Ornidazole compound in new path
A technology of ornidazole and compound, applied in the field of drug synthesis, can solve the problems of high cost, low yield, unsuitable production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
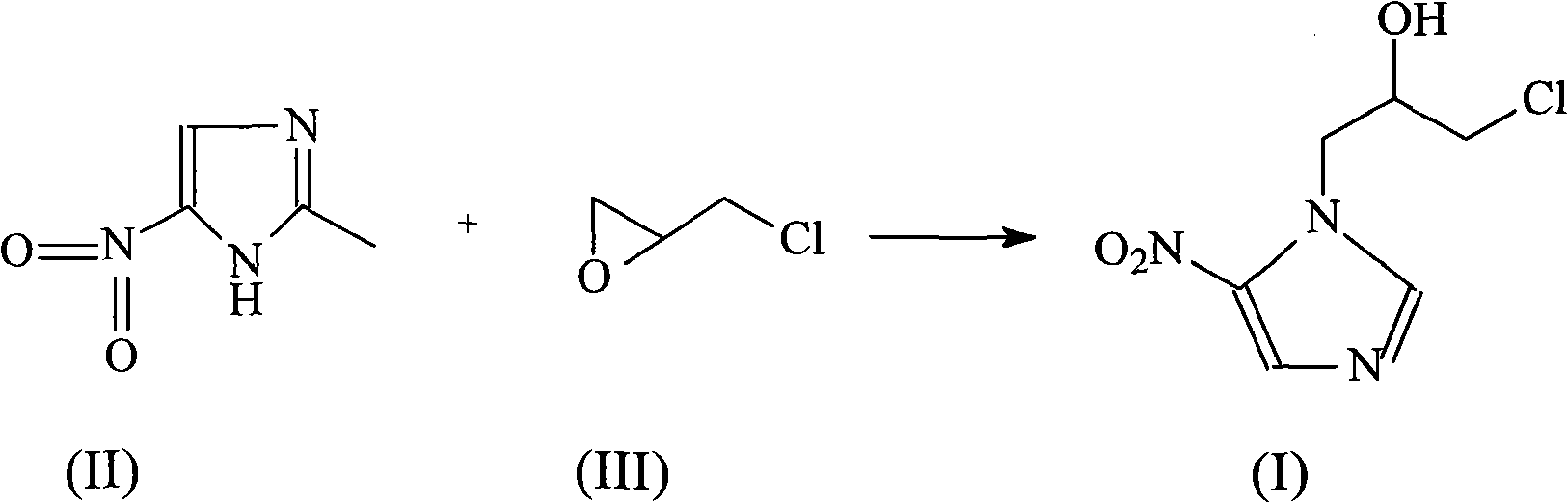
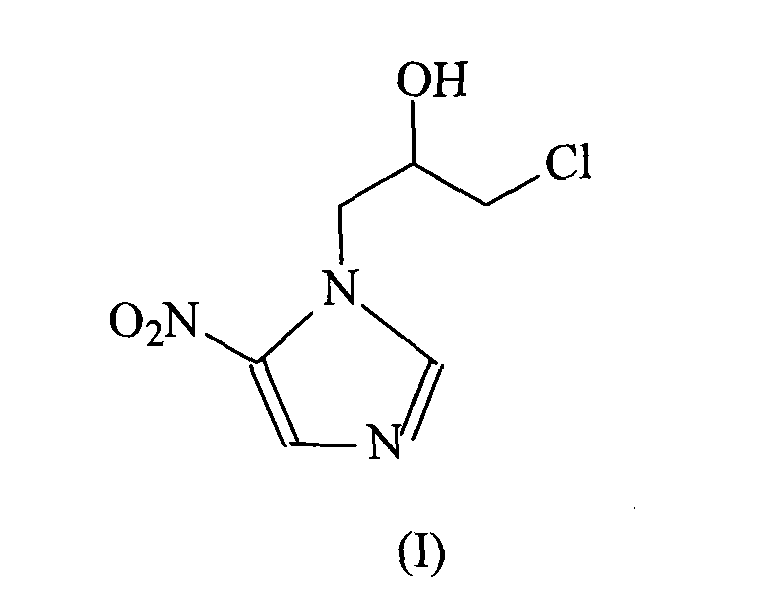
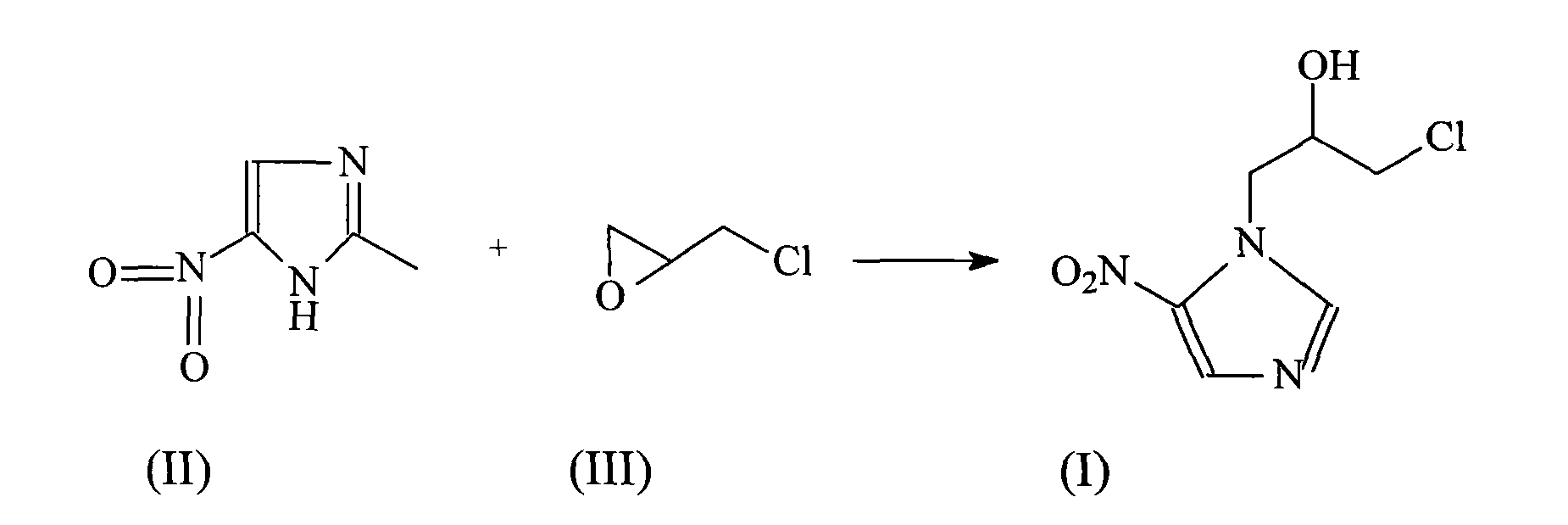
[0024] The synthesis of embodiment 1 ornidazole
[0025] In 1500ml of ethyl acetate, add 127 grams (1mol) of 2-methyl-5-nitroimidazole, cool to 2°C, slowly add 100ml of boron trifluoride ether solution, keep the temperature of the reaction system not exceeding 5°C, and then Add 139 grams (1.5 mol) of epichlorohydrin dropwise to the reaction system, while keeping the reaction temperature not exceeding 5°C, continue to react at this temperature for 5 hours after the addition, after the reaction, slowly add 600ml of ice-water mixture, Vigorously stir, the reaction temperature does not exceed 25°C, then use concentrated hydrochloric acid to adjust the pH of the system to 1, let it stand, and separate layers, adjust the water phase to pH = 7.3 with concentrated ammonia water, extract with 2000ml ethyl acetate, dry over anhydrous sodium sulfate, and filter , concentrated, and then recrystallized from toluene to obtain 187 g of the product, with a yield of 85%, and mp: 77°C.
[0026...
Embodiment 2
[0028] The synthesis of embodiment 2 ornidazole
[0029] In 3000ml of ethyl acetate, add 254 grams (2mol) of 2-methyl-5-nitroimidazole, cool to 5°C, slowly add 200ml of boron trifluoride ether solution, keep the temperature of the reaction system not exceeding 5°C, and then Add 278 grams (3 mol) of epichlorohydrin dropwise to the reaction system while keeping the reaction temperature not exceeding 5°C. After the addition, continue to react at this temperature for 5 hours. After the reaction, slowly add 1100ml of ice-water mixture, vigorously Stir, the reaction temperature does not exceed 25°C, then use concentrated hydrochloric acid to adjust the pH of the system to 1, let stand, separate layers, adjust the water phase to pH = 7.4 with concentrated ammonia water, extract with 3000ml ethyl acetate, dry over anhydrous sodium sulfate, filter, Concentrate, and then recrystallize with toluene to obtain 387 g of product, yield 88%, mp: 78°C.
[0030] Elemental analysis theoretical ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com