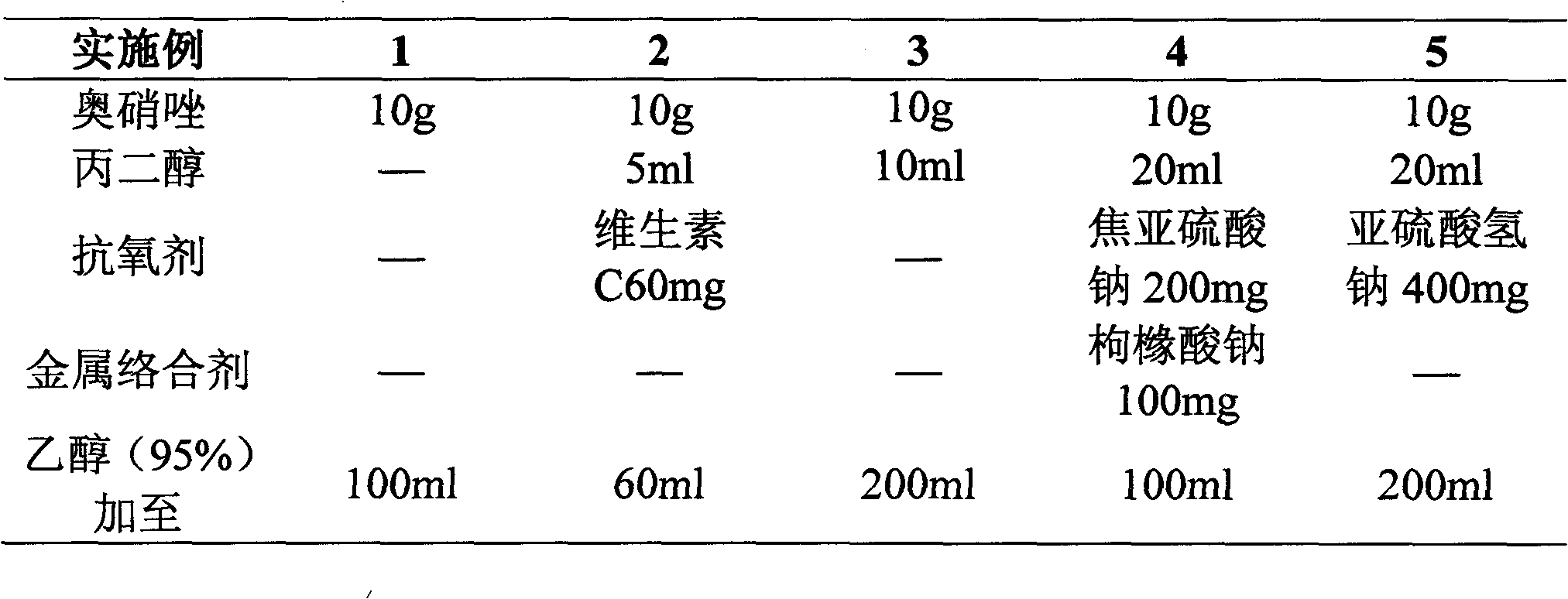
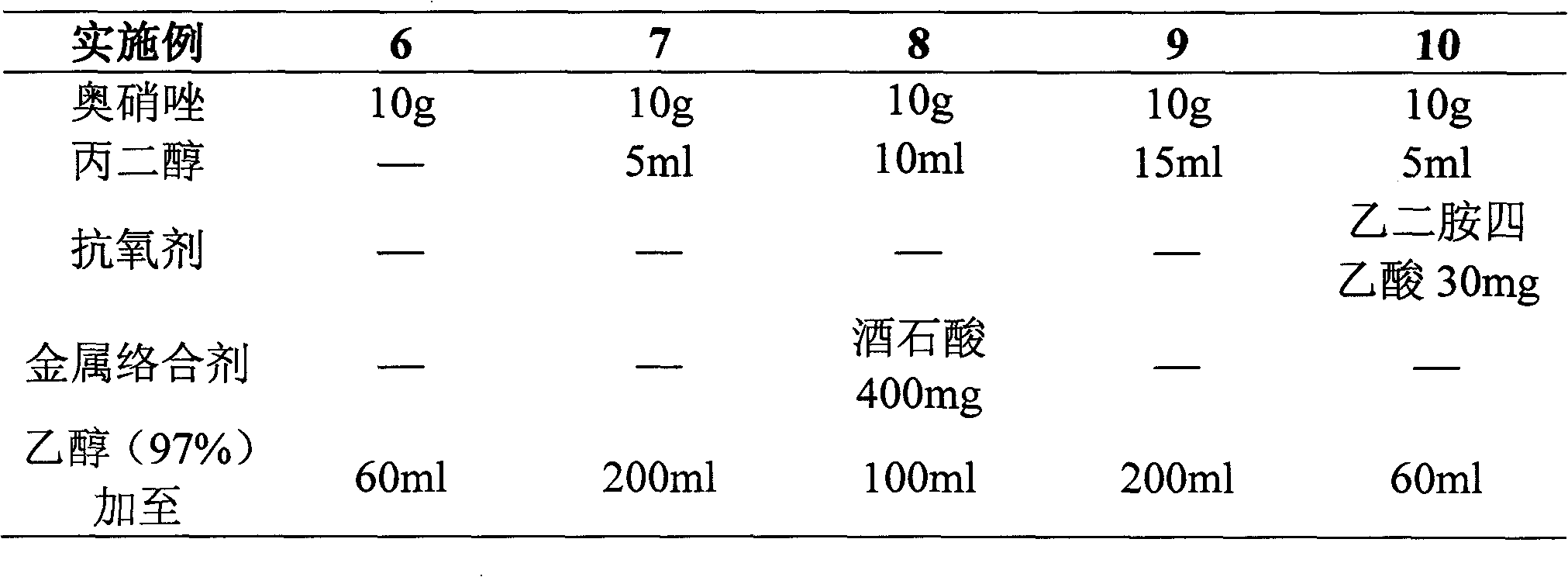
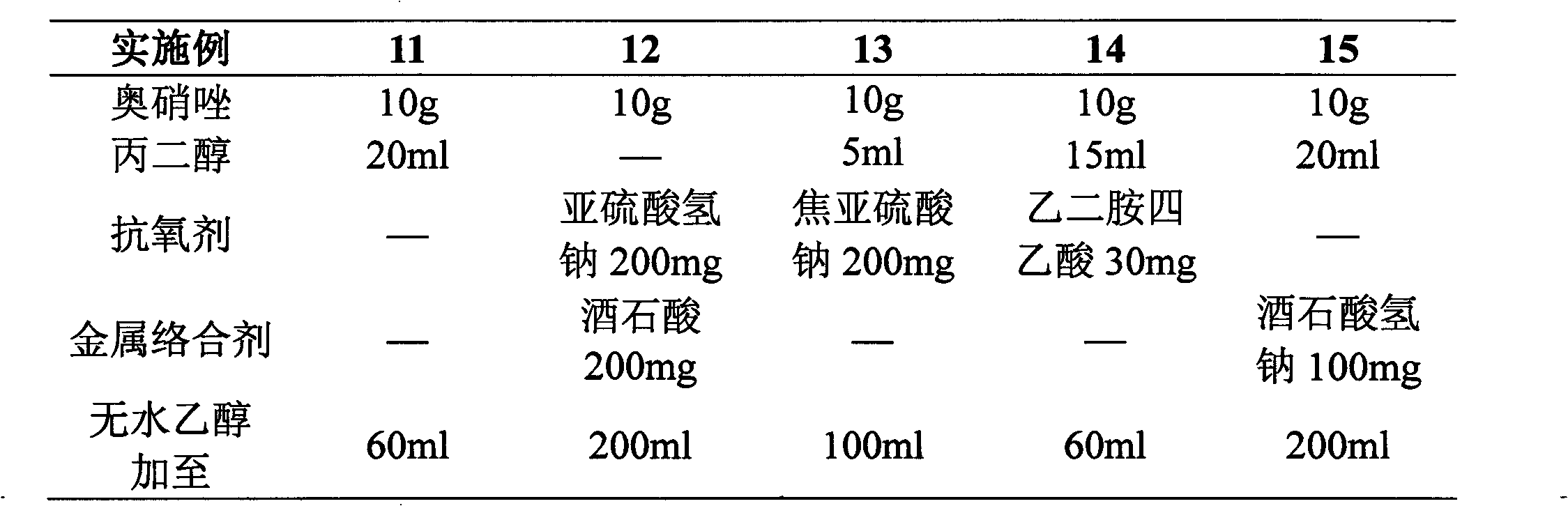
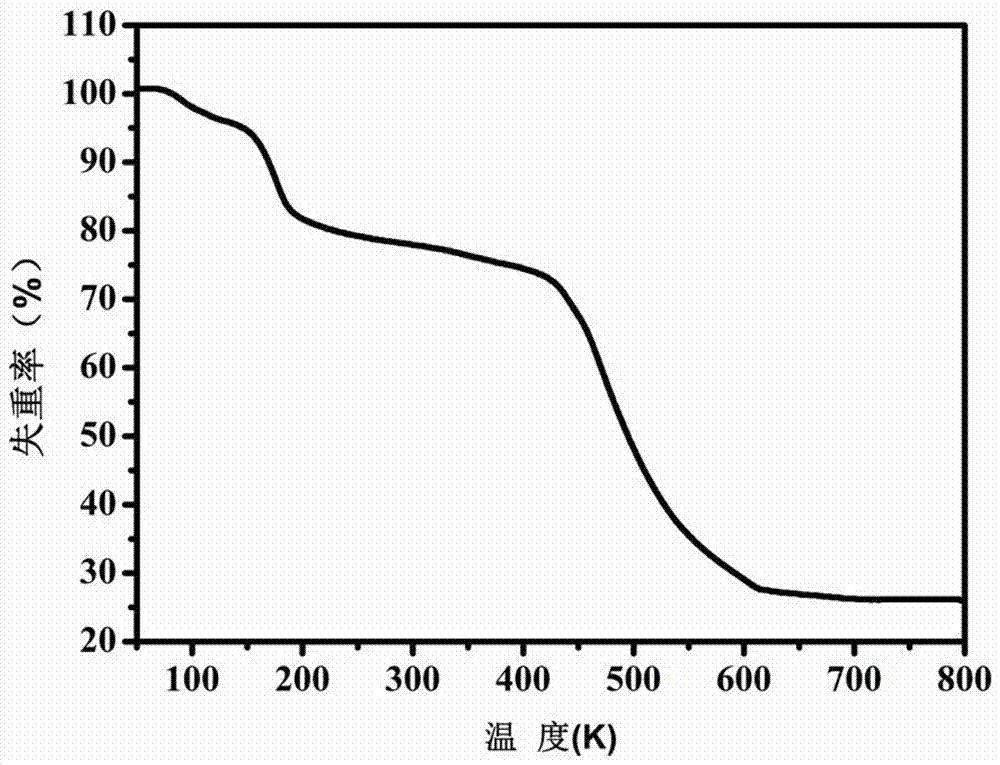
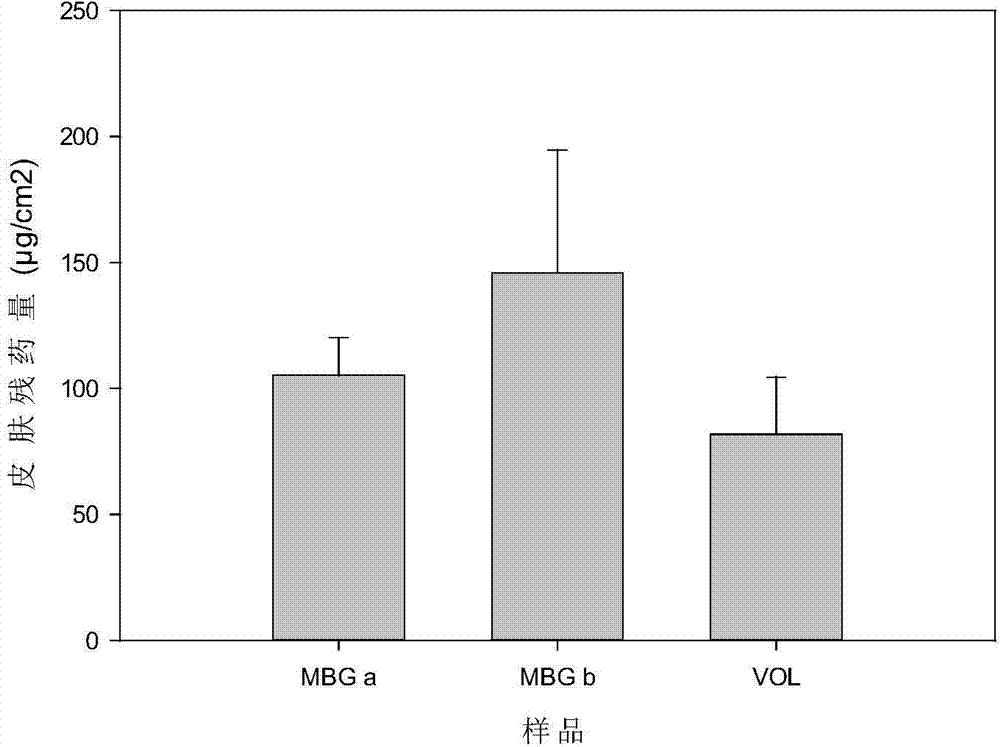
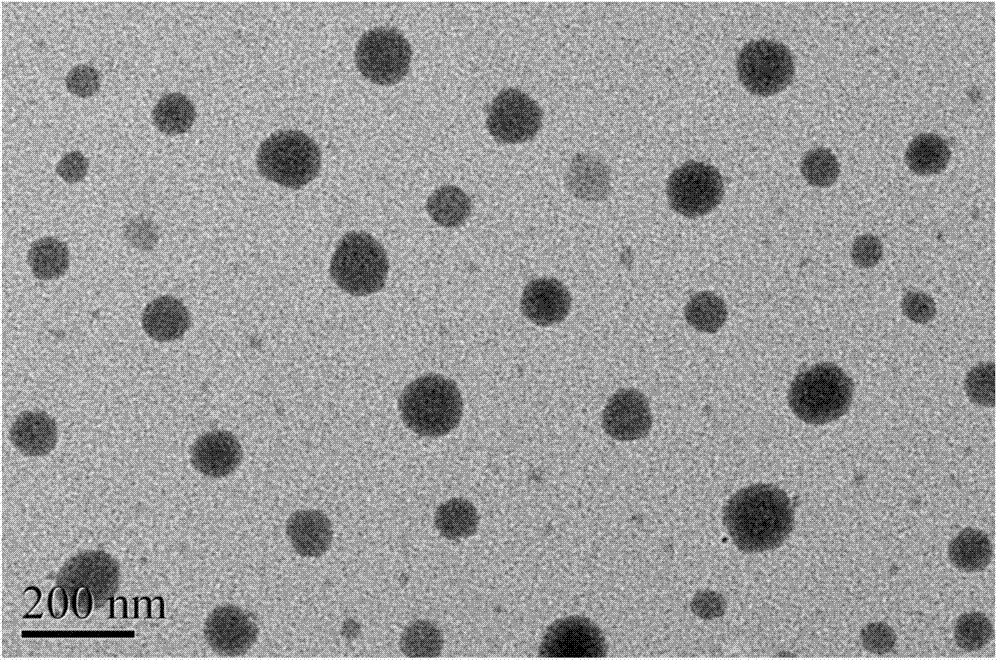
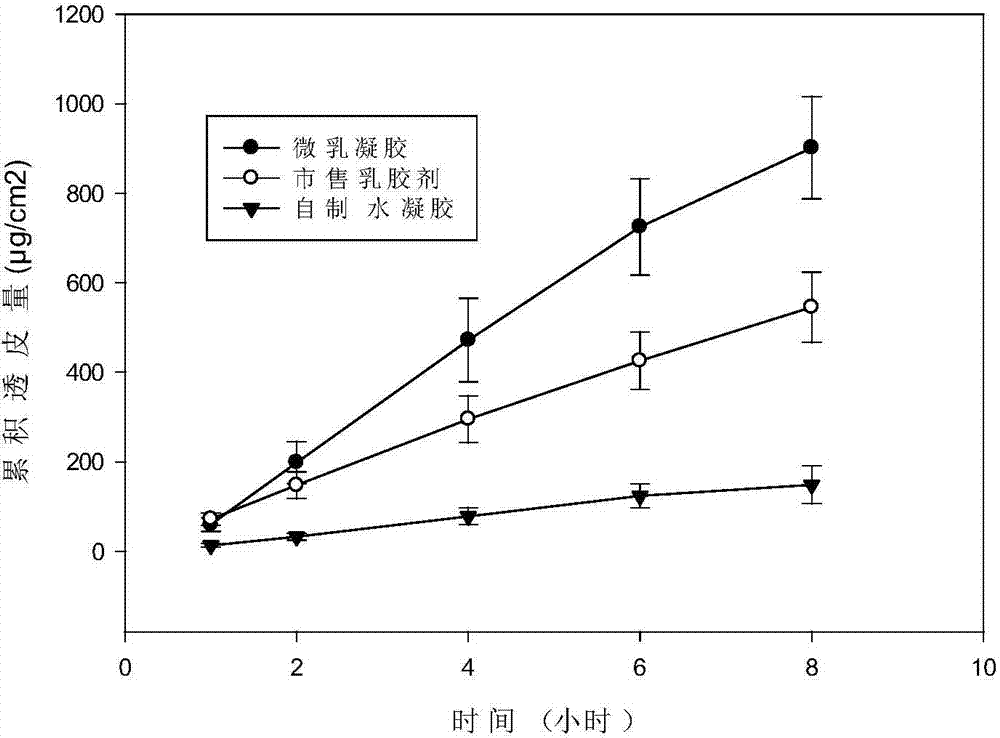
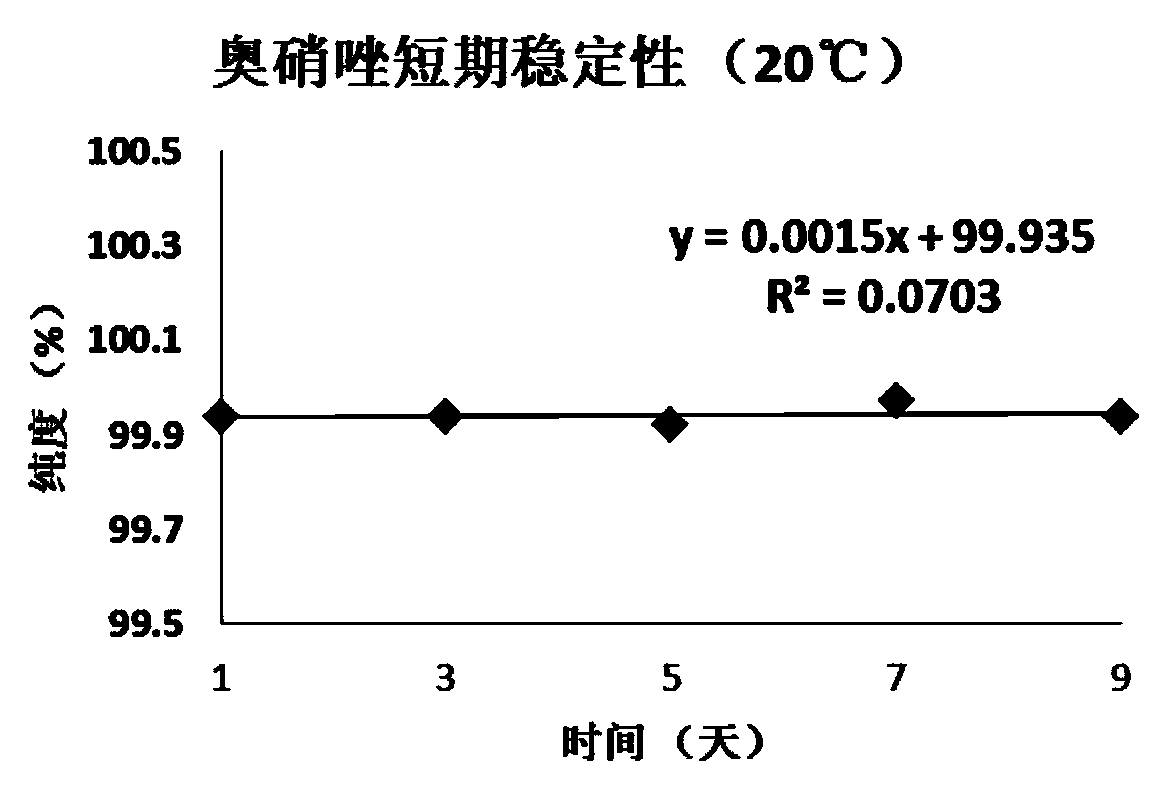
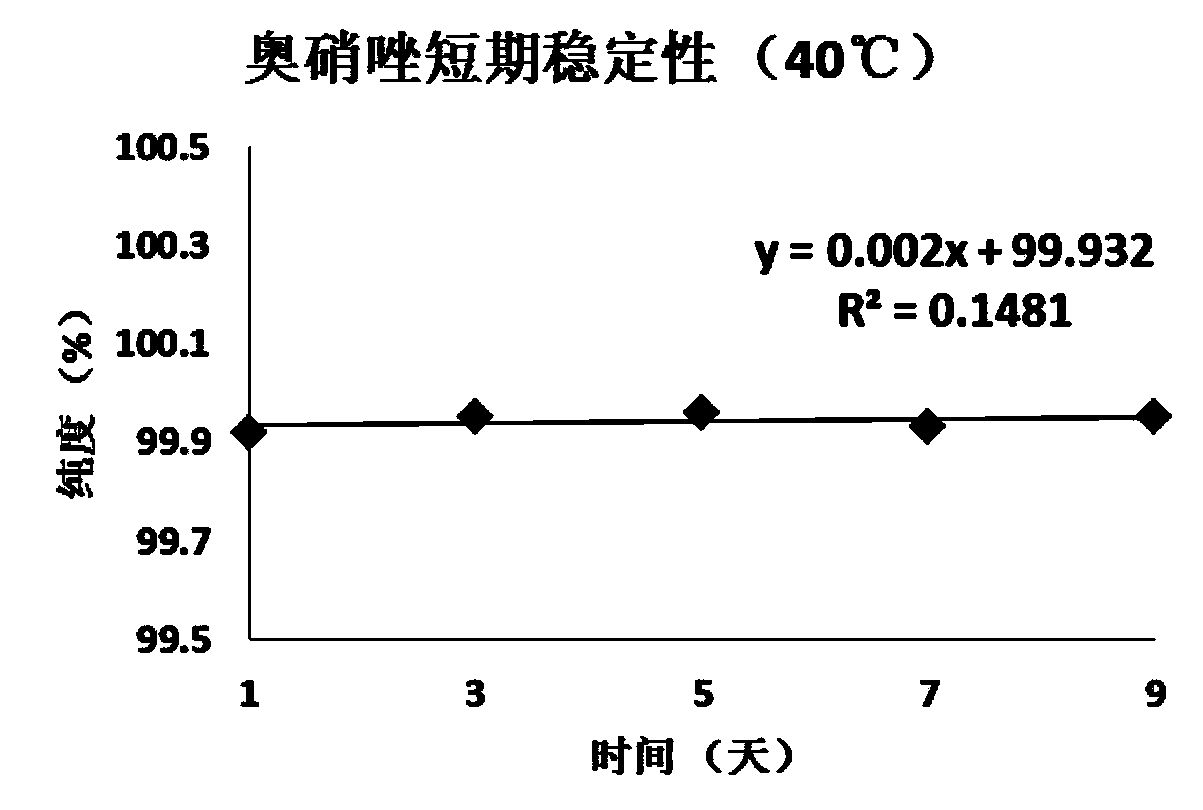
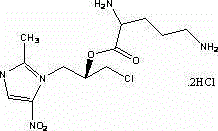
Patents
Literature
176 results about "Ornidazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
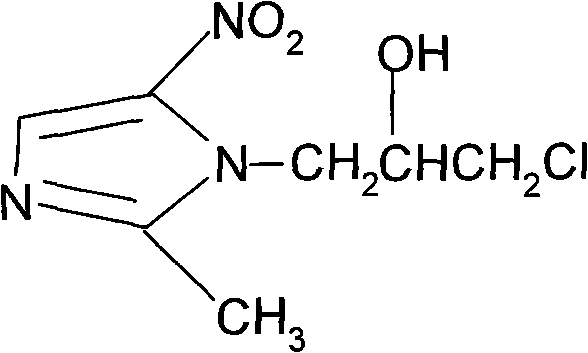
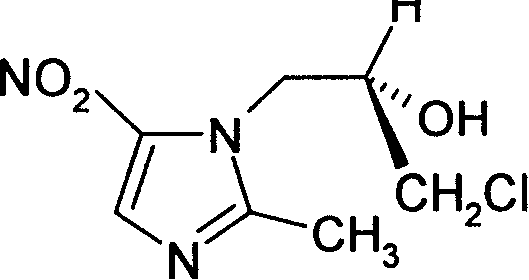
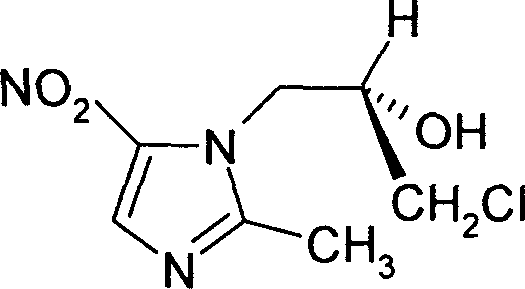
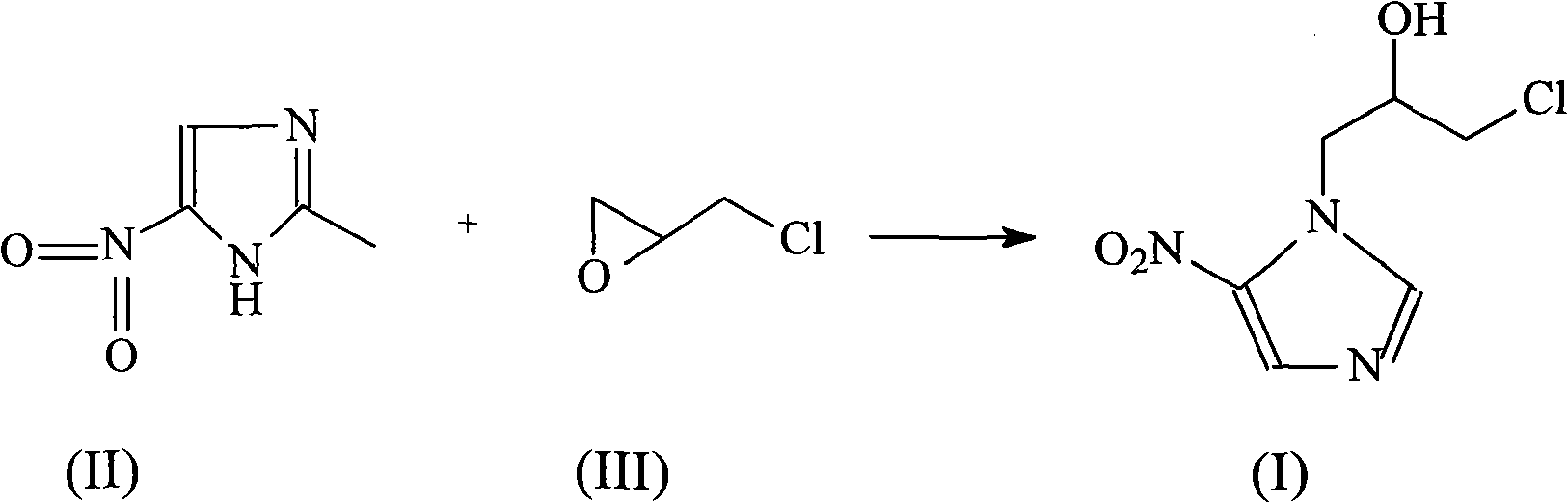
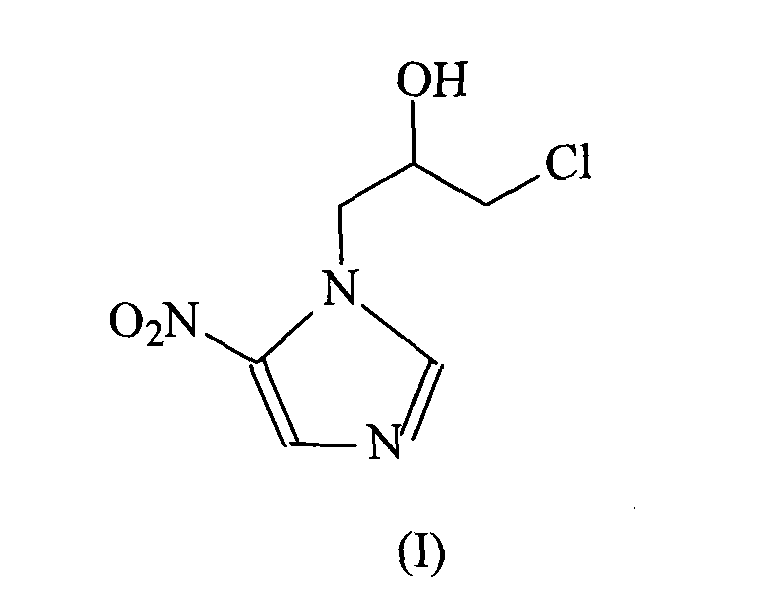
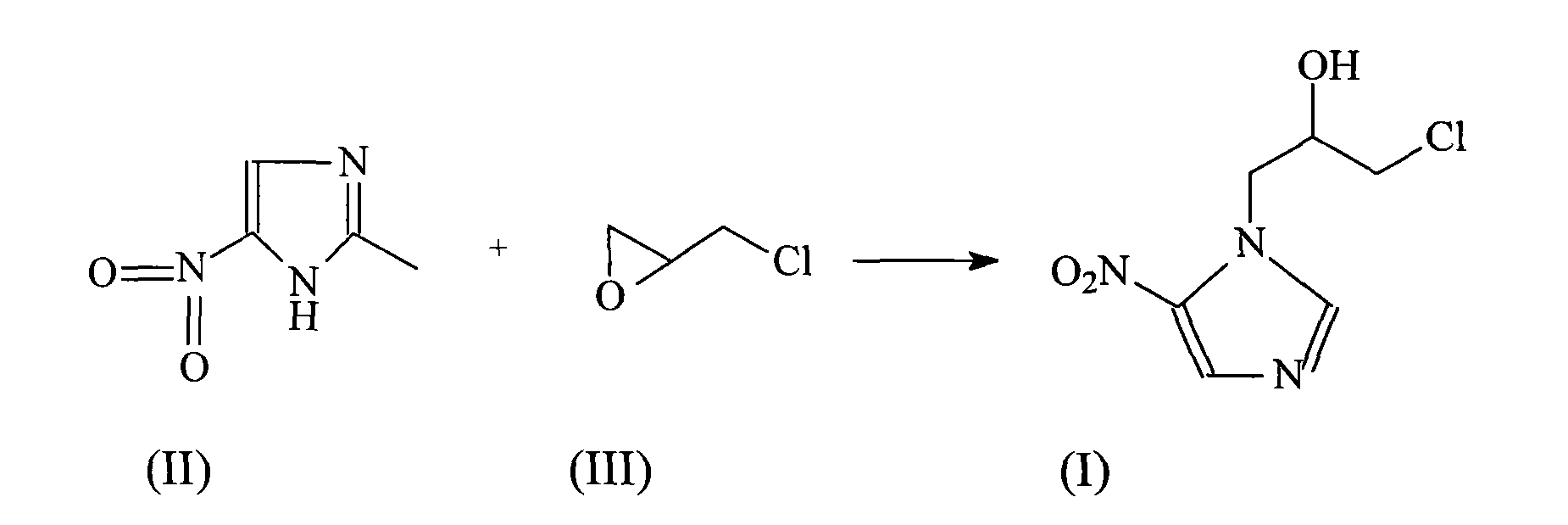
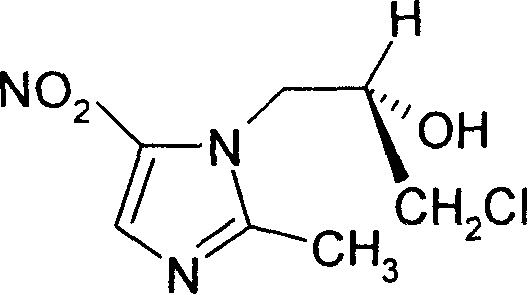
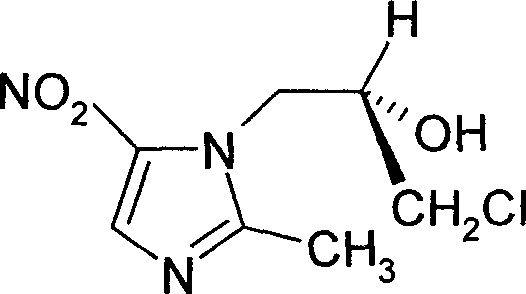
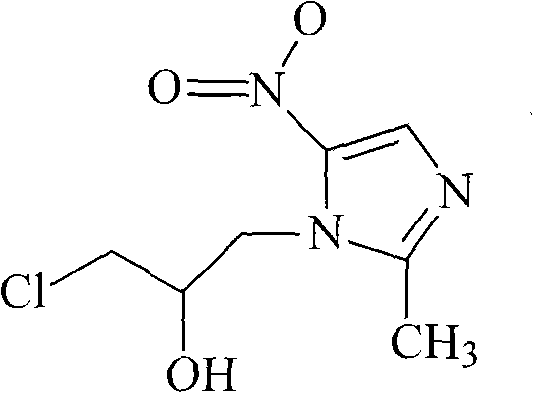
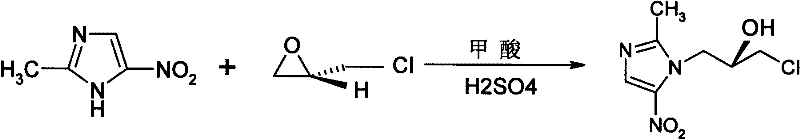
Ornidazole is an antibiotic used to treat some protozoan infections. It has also been investigated for use in Crohn's disease after bowel resection. Synthesis is a straightforward reaction between 2-methyl-nitroimidazole and epichlorohydrin under acid catalyst conditions.
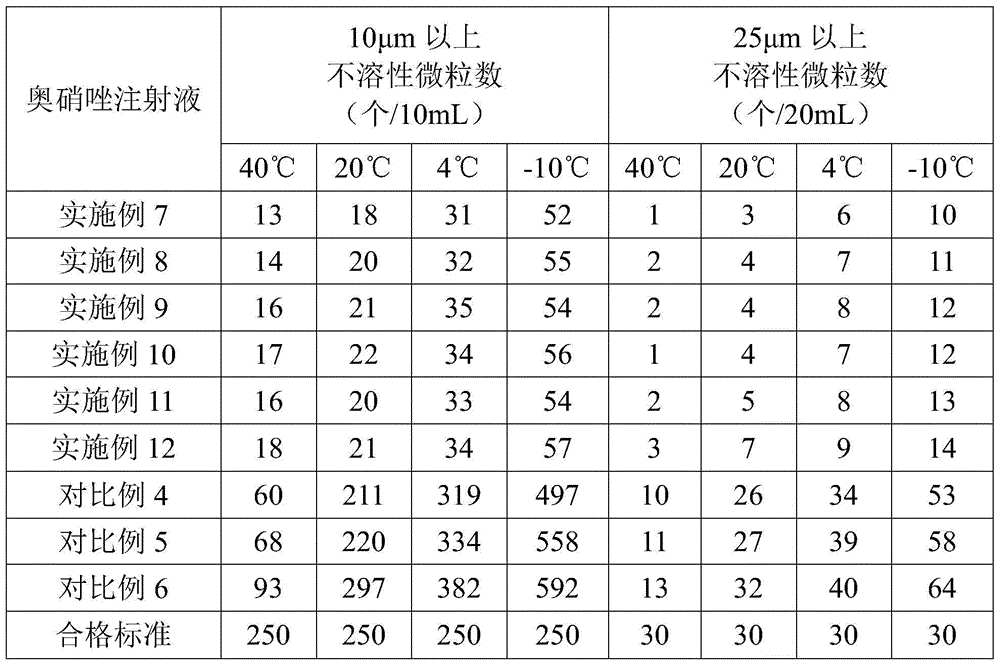
Ornidazole injection
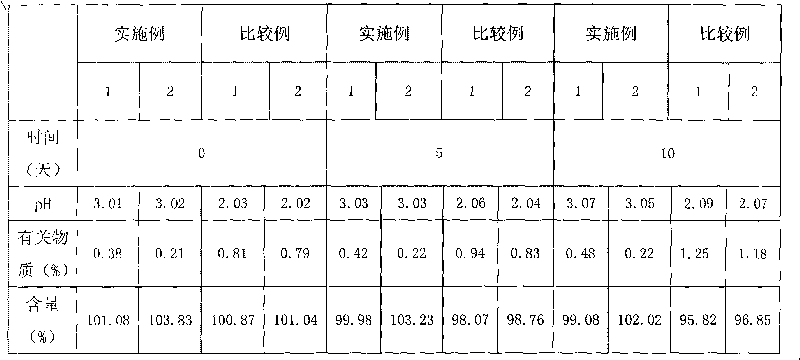
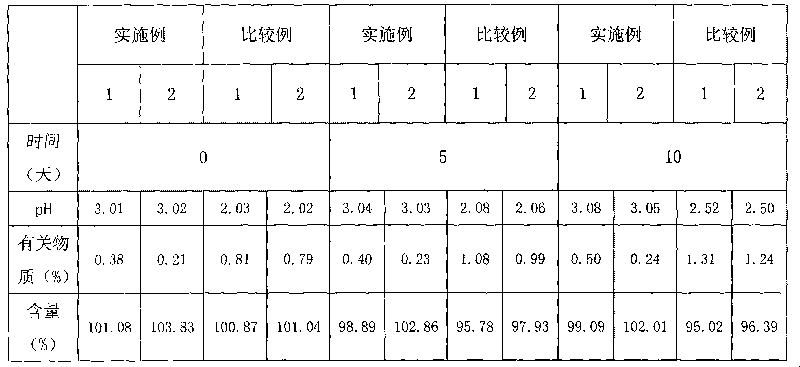
ActiveCN102552127ALow impurity contentImprove stabilityOrganic active ingredientsPharmaceutical delivery mechanismSuperficial phlebitisOrnidazole
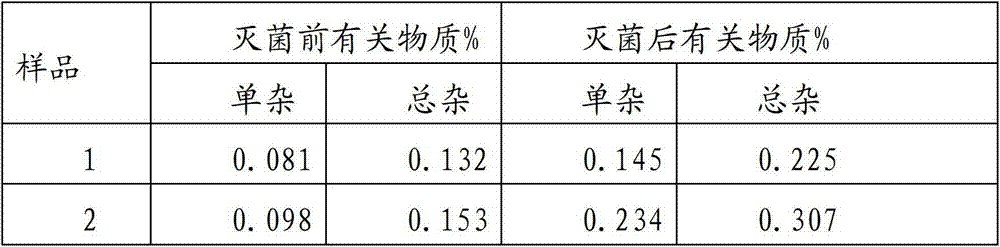
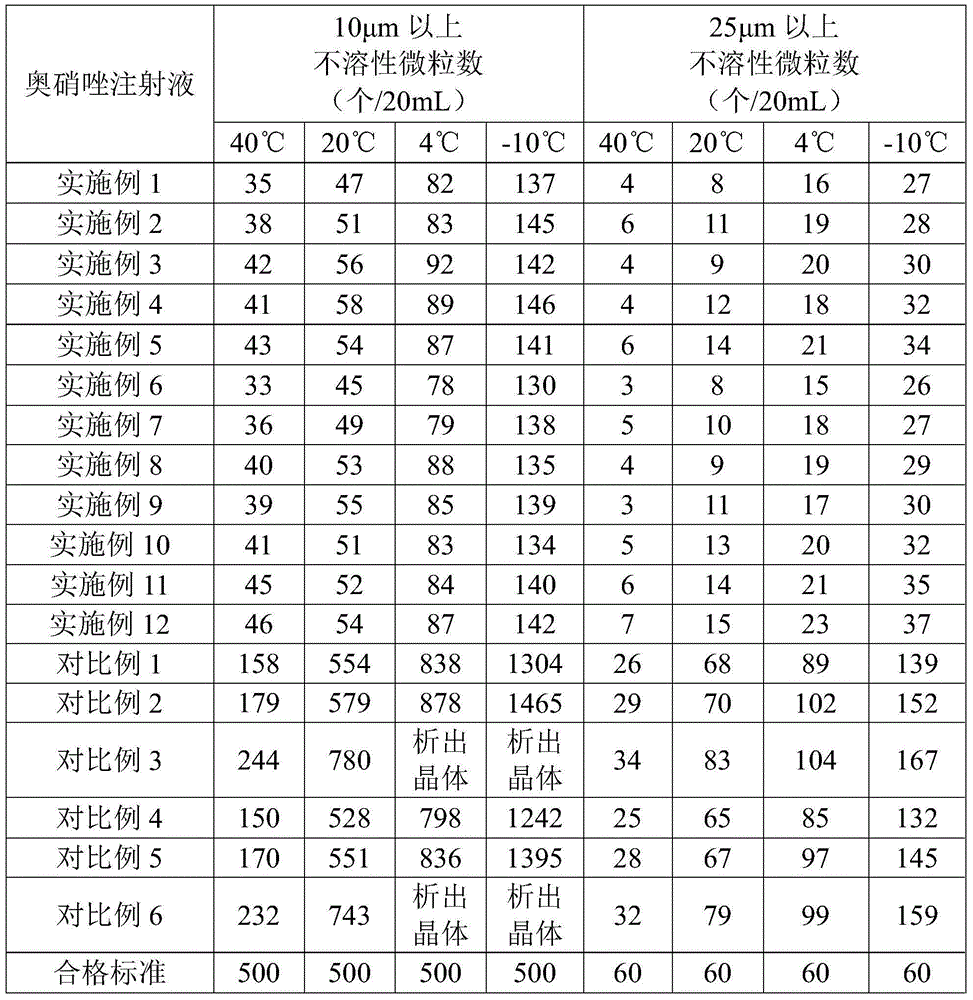
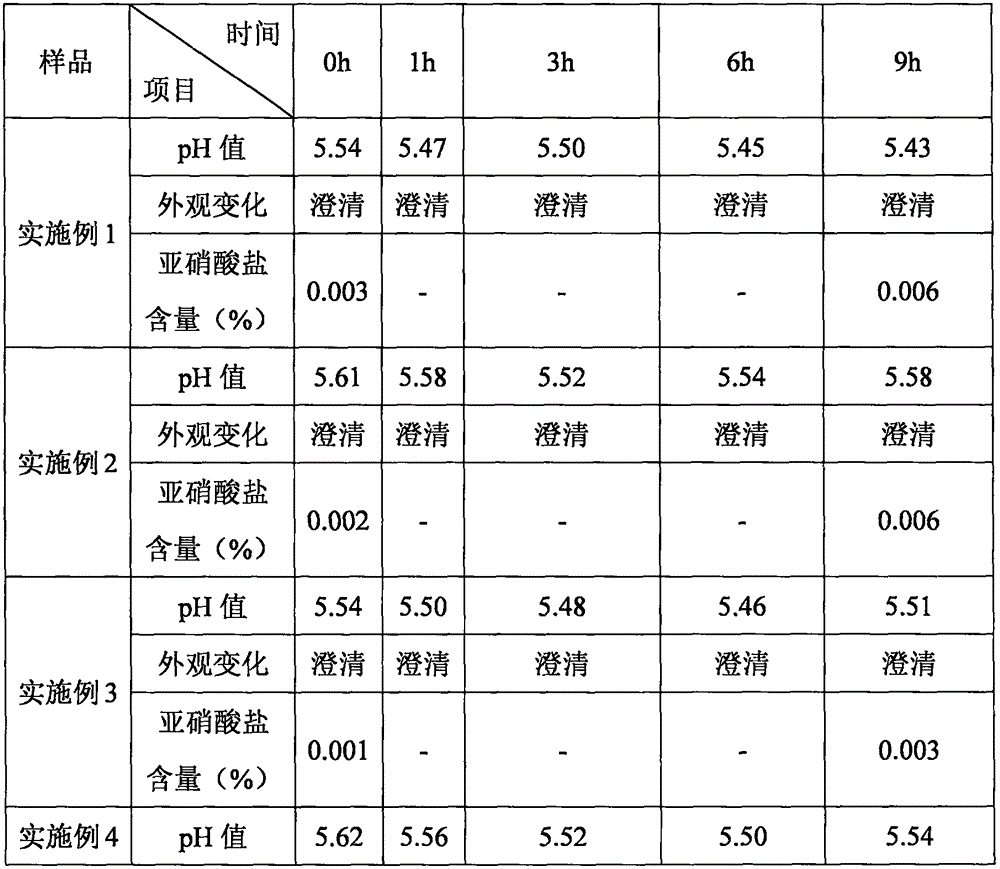
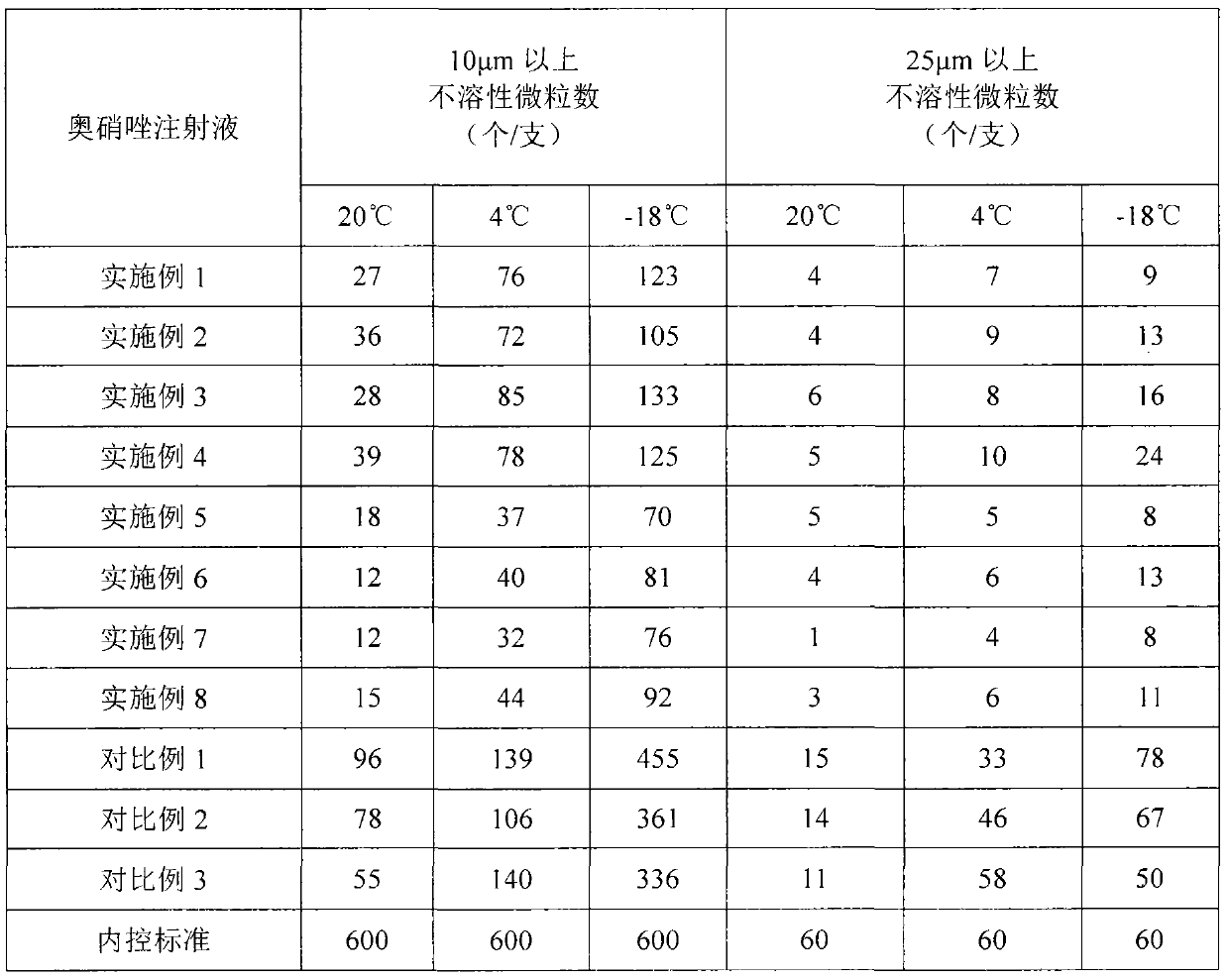
The invention provides an ornidazole injection. The ornidazole injection has the advantages of high stability, low impurity content, pH value that is closer to the human physiological pH value, low incidence rate of infusion pain, infusion phlebitis and other adverse reactions, etc.
Owner:HEBEI RENHE YIKANG PHARMA
Levo ornidazole vein administration agent and its preparation method
ActiveCN1686116AThe preparation process is feasibleImprove stabilityAntibacterial agentsOrganic active ingredientsActivated carbonTitanium
An intravenous injection or perfusion of levo-ornidazole is prepared from levo-ornidazole, auxiliary and the water for injection through proportionally mixing, stirring for dissolving, regulating pH=3.5-5.5, adding the water and activated carbon for injection, stirring, laying aside, decarbonizing by titanium rod, fine filtering, and sterilizing.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Intravenous formulation of ornidazole and its preparation method
InactiveCN1768742AThe preparation process is feasibleImprove product qualityAntibacterial agentsOrganic active ingredientsAdjuvantFiltration
The invention provides the process for preparing Ornidazole intra-vascular preparations in the dosage forms of freeze-dried injections, which comprises, (1) weighing prescription amount of Ornidazole and adjuvant, charging water for injection, stirring and dissolving, (2) adjusting pH to 3.5-6.0 with acids, charging 35-55 deg C. water for injection to full amount, charging 0.05-0.2% of activated charcoal for injection, homogenizing, removing charcoal and carrying out refined filtration, (3 loading, (4) making preparations through freeze drying.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
Ornidazole medicinal composition and preparation method thereof
InactiveCN101697969AImprove stabilityMeet quality requirementsAntibacterial agentsOrganic active ingredientsActivated carbonGram
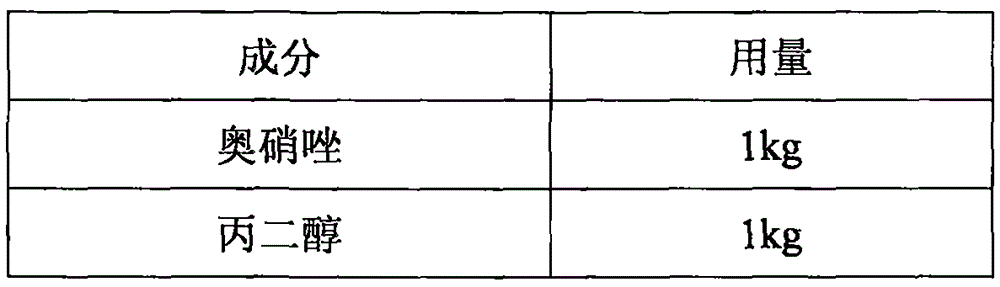
The invention discloses an ornidazole medicinal composition. Each 100 milliliters of ornidazole medicinal composition contains 5 grams of ornidazole, 35 to 60 milliliters of 1,2-propylene glycol and the balance of water for injection. The pH value of the ornidazole medicinal composition is 2.5 to 4.5. The composition can be made into a small-volume injection, wherein the injection can be a 5-milliliter injection containing 0.25 gram of the composition or a 10-milliliter injection containing 0.5 gram of the composition. The invention also provides a preparation method of the ornidazole medicinal composition, which comprises: adding ornidazole into 1,2-propylene glycol, keeping the temperature to be 45 to 60 DEG C and stirring the mixture till the ornidazole dissolves completely; adding disodium ethylenediamintetraacetate aqueous solution at a concentration of 40-70mg / 100ml; and using needle active carbon for absorption and microporous filter membrane for filtration decarbonization, supplying water for injection and adjusting the pH value to 2.5 to 4.5 to obtain the ornidazole medicinal composition. The method provided by the invention has simple process and the prepared product has high stability.
Owner:雷绍青 +2
Use of levo-ornidazole for preparing anti-parasitic-infectious drug
The present invention provides an application of levo-orinidazole in preparation of medicine for resisting parasitization. The tests show that the levo-orinidazole is superior to dextro-orinidazole and racemic orinidazole in therapeutic action for curing parasitization (special for curing infection of trichomonas vaginalis and cecal amebic infection). Said invention also relates to the conventional dosage forms for clinical application, including oral preparation, venous administration preparation and vaginal medication preparation, etc.
Owner:NANJING SANHOME PHARMACEUTICAL CO LTD
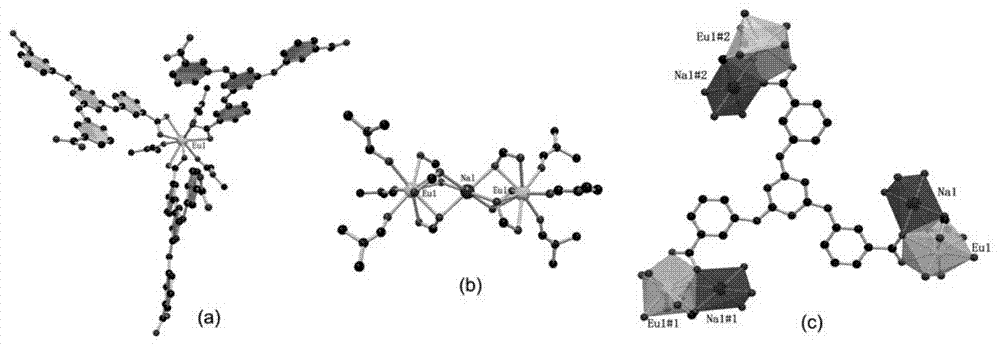
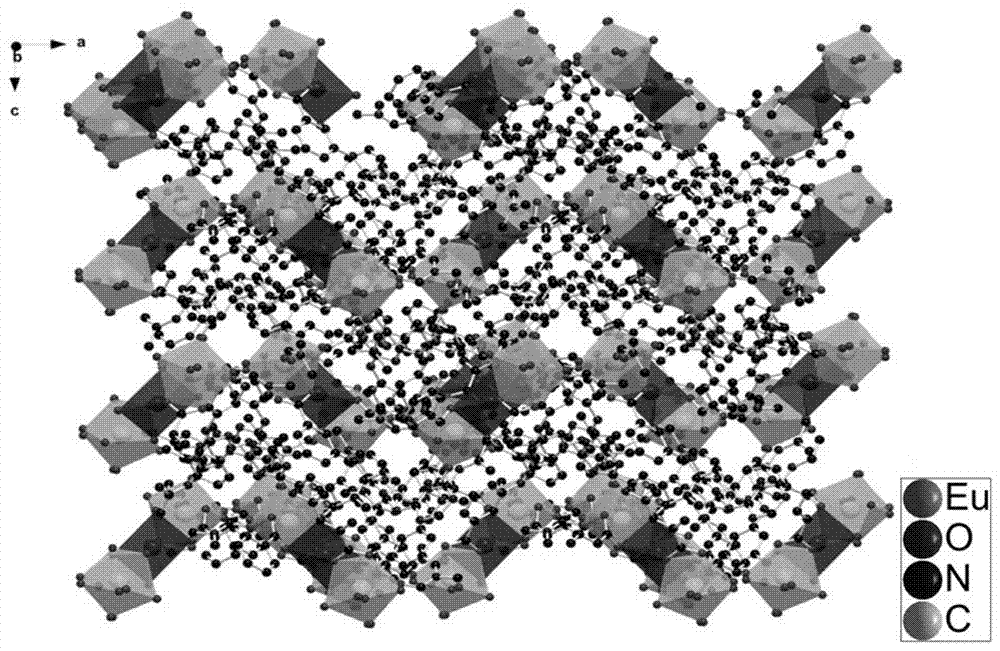
Organic crystalline state material on the basis of rare earth metal europium, preparation, and application thereof in fluorescent recognition of antibiotics
ActiveCN106916317ALow temperature requirementEasy temperature controlFluorescence/phosphorescenceLuminescent compositionsFluorescenceRare earth
An organic crystalline state material on the basis of rare earth metal europium, preparation, and fluorescent recognition of antibiotics. The invention belongs to the technical field of crystalline state materials. The chemical molecular formula of the organic crystalline state material is {H3O<+>[EuNa<0.5>(TATAB)(DMF)].(solvent)<x>}n, wherein x and n refers to positive infinity, and H3TATAB refers to 4,4',4"-s-triazine-1,3,5-tri-m-aminobenzoic acid. One Eu<3+> ion and 0.5 Na<+> ion are connected to form a trimetal-cluster basic unit (Eu-Na-Eu) through six oxygen atoms on three TATAB ligands, wherein the binuclear unit is connected to form a three-dimensional network frame through the ligands. The metal-organic frame material has simple synthesis process and high crystalline purity, has a novel structure, is high in stability in a water solution, and can recognize an antibiotic, ornidazole, from a plurality of antibiotic water solutions.
Owner:CHINA THREE GORGES UNIV
Multifunctional microemlusion gel preparation and preparation process thereof
InactiveCN103655459AImprove skin penetrationImprove performanceAntimycoticsAntipyreticIndometacinActive agent
The invention discloses a multifunctional microemlusion gel preparation and a preparation process thereof, and belongs to the technical field of medicines. The preparation mainly comprises bulk pharmaceutical chemicals (such as non-steroidal anti-inflammatory drugs-diclofenac sodium, ibuprofen, indometacin, antifungal drugs-ornidazole, antiviral drugs-ganciclovir, hormone drugs-dexamethasone, local anesthesia drugs-lidocaine and irritants-menthol), a cationic polymer and a microemlusion, can further comprise gel or a thickener, and can be used for transdermal drug delivery and local drug delivery. The preparation process is simple, convenient, good in stability and pollution-free. Compared with existing cream and gel, the preparation has the advantages that a novel action mechanism is adopted, the accumulative penetration amount of unit area of drugs is remarkably increased, a certain slow-release effect is achieved, and the drug delivery frequency and the drug delivery amount can be reduced; a chemical penetration enhancer and a conventional preservative are not added, a certain bacterial inhibition effect is achieved, the skin irritation is avoided, and the use safety of the drugs is improved.
Owner:CHINA PHARM UNIV
Ornidazole injection and preparation technology thereof
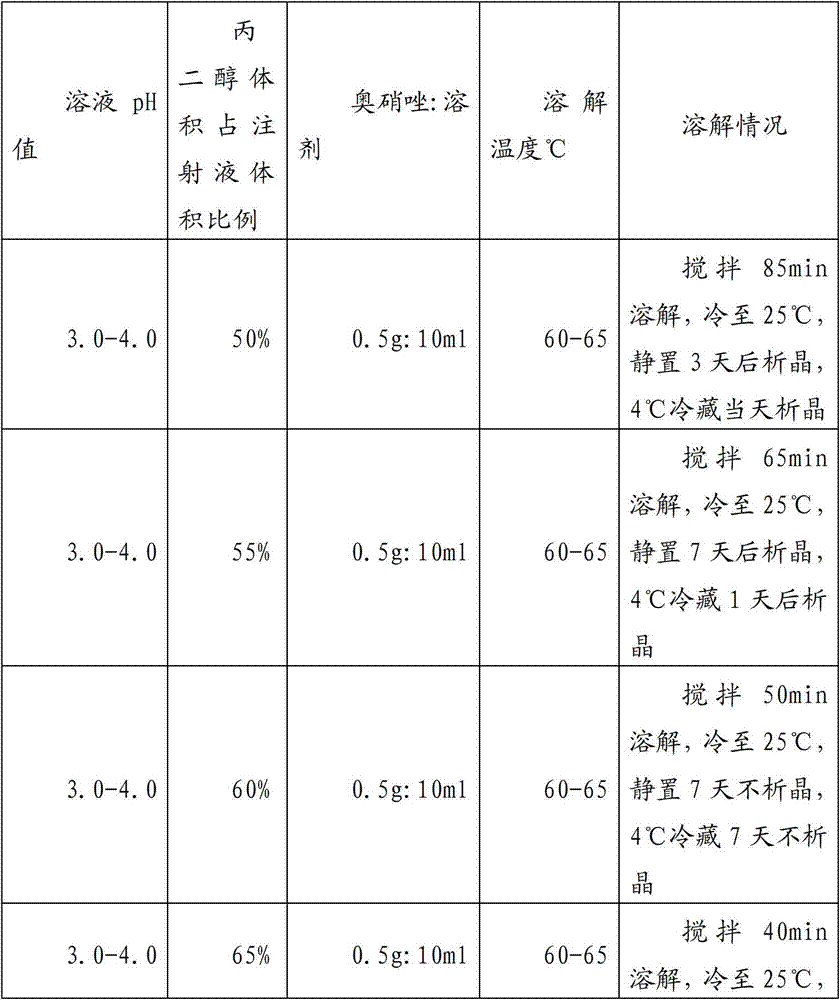
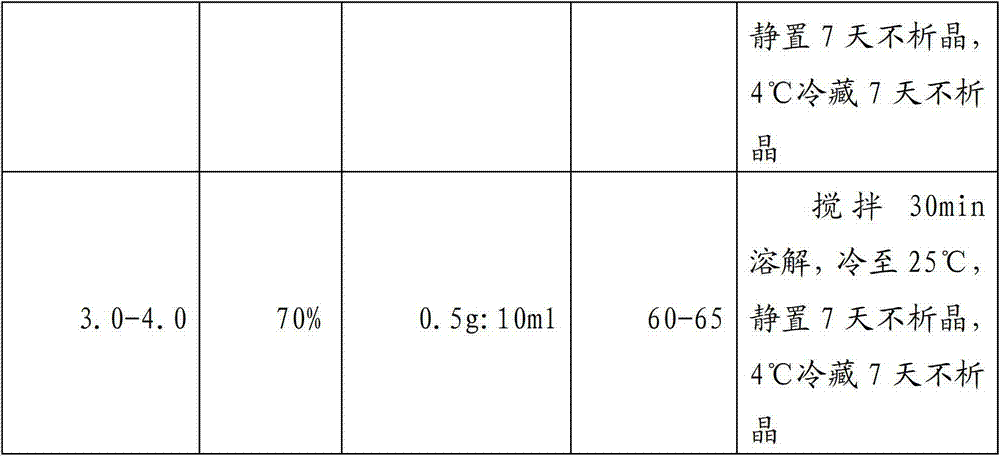
The invention provides an ornidazole injection which comprises ornidazole, propylene glycol and water for injection, wherein the ornidazole can be well dissolved when the volume of the propylene glycol is 60%-70% of that of the injection. According to a preparation technology for the ornidazole injection, oxygen in the solution can be completely removed by utilizing carbon dioxide gas, and the saturated carbon dioxide gas in the injection can be used for not only reducing the oxidized by-product of the ornidazole injection, but also improving the heat stability of the ornidazole injection.
Owner:SHANXI PUDE PHARMA CO LTD
Ornidazole injection liquid
InactiveCN104127410AGood storage stabilityRaise the pHAntibacterial agentsOrganic active ingredientsAcetic acidSolvent
The invention provides a preparation method of an ornidazole injection liquid. The preparation method comprises the steps: evenly mixing ornidazole and a solvent, wherein the solvent is a mixture of propylene glycol and at least a part of injection water, or the solvent is a mixture of propylene glycol, ethanol and at least a part of injection water; adjusting the pH value of the obtained mixture to 3-6 with acetic acid; and then carrying out at least two times of heating cooling circulation on the material after the pH value is adjusted. The invention also provides the ornidazole injection liquid, wherein the ornidazole injection liquid is prepared according to the above method. Through the technical scheme, the storage stability of the ornidazole injection liquid under a condition of relatively low temperatures (-10 DEG C to 20 DEG C) is significantly improved, and irritation is relatively small after injection.
Owner:BEIJING JINGKE TAILAI TECH
Ornidazole injection and preparing method thereof
ActiveCN104013571AThe finished product has less impuritiesImprove stabilityAntibacterial agentsOrganic active ingredientsInjection volumeGlycerol
The invention discloses an ornidazole injection and a preparing method thereof. The metric injection volume of the ornidazole injection is 1-2 mL. The ornidazole concentration is 0.25-0.5 g / mL. The solvent of the injection is absolute ethyl alcohol or a mixture of absolute ethyl alcohol and glycerol. A finished product of the ornidazole injection is extremely low in impurity content, good in stability and extremely stable in guarantee period. Compared with originally developed ornidazole injections, the ornidazole injection provided by the invention is free of propylene glycol, and is better in clinical using safety, less in medical risk and simpler in preparation process.
Owner:江苏凌霄科技发展有限公司
Ornidazole purity standard substance and preparation method and application thereof
InactiveCN107632105AGuaranteed traceabilityComponent separationMaterial analysis by electric/magnetic meansDrugChemistry
The invention discloses an ornidazole purity standard substance, a preparation method and an application thereof. The preparation method comprises the following steps: 1) sampling ornidazole raw material powder, employing a high performance liquid chromatography area normalization method to measure the content to obtain a purity fixed value of the ornidazole raw material powder; 2) measuring masspercentage of water, non-volatile impurity and organic volatile impurity in the ornidazole raw material powder, calculating according to a formula I to obtain the purity fixed value 1 of the ornidazole purity standard substance, and labeling as PHPLC-AN; wherein PHPLC-AN=P0*[100%-Xw-Xn-Xv] in the formula I; 3) taking the ornidazole raw material powder and employing a quantitative nuclear magneticmethod to measure the content to obtain the purity fixed value 2 of the ornidazole purity standard substance, and labeling as PNMR; and 4) calculating the average value of PHPLC-AN and PNMR to obtainthe purity fixed value of the ornidazole purity standard substance. The ornidazole purity standard substance can be used for detecting drug residue in livestock and poultry products, and provides technical support and substance guarantee for establishing a national agricultural product quality and safety risk assessment as well as monitoring traceablility system.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
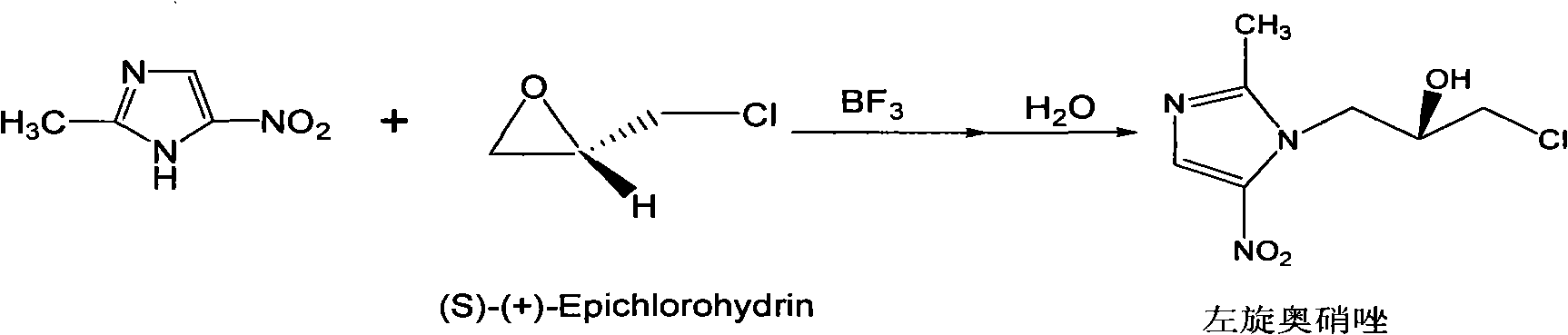
Method for preparing (S)-ornidazole
The invention provides a method for preparing (S)-ornidazole. The method comprises the following steps of: adding an organic solvent and 2-methyl-5-ornidazole, slowly adding a catalyst dropwise with stirring first, then slowly dripping S-(+)-epichlorohydrin into the mixed solution, and after the S-(+)-epichlorohydrin is dripped completely, performing a reaction for 2 to 10 hours at the temperature of between 0 and 20 DEG C; slowly adding the reaction solution into the ice-water mixture, stirring the mixed solution for a reaction for 10 minutes and 5 hours at the temperature of no less than 30 DEG C, adjusting a pH value of the mixed solution to 1.0 to 2.0 with acid, removing an organic layer, adjusting the pH value of a water layer to 6.0 to 8.0 with alkali, stirring the mixed solution for 1 to 24 hours for crystallization, filtering the reaction solution, washing the product obtained by water, and drying the product to obtain the (S)-ornidazole. The method has the advantages of high product purity, simplified procedures and the suitability for industrial production.
Owner:HC SYNTHETIC PHARMA CO LTD
L-ornidazole injection prepn
InactiveCN1739505AStrong pharmacodynamic activityGood curative effectAntibacterial agentsOrganic active ingredientsNitroimidazoleFreeze-drying
The present invention discloses L-ornidazole injection preparation as one kind of nitroimidazole antibiotics. The L-ornidazole injection preparation contains L-ornidazole or its salt or their hydrate. L-ornidazole is named chemically as S-(+)-1-(3-chloro-2-hydroxypropyl)-2-methyl-5- nitroimidazole. The L-ornidazole injection preparation is prepared with L-ornidazole, supplementary material and injection water, and may be prepared into injection for immediately injection, concentrated solution for injection after clinical re-compounding or powder for injection through further freeze drying. Compared with similar preparations, the L-ornidazole preparation has high pharmacodynamic activity, lower toxicity and stable quality.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Stable ornidazole and S-ornidazole injections and a preparing method thereof
ActiveCN107041868ACompatibility is goodStable materialAntibacterial agentsOrganic active ingredientsSodium acetateAcetic acid
Stable ornidazole and S-ornidazole injections are disclosed. Based on 1 g of ornidazole or S-ornidazole, the injections comprise 1 g of the ornidazole or the S-ornidazole, 2-4 g of 1,2-propanediol, 1-3 g of absolute alcohol, acetic acid and sodium acetate, wherein the acetic acid and the sodium acetate are pH adjusting agents, and the using amounts of the acetic acid and the sodium acetate are amounts allowing the pH value of the ornidazole and S-ornidazole injections to be 5.4-6.5. In addition, a preparing method of the injections is also disclosed. Compared with the prior art, stability of related compounds and contents of the injections under filtration and sterilization conditions is good, compatibility with other products is good and irritation is low.
Owner:NANJING CAVENDISH BIO ENG TECH +1
Omidazole injection
InactiveCN106309359ALow organic solvent contentCompatibility is goodAntibacterial agentsOrganic active ingredientsNitriteAlcohol
The invention provides an over-saturated omidazole injection which comprises 1 g of omidazole, 0.7-1.1 g of propylene glycol and alcohol added to 4ml. Content of propylene glycol is 0.7-1.02g preferably, 0.78-0.91g further preferably and 0.78g optimally. The over-saturated omidazole injection is high in combining stability and low in content of nitrite in the process of storage.
Owner:BEIJING JINGKE TAILAI TECH
Ornidazole injection and preparation method thereof
InactiveCN104188903AHigh clarityAntibacterial agentsOrganic active ingredientsMixed materialsOrnidazole
The invention discloses an ornidazole injection, which comprises 1g of ornidazole, 1-1.5g of propylene glycol and 2-2.5g of ethanol. The preparation method of the ornidazole injection comprises the following steps: (1) uniformly mixing ornidazole, propylene glycol and ethanol; (2) heating and cooling the uniformly mixed materials in a circulating manner at least twice, wherein the cycle of heating and cooling comprises steps of heating to 40-60 DEG C from 10-30 DEG C and cooling to 10-30 DEG C from 40-60 DEG C; and (3) filtering, filling and sealing, wherein specification of 2-5ml.
Owner:BEIJING LANDAN PHARMA TECH
Intelligent temperature-sensitive sustained-release gel and preparation method thereof
InactiveCN103784394AFully contactedEasy to acceptAerosol deliveryAntisepticsPolyethylene glycolOrnidazole
The invention discloses an intelligent temperature-sensitive sustained-release gel and a preparation method thereof. The intelligent temperature-sensitive sustained-release gel comprises the following components in percentage by weight: 15-25 percent basic remedy, in-situ gel material and water, 2-3 percent of poloxamer 188, 0.5-1 percent of polyethylene glycol-6000, 5-7 percent of ornidazole and 60-70 percent of distilled water. An intelligent temperature-sensitive sustained-release in-situ gel drug-loading system is prepared by combining medicaments with different types of high molecular carrier materials, and is applied to root canal treatment for the first time, so that the defects that the lasting time of the conventional root canal disinfecting and sterilizing effects is short, root canals cannot be fully filled with medicaments under the limitation of dosage form and the like are overcome, the root canal disinfecting operation is simplified, and the double effects of sustained-release long-acting treatment and root canal enclosing are achieved. The one-time success rate of root canal treatment is increased, and the intelligent temperature-sensitive sustained-release gel has an important practical value on the treatment of pulposis, pulpitis and periapical periodontitis and the like.
Owner:凌春生
Preparation method and application of controlled-release antibiotic composite hydrogel
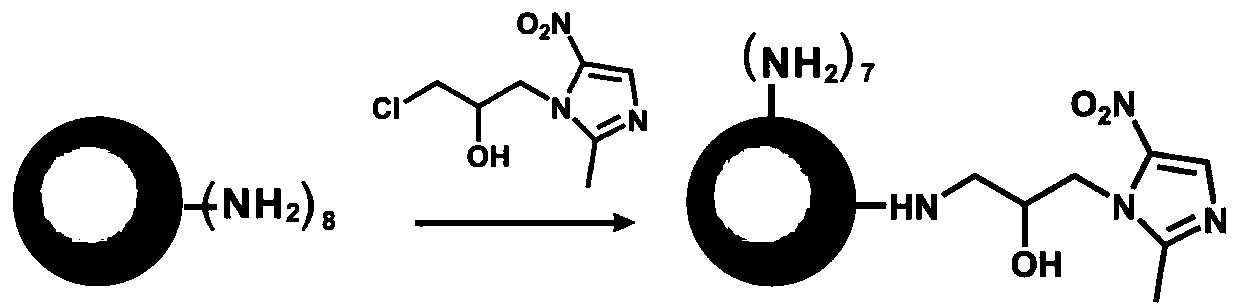
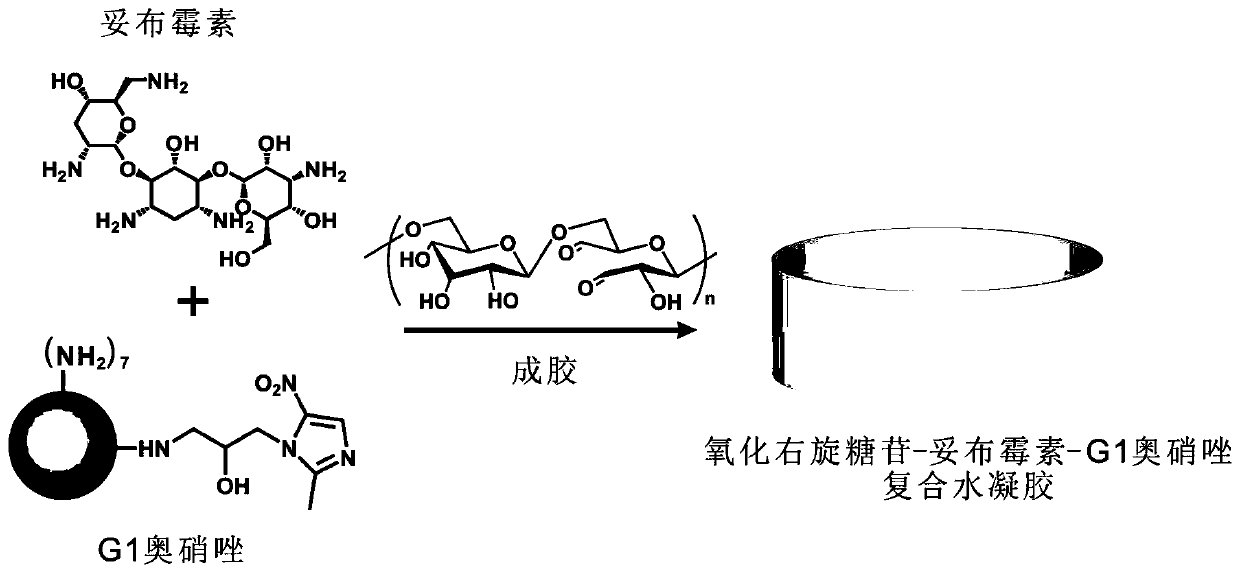
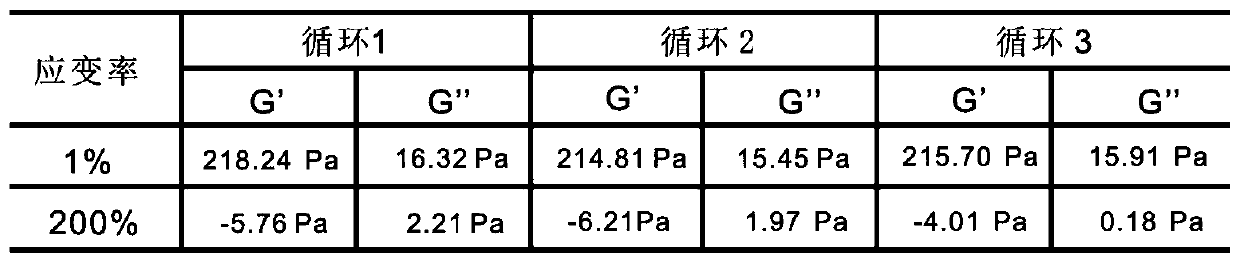
ActiveCN110314242AEasy to synthesizeHigh antibacterial efficiencyAntibacterial agentsOrganic active ingredientsDendrimerAnaerobic bacteria
The invention provides composite antibiotic hydrogel based on controlled release of aminoglycoside antibiotics and ornidazole. The composite antibiotic hydrogel is obtained by crosslinking an oxidizednatural polysaccharide polymer, the aminoglycoside antibiotics and the ornidazole through an acid-sensitive Schiff base bond, wherein the ornidazole is modified by a first-generation polyamidoamine dendrimer with an amino terminal. The Schiff base bond is broken in an acidic environment caused by bacterial infection, so that gel degradation is caused, and the on-demand release of the antibioticsis realized. The composite antibiotic hydrogel is easy to prepare and low in cost. According to the prepared controlled-release composite antibiotic hydrogel, the strength, morphology and degradationof the gel, the release rate of drugs and the like can be controlled based on the content of antibiotics, the composite antibiotic hydrogel has broad-spectrum high-efficiency antibacterial properties,and the bacteriostatic effect is superior to that of various kinds of commercial antibacterial gel on the market. The hydrogel is expected to be prepared into external dressings, ointment preparations, implants, coatings on medical apparatuses and instruments and the like and used for resisting infection caused by Gram-negative bacteria, Gram-positive bacteria, anaerobic bacteria and the like.
Owner:SHANGHAI CHANGZHENG HOSPITAL +1
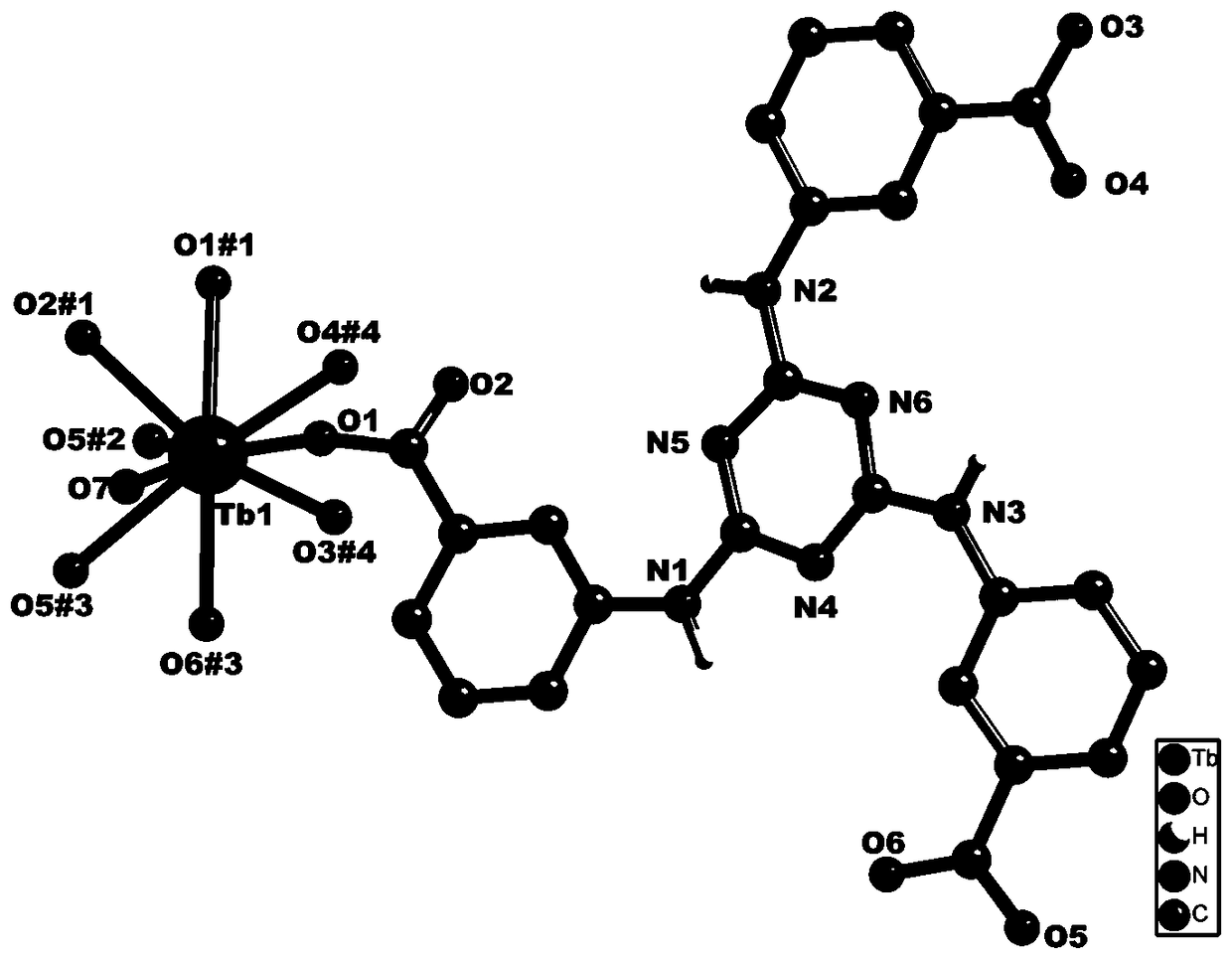
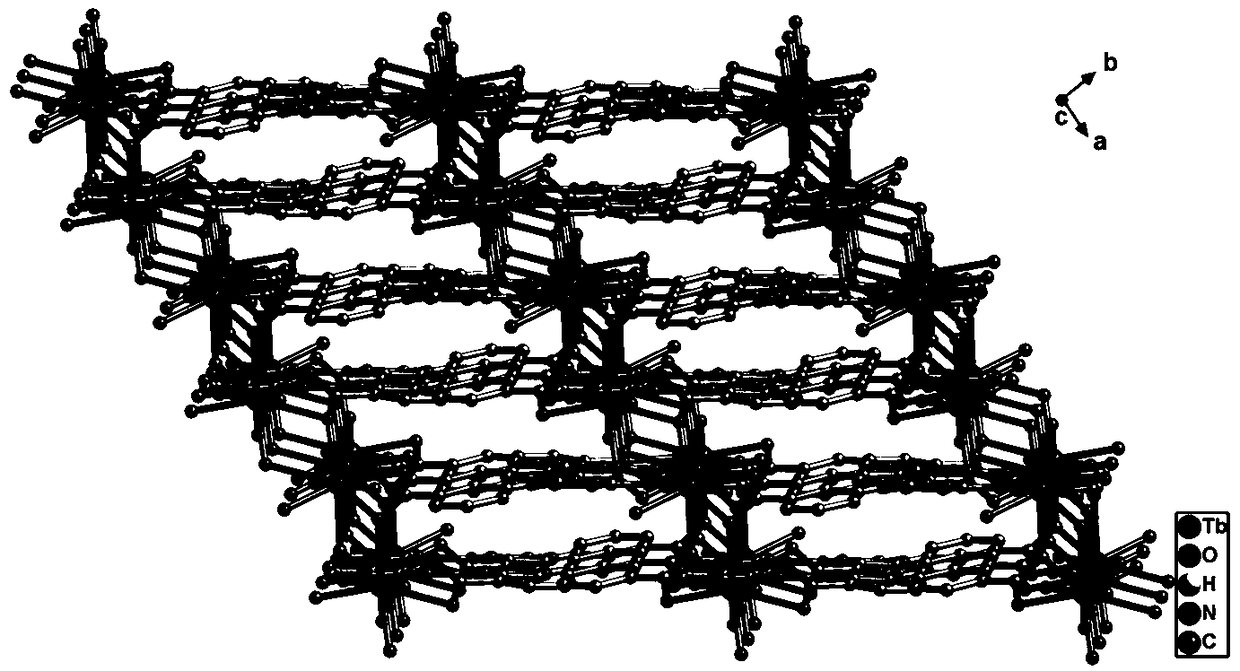
Rare-earth terbium-based metal organic framework material, synthesis method and application thereof to antibiotics identification
ActiveCN109400895ASimple reaction conditionsEasy to repeat a large number of synthesisFluorescence/phosphorescenceLuminescent compositionsSynthesis methodsRare earth
The invention relates to a rare-earth terbium-based metal organic framework material, a synthesis method and application thereof to antibiotics identification. A chemical molecular formula of the metal organic framework material is {[Tb(TATAB)(H2O)].2H2O}n, wherein TATAB is an organic ligand 4,4',4''-s-triazine-1,3,5-tri-m-aminobenzoic acid. A basic structure unit contains one free terbium ion andone complete 4,4',4''-s-triazine-1,3,5-tri-m-aminobenzoic acid; protons of three carboxyl groups in the ligand are removed and then the carboxyl groups are bridged with the adjacent terbium ion; eachterbium ion is coordinated with five ligands and one water molecule, wherein two carboxyl groups are connected with two different terbium ions respectively to form a three-dimensional network structure. The material is simple to prepare, novel in structure and is stable in a water solution; the material can be used for rapidly detecting metronidazole (MDZ), ornidazole (ODZ), ronidazole (RDZ) anddimetridazole (DTZ) in the water solution.
Owner:CHINA THREE GORGES UNIV
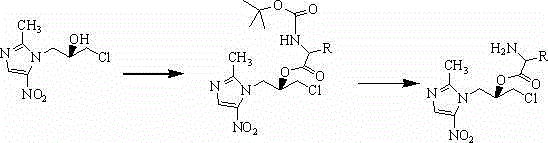
Amino-acid ester water-soluble derivative of (S)-ornidazole and application thereof
The invention relates to an amino-acid ester water-soluble derivative of (S)-ornidazole and application thereof. the amino-acid ester of (S)-ornidazole has a structure as shown in the formula (I), wherein R and (HnX)m are defined in the claims. The invention also relates to a preparation method of the compound as shown in the formula (I), pharmaceutical salt thereof, a pharmaceutical composition containing the compound and the pharmaceutical salt and application of the pharmaceutical composition used as a medicine, especially application of the pharmaceutical composition used for treatment of anaerobic infection or protozoan infection.
Owner:HC SYNTHETIC PHARMA CO LTD
Compound prepn for treating women's inflammation
The present invention is compound preparation for treating womení»s inflammation, and belongs to the field of medicine technology. Each 1000 application units of the compound preparation consists of ornidazole 100-1000 g, butoconazole nitrate 10-500 g and policresulen 50-300 g. The compound preparation may be prepared into different forms, including vaginal suppository, vaginal effervescent tablet, vaginal tablet, vaginal gel, etc. The compound preparation has the functions of resisting anaerobic bacteria, resisting protoplasm, resisting mildew and resisting aerobion, and may be used in treating various womení»s infectious diseases, such as bacterial vaginitis, mycotic vaginitis, protozoal vaginitis, non-specific vaginitis, mixed infectious vaginitis, pruritus vulvae, etc.
Owner:山东特瑞林医药科技发展有限公司
Ornidazole compound in new path
InactiveCN101633643ALow costHigh reaction yieldOrganic chemistryPhysical/chemical process catalystsIce waterCombinatorial chemistry
The invention provides an ornidazole compound in new path. The preparation method comprises the following steps: allowing 2-methyl-5-nitromidazole to react with epichlorohydrin in solvent, adding an ice-water mixture, adjusting pH value, and recrystallizing to obtain the ornidazole. The preparation method can help obtain products with high yield up to more than 85%.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Levo-ornidazole freeze-dried powder injection
InactiveCN1820748AImprove stabilityEasy to transportAntibacterial agentsPowder deliveryAnaerobic infectionFreeze-drying
The present invention discloses freeze dried levo-ornidazole powder for injection as one anaerobic bacteria infection resisting medicine. The freeze dried levo-ornidazole powder for injection includes levo-ornidazole as main medicinal component and pharmaceutically acceptable carrier, includes solvent selected from one or several of ethanol, propylene glycol, glycerin, polyethylene glycol, Tween-80, etc. The freeze dried levo-ornidazole powder for injection may be prepared into specification containing levo-ornidazole of 1-2000 mg, preferably 0.25-1.5g and especially 0.25g, 0.5g and 1.0g. It has high stability, high curative effect and other features.
Owner:GUANGDONG XIANQIANG PHARMA
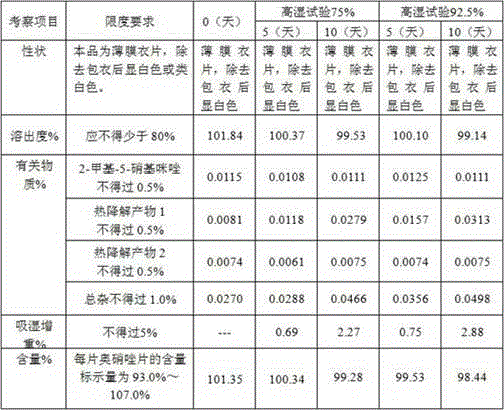
Quality control method for ornidazole
InactiveCN102565271APromote absorptionSensitive and accurate detectionComponent separationNitroimidazoleElution
The invention discloses a quality control method for ornidazole, which adopts the high performance liquid chromatography for on-line detecting ornidazole and impurity constituent such as 2-methyl-5-nitroimidazole at the same time. According to the invention, the optimal mobile phase composition, elution procedure, flow velocity, detection wave length, chromatographic columns and other analysis conditions can be preferably selected through plenty of experiments, and as verified and proved through many experiments, the quality control method for ornidazole provided by the invention has excellent stability and repeatability, high analysis efficiency and excellent separation degree, and can be used for qualitative and quantitative detections for compounds sensitively and accurately, thereby being used for objectively, comprehensively and accurately evaluating the quality of ornidazole injection, and having vital significances to the quality control of ornidazole injection and assurance of the clinical curative effect.
Owner:YANGTZE RIVER PHARM GRP NANJING HAILING PHARM CO LTD
Ornidazole injection liquid
InactiveCN104127373AGood storage stabilityLess irritatingAntibacterial agentsOrganic active ingredientsOrganic solventIrritation
The invention provides a preparation method of an ornidazole injection liquid. The preparation method comprises the steps: (1) mixing evenly ornidazole and an organic solvent, wherein the organic solvent is ethanol or is a mixture of ethanol and propylene glycol; (2) carrying out at least two times of heating cooling circulation on the evenly-mixed material, wherein the heating cooling circulation comprises the steps of firstly heating from the temperature of 10-30 DEG C to the temperature of 40-60 DEG C, and then cooling from the temperature of 40-60 DEG C to the temperature of 10-30 DEG C. The invention also provides the ornidazole injection liquid, wherein the ornidazole injection liquid is prepared according to the above method. Through the technical scheme, the storage stability of the ornidazole injection liquid under a condition of relatively low temperatures (-10 DEG C to 20 DEG C) is significantly improved, and irritation is relatively small after injection.
Owner:BEIJING JINGKE TAILAI TECH
L-ornidazole prepn
InactiveCN1739504AImprove antibacterial propertiesExtended half-lifeOrganic active ingredientsAntimycoticsCoated tabletsNitroimidazole
The present invention discloses L-ornidazole preparation as one kind of nitroimidazole antibiotics. The L-ornidazole preparation contains L-ornidazole or its salt or their hydrate. L-ornidazole is named chemically as S-(+)-1-(3-chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole. The L-ornidazole preparation is prepared with L-ornidazole and supplementary material, and may be prepared in tablet, dispersant tablet, coated tablet, enteric tablet, capsule or granule. Compared with similar preparations, the L-ornidazole preparation has high pharmacodynamic activity, lower toxicity and stable quality.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Ornidazole oral preparation and preparation method thereof
ActiveCN103142494AReduce typesImprove bioavailabilityAntibacterial agentsOrganic active ingredientsCelluloseAdhesive
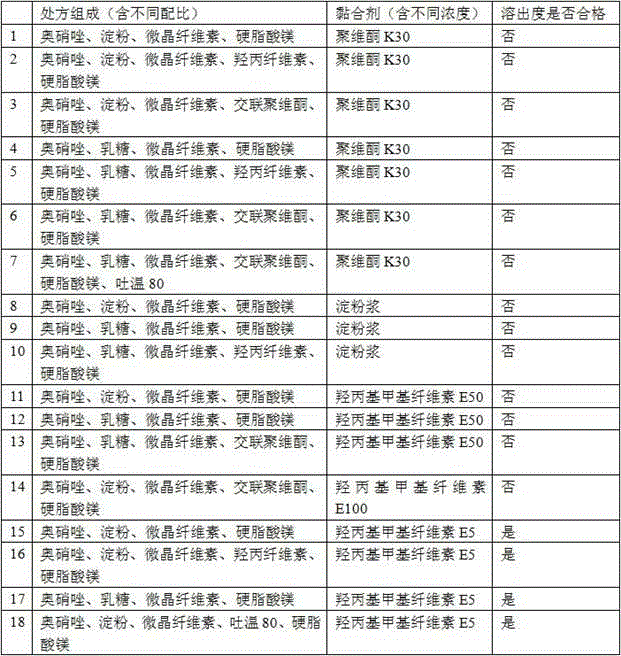
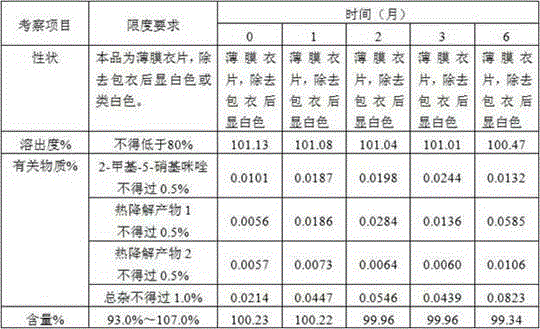
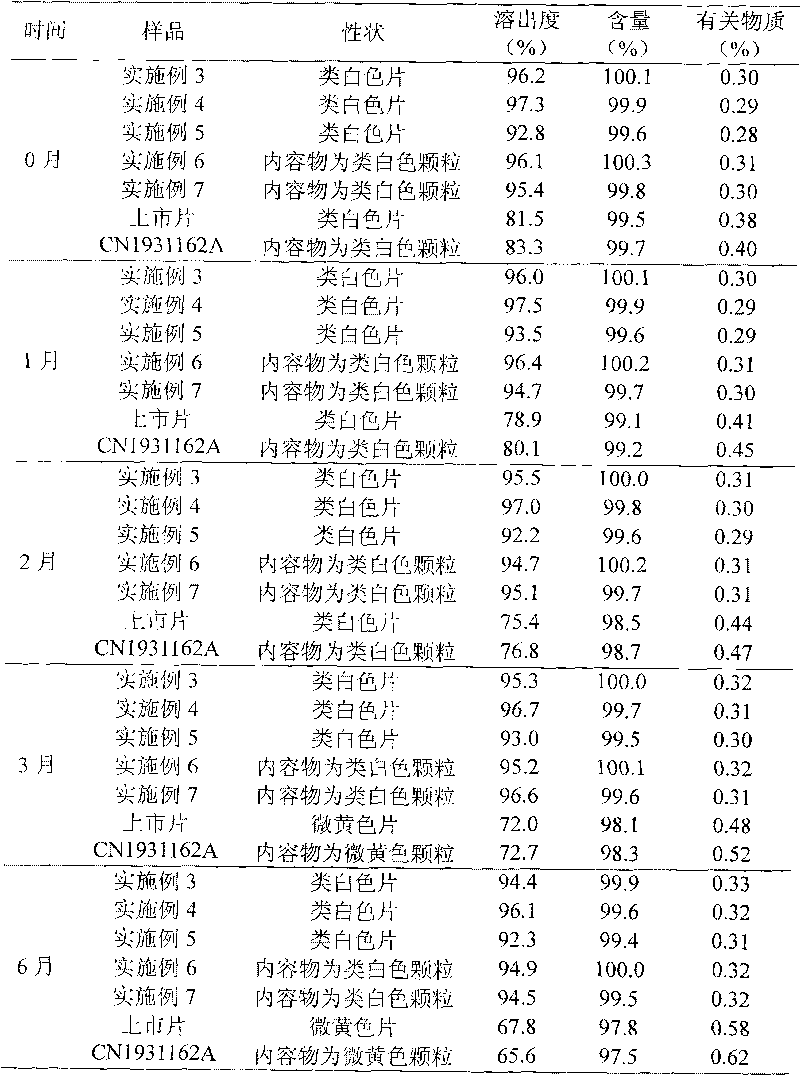
The invention discloses an ornidazole oral preparation and a preparation method thereof. The prescription of the oral preparation comprises ornidazole, filler, an adhesive and a lubricant, and also can contain film coating powder. According to the preparation method, a disintegrating agent is not required to be added, and the problem of low dissolution rate of the ornidazole oral preparation can be solved by selecting a proper adhesive (hydroxypropyl methyl cellulose E5 aqueous solution or alcohol solution). The ornidazole oral preparation is simple in prescription, low in cost and high in dissolution rate, and is easy to industrialize.
Owner:河北凯盛医药科技有限公司
Ornidazole ester microsphere solid preparation
InactiveCN101716153AImprove therapeutic indexLow toxicityOrganic active ingredientsAntiinfectivesYolkSide effect
The invention discloses an ornidazole ester microsphere solid preparation. Specifically, ornidazole ester microspheres are prepared by adopting film dispersion technology through the combination of hydrogenated yolk lecithin, cholesterol, poloxamer 188, sodium glycyl-cholate and ornidazole serving as active component with certain contents, and then the ornidazole ester microspheres are mixed with certain accessories to obtain the solid preparation such as tablets and capsules. The solid preparation has the surprising technical effects of high dissolution degree, high bioavailability, small toxic and side effect and the like.
Owner:HAINAN YONGTIAN PHARMA INST
Toothpaste composition with oral cavity nursery function
InactiveCN1994267AIncreased Antibacterial Enzyme ContentInhibitionCosmetic preparationsToilet preparationsToothpasteGINSENG EXTRACT
The invention provides a toothpaste composition which is prepared from the following constituents (by weight portions): sorbitol 55-60%, methyl glycol 2-5%, sodium carboxymethylcellulose 0.5%, gluside 0.3%, sodium dodecylsulfate 2%, gourmet powder 1-1. 5%, strontium chloride 0.2-0.5%, medicinal guan-yin tea 0.5-1. 5%, pseudo-ginseng extract 0.5-1. 5%, silicon abrasive 25%, SOD complex enzyme 0.02%, biological lysozyme 0.01%, Ornidazole 0.5-1. 5% and balancing water.
Owner:TIANJIN LANTIAN GRP
Preparation and purification method for new ornidazole optical antimer
The invention provides a preparation and refining method for a new ornidazole optical antimer. The method comprises the following steps of: adding 2-methyl-5-nitroimidazole into an acid solution with a catalyst to react with chiral epoxy chloropropane to generate an ornidazole optical antimer, performing re-crystallization on the ornidazole optical antimer by using a mixed solution of an organic solvent and water, and thus obtaining the high-purity ornidazole optical antimer.
Owner:HC SYNTHETIC PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com