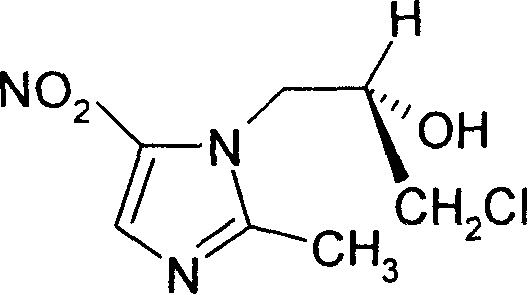
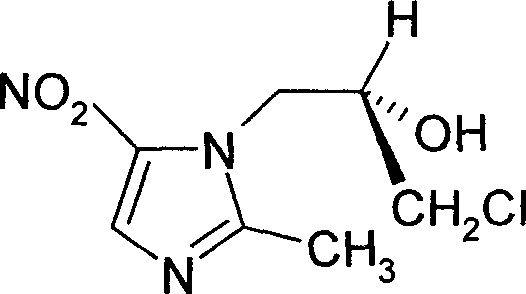
L-ornidazole prepn
A preparation technology of L-ornidazole, which is applied in the field of preparation of nitroimidazole antibiotics L-ornidazole, can solve the problems of high toxicity and poor activity of ornidazole racemate, and achieve long half-life, no special toxicity, The effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Make 1000 L-ornidazole tablets with the raw materials in the following weight ratio, and the tablet weight is 435mg
[0019] Levonidazole 250g
[0020] Pregelatinized starch 50g
[0021] Microcrystalline Cellulose 100g
[0022] 3% HPMC 40% ethanol solution appropriate amount
[0023] Sodium carboxymethyl starch 30g
[0025] Preparation Process:
[0026] 1. Raw materials and auxiliary materials of L-ornidazole are pulverized separately and passed through a 100-mesh sieve.
[0027] 2. Take L-ornidazole, pregelatinized starch, and microcrystalline cellulose and mix well, use 3% HPMC 40% ethanol solution to make soft material, granulate, and granulate after drying.
[0028] 3. Add sodium carboxymethyl starch and magnesium stearate, mix well, and press into tablets.
Embodiment 2
[0030] 1. Prepare 6% ethanol solution of Opadry enteric coating solution;
[0031] 2. Take the plain tablet obtained in Example 1 and pour it into a coating pan for coating to obtain L-ornidazole enteric-coated tablet. Coating powder relative to tablet core weight gain: 4.0-5.0%.
Embodiment 3
[0033] Prepare 1000 L-ornidazole tablets with the raw materials in the following weight ratio, and the tablet weight is 230 mg.
[0034] Levonidazole 125g
[0035] 30g pregelatinized starch
[0036] Microcrystalline Cellulose 50g
[0037] 3% HPMC 40% ethanol solution appropriate amount
[0038] Sodium carboxymethyl starch 20g
[0040] Preparation Process:
[0041] 1. Separately pulverize the L-ornidazole raw material and auxiliary materials, and pass through a 100-mesh sieve.
[0042] 2. Take L-ornidazole, pregelatinized starch, and microcrystalline cellulose and mix well, use 3% HPMC 40% ethanol solution to make soft material, granulate, and granulate after drying.
[0043] 3. Add sodium carboxymethyl starch and magnesium stearate, mix well, and press into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sheet weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com