.
[0032] B. Preparation of coating film:
[0033] Preparation of coating buffer: 0.02M phosphate buffer (PB) with pH 7.2 was used as coating buffer, sterilized by 0.22 μm microporous membrane filter and stored at 4°C for later use, valid for two weeks.
[0034] Preparation of blocking solution: 0.5% BSA, 0.02M phosphate buffered saline (PBS), 0.22 μm microporous membrane filtration and sterilization, and store at 4°C for later use, valid for one week.
[0035] Preparation of coating membrane: Dilute total PSA+fPSA antibody to 0.5mg / ml and 0.8mg / ml with coating buffer (0.02M PB...
Embodiment 2
[0062] Except for the preparation step of the magnetic particles: the molecular ratio of adding the total PSA antibody to the magnetic particles is 2:1. Other steps are with embodiment 1,
Embodiment 3
[0064] In addition to the step of preparing the magnetic particles: adding the molecular ratio of the total PSA antibody to the magnetic particles is 10:1. Other steps are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com