Preparation and purification method for new ornidazole optical antimer
An ornidazole optics and purification method technology, applied in organic chemistry and other fields, can solve problems such as discomfort, low water solubility, autoimmune hepatitis, peripheral neuropathy, etc., and achieve the effects of reducing pollution, simple operation, and easier control of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: Preparation of L-ornidazole
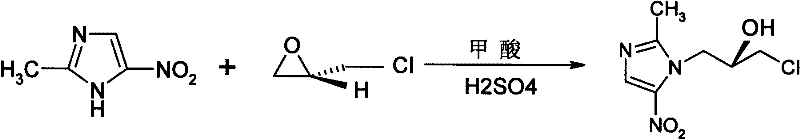
[0019] Add 3L of formic acid into a 5L reaction flask, add 171ml of concentrated sulfuric acid while stirring, and continue stirring for 1 hour. Add 540 g of 2-methyl-5-nitroimidazole, stir and lower the temperature to -5°C. Slowly add 1.08 L of S-(+)-epichlorohydrin dropwise, and control the reaction temperature below 5°C. After dropping, stir the reaction, control the temperature of the reaction solution at 10°C to 15°C, and monitor the reaction by HPLC. After the reaction was stopped (increase of reaction degree in 2 hours was less than 0.3%), the formic acid and excess S-(+)-epichlorohydrin in the reaction solution were evaporated under reduced pressure, and the residue was added to 2000ml of ice-water mixture to adjust the pH to 3-4, the filtrate was extracted with ethyl acetate (2L×3), the ethyl acetate was combined, dried with anhydrous sodium sulfate, filtered, 5L of water was added to the filtrate, and hydrochloric ...
Embodiment 2
[0022] Embodiment 2: Preparation of dex-ornidazole
[0023] Add 3L of acetic acid into the 5L reaction flask, add 171ml of concentrated sulfuric acid while stirring, and continue stirring for 1 hour. Add 540 g of 2-methyl-5-nitroimidazole, stir and lower the temperature to 15°C. Slowly add 1.08 L of R-(-)-epichlorohydrin dropwise, and control the reaction temperature to 15°C. After dropping, stir the reaction, control the temperature of the reaction solution at 10°C to 20°C, and monitor the reaction by HPLC. After the reaction was stopped (increase of reaction degree in 2 hours was less than 0.3%), the formic acid and excess R-(-)-epichlorohydrin in the reaction solution were evaporated under reduced pressure, and the residue was added to 2000ml of ice-water mixture to adjust the pH to 3-4, the filtrate was extracted with ethyl acetate (2L×3), the ethyl acetate was combined, dried with anhydrous sodium sulfate, filtered, 5L of water was added to the filtrate, and hydrochlori...
Embodiment 3
[0026] Embodiment 3: Preparation of L-ornidazole
[0027] Add 3L of formic acid into a 5L reaction flask, add 120ml of perchloric acid under stirring, and continue stirring for 1 hour. Add 540 g of 2-methyl-5-nitroimidazole, stir and lower the temperature to -5°C. Slowly add 1.08 L of S-(+)-epichlorohydrin dropwise, and control the reaction temperature below 5°C. After dropping, stir the reaction, control the temperature of the reaction solution at 10°C to 15°C, and monitor the reaction by HPLC. After the reaction was stopped (increase in the degree of reaction in 2 hours was less than 0.3%), the formic acid and excess S-(+)-epichlorohydrin in the reaction solution were evaporated under reduced pressure, and the residue was added to 2000ml of ice-water mixture to adjust the pH to 3-4, the filtrate was extracted with ethyl acetate (2L×3), the ethyl acetate was combined, dried with anhydrous sodium sulfate, filtered, 5L of water was added to the filtrate, and hydrochloric acid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com