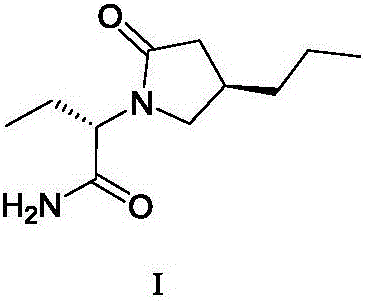
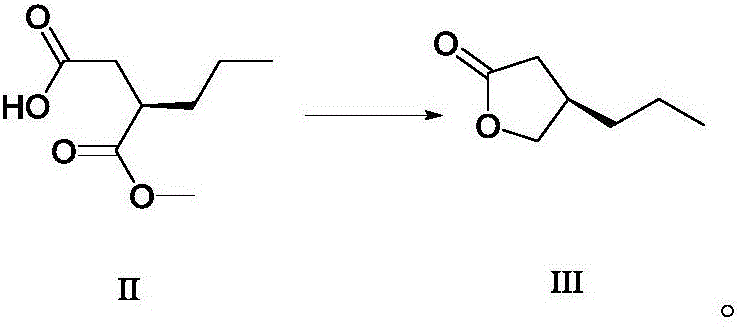
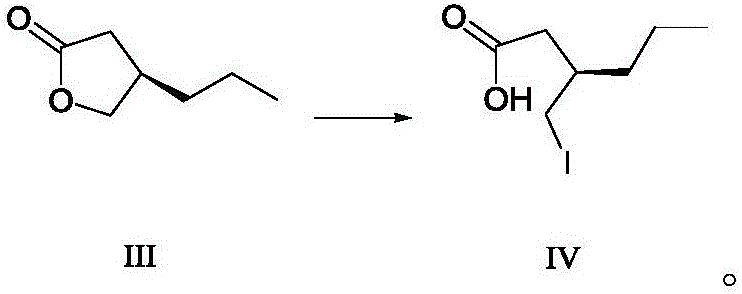
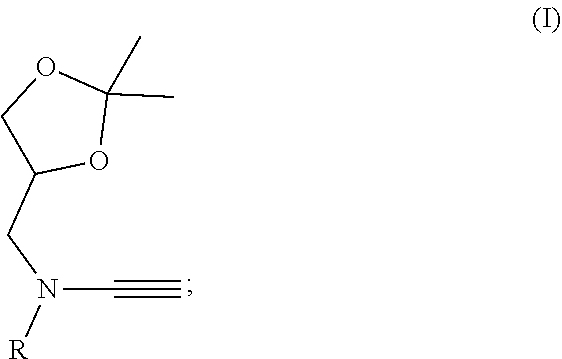
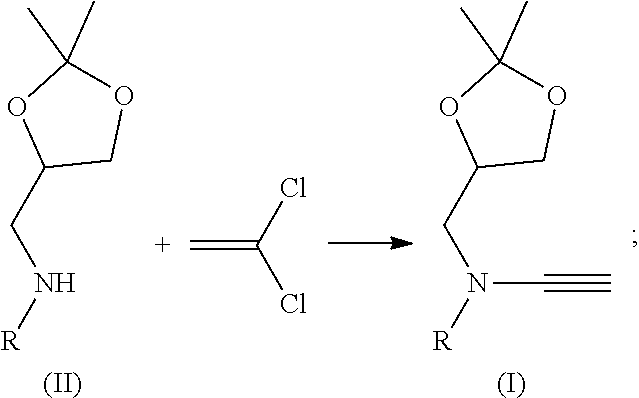
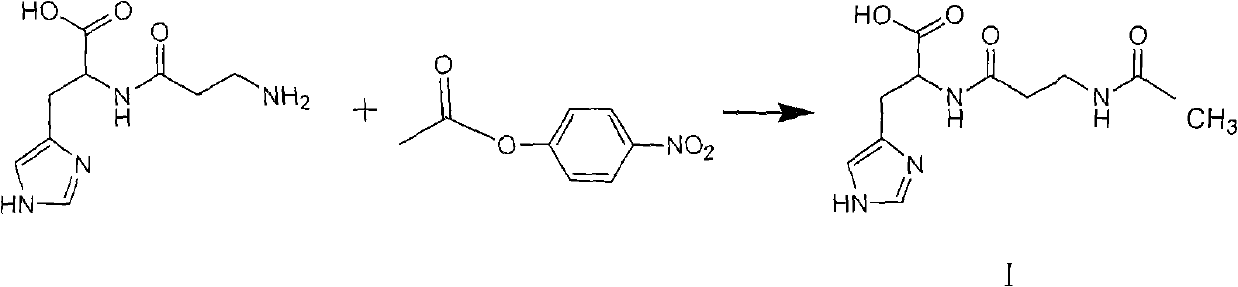
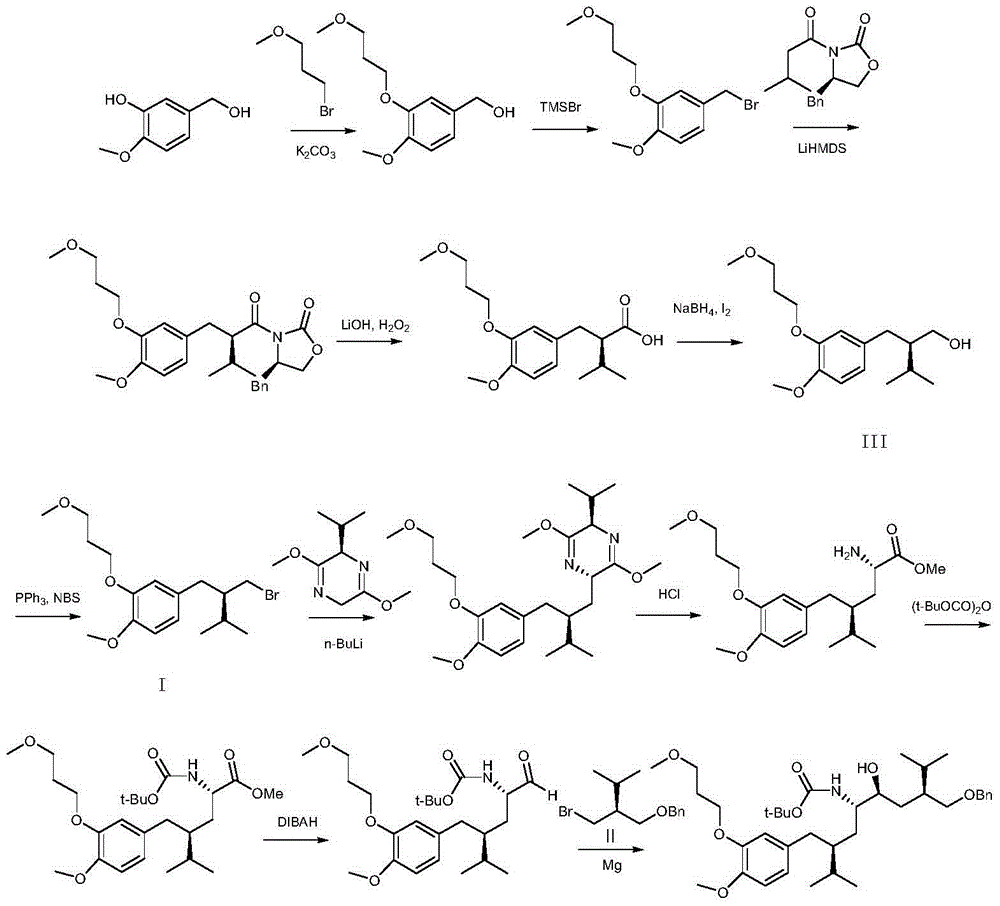
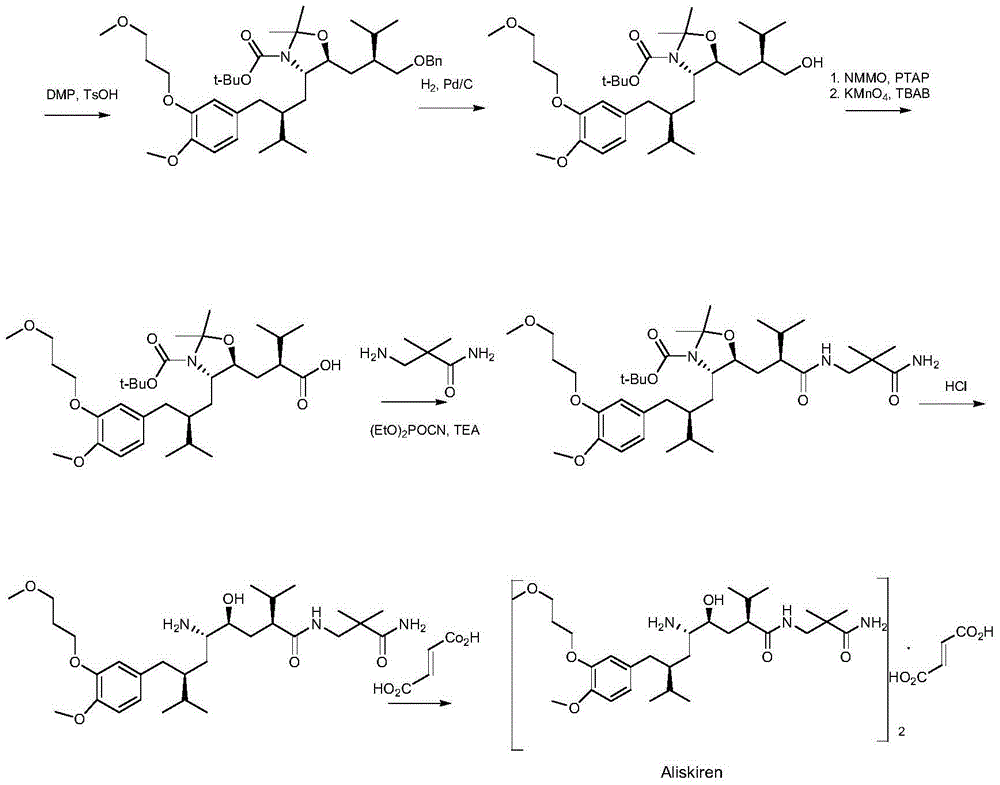
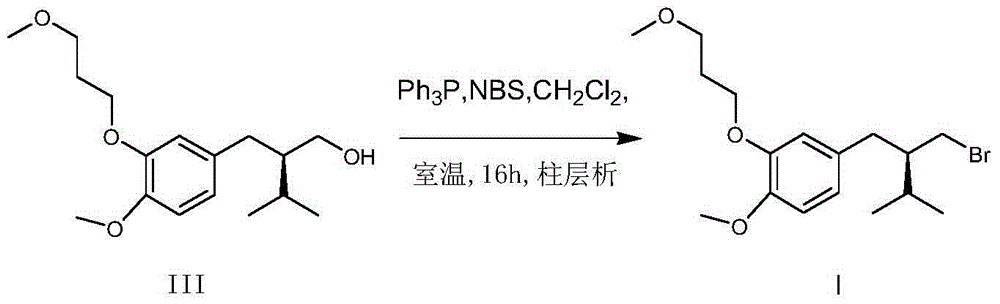
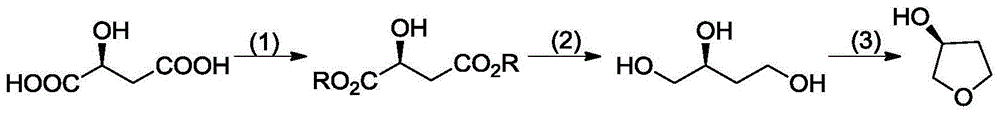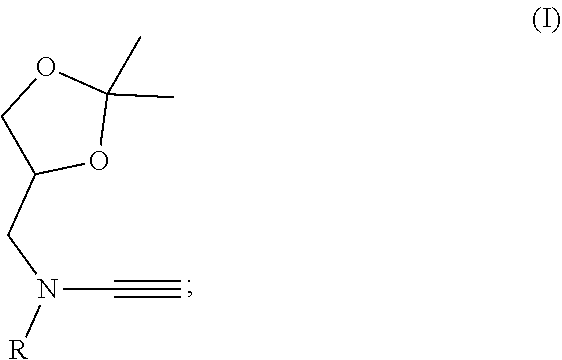Patents
Literature
39results about How to "No racemization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for preparing and purifying (L)-pantoprazole sodium
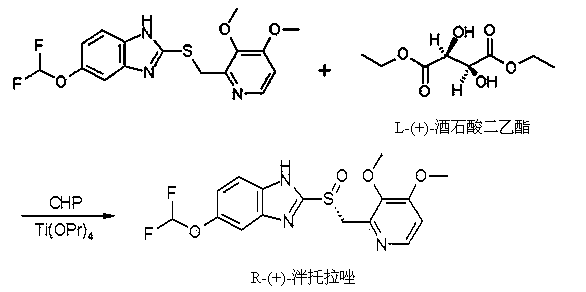
The invention provides a method for preparing (L)-pantoprazole sodium, which comprises the following steps of: oxidizing 5-difluoromethoxy-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]thio}-1H-benzimidazole by using 3,5-diisopropylbenzene hydroperoxide under the catalysis of a tetraisopropyl titanate, D-(-)-diethyl tartrate and N,N-diisopropylethylamine system to obtain S-(-)-5-difluoromethoxy-2-{[(3,4-dimethoxy-2-pyridinyl)methyl]sulfinyl}-1H-benzimidazole, namely (L)-pantoprazole, refining the (L)-pantoprazole, and preparing a salt to obtain the (L)-pantoprazole sodium.
Owner:HC SYNTHETIC PHARMA CO LTD
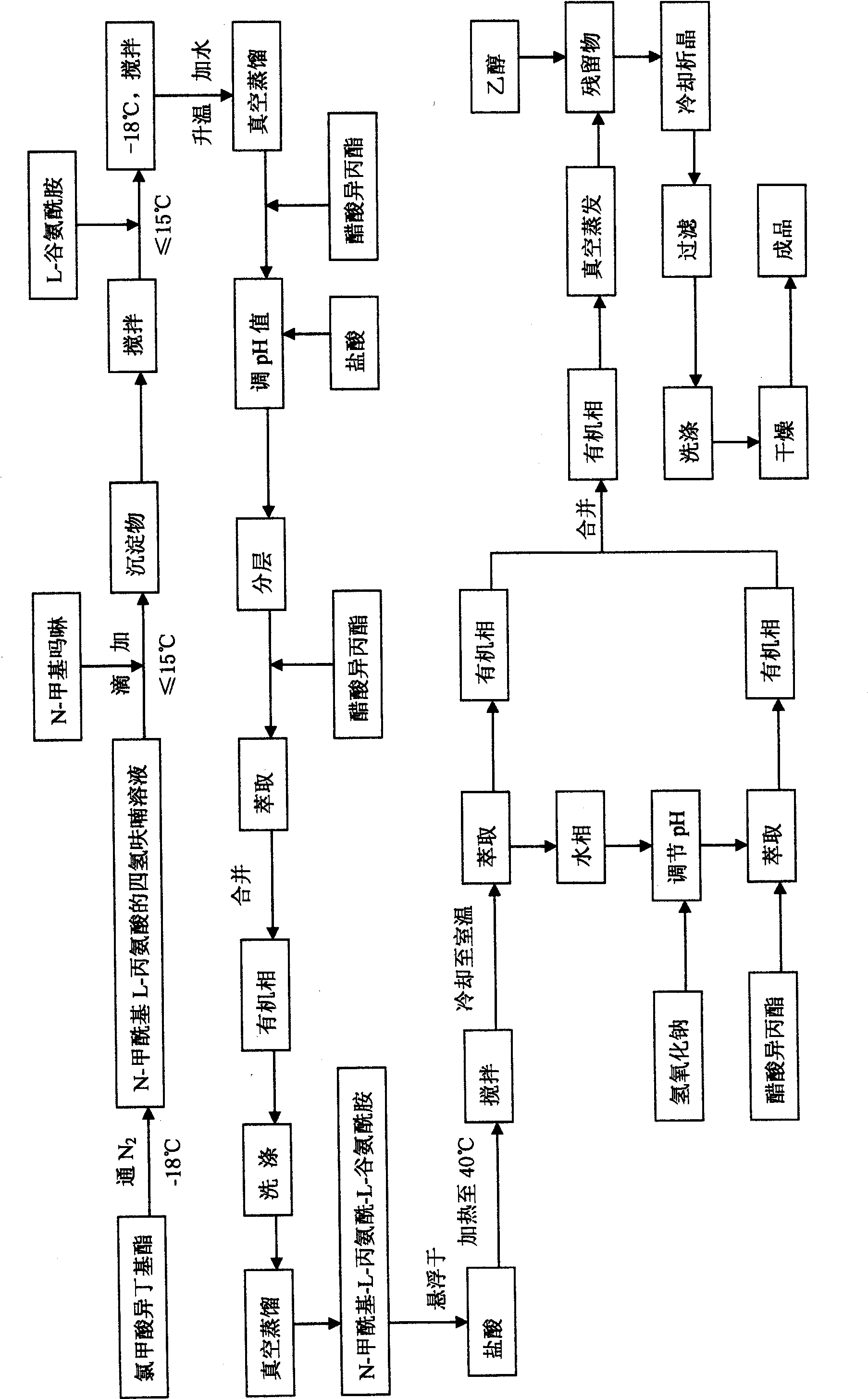
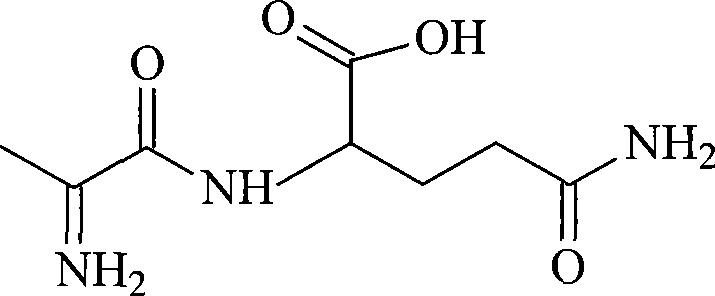
L-alanyl-L-glutamine compound and synthetic method thereof
InactiveCN101519428AThe synthesis process is simpleThe synthesis process is easy to operatePeptidesChemistryL-glutamine
The invention aims to overcome the defects of complicated synthetic process, great harm to human body and high production cost of L-alanyl-glutamine compound in the prior art, and the synthetic method of the L-alanyl-glutamine compound with simple process, low cost, high productivity and less harm is provided. The invention provides the synthetic method of the L-alanyl-L-glutamine compound, which comprises the following steps: (1) adding a chloroformate isobutyl ester to a tetrahydrofuran solution of N-formacyl L-alanine to obtain N-formacyl-L-alanyl-L-glutamine, and (2) suspending the N-formacyl-L-alanyl-L-glutamine in hydrochloric acid to prepare the L- alanyl-L-glutamine compound.
Owner:HAINAN LINGKANG PHARMA CO LTD
Method for preparing levodopa by virtue of enzymic method
ActiveCN104726513ARich sourcesReduce manufacturing costMicroorganism based processesFermentationBiotechnologyStenotrophomonas maltophilia
The invention discloses a method for preparing levodopa by virtue of an enzymic method. The method comprises the following steps: picking up an annular stenotrophomonas maltophilia strain slant for inoculation into a seed culture medium for culturing, thereby obtaining a primary seed liquid; inoculating a fermentation culture medium with the primary seed liquid at the inoculum size of 3-10% for fermentation culturing, centrifuging after the fermentation is ended and collecting bacterial cells; and adding tyrosine and the bacterial cells to a buffer solution to have an enzymatic reaction under the conditions of 18-30 DEG C and the pH of 5.0-6.0, thereby converting the tyrosine into the levodopa. According to the method, due to the resting cell transformation technology, and the preferred intermittent weak ventilation technology, adopted in the transformation process, the catalytic efficiency of the enzyme is improved; the concentration of the levodopa is 27g / L and the molar transformation rate of the tyrosine is above 99%; and besides, the method is mild in conditions, high in enantio-selectivity and free from racemization effect, is more suitable for industrial production, and has remarkable economic benefit and industrial application value.
Owner:SHANDONG YANGCHENG BIOLOGY TECH CO LTD
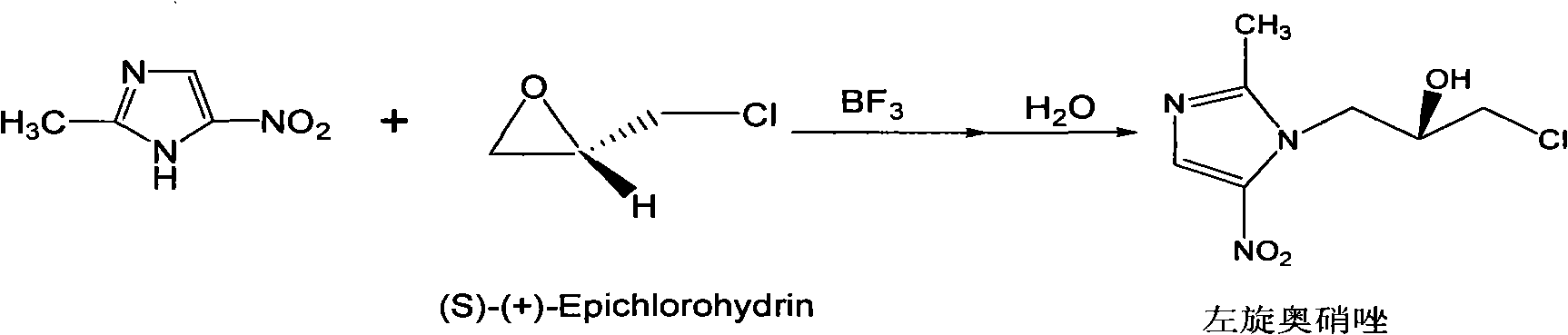
Method for preparing (S)-ornidazole
The invention provides a method for preparing (S)-ornidazole. The method comprises the following steps of: adding an organic solvent and 2-methyl-5-ornidazole, slowly adding a catalyst dropwise with stirring first, then slowly dripping S-(+)-epichlorohydrin into the mixed solution, and after the S-(+)-epichlorohydrin is dripped completely, performing a reaction for 2 to 10 hours at the temperature of between 0 and 20 DEG C; slowly adding the reaction solution into the ice-water mixture, stirring the mixed solution for a reaction for 10 minutes and 5 hours at the temperature of no less than 30 DEG C, adjusting a pH value of the mixed solution to 1.0 to 2.0 with acid, removing an organic layer, adjusting the pH value of a water layer to 6.0 to 8.0 with alkali, stirring the mixed solution for 1 to 24 hours for crystallization, filtering the reaction solution, washing the product obtained by water, and drying the product to obtain the (S)-ornidazole. The method has the advantages of high product purity, simplified procedures and the suitability for industrial production.
Owner:HC SYNTHETIC PHARMA CO LTD
Water-soluble amino acid fertilizer and preparation method thereof
InactiveCN106008085ARich varietyReasonable collocationCalcareous fertilisersMagnesium fertilisersFermentationWheat Brans
The invention provides water-soluble amino acid fertilizer and a preparation method thereof. Raw materials of the water-soluble amino acid fertilizer comprise one or a mixture of bone meal, soybean cakes, rice bran, wheat bran, fish and shrimps. According to the water-soluble amino acid fertilizer, protein waste is used sufficiently, waste is changed into wealth, reaction conditions in a preparation process are mild, amino acid deterioration is avoided, and a racemization effect is not produced. Meanwhile, by the aid of a microbial fermentation technology, soil is conditioned, microorganisms in the soil are activated, soil hardening is avoided, and the air permeability of the soil is improved. Drought pressure of the soil can be relieved, use of chemical fertilizer can be reduced, damage of salt and alkali to the soil can be reduced, and the soil fertility can be improved. Meanwhile, the yield of food crops, cash crops, vegetables, melons and fruits can be increased greatly.
Owner:吴健
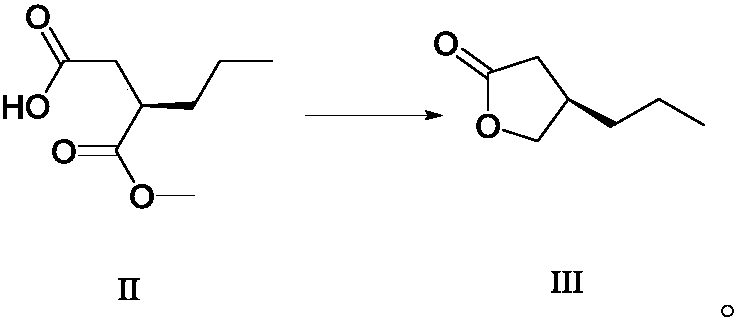
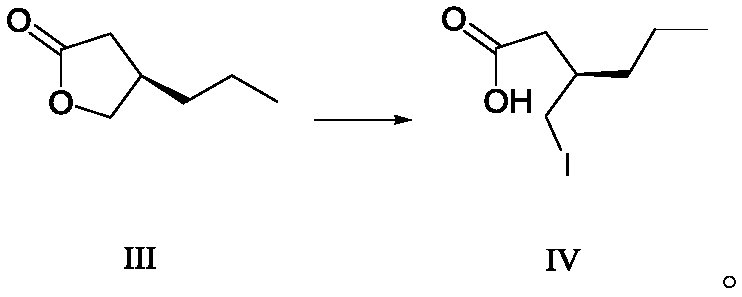
Method for preparing furanone compounds
ActiveCN106588831AHigh chiral purityShort synthetic routeOrganic compound preparationPreparation from carboxylic acid esters/lactonesInorganic saltsSolvent
The invention discloses a method for preparing furanone compounds, and provides a method for preparing a furanone compound III. The method includes the following steps that in a solvent, in the presence of inorganic salt, a compound II and a reducing agent are subjected to a reduction reaction, and the furanone compound III is obtained; the solvent is a fatty alcohol solvent or a mixed solvent of a fatty alcohol solvent and water. The brivaracetam can be prepared with the furanone compound III only with the three steps, and the synthetic route is short; the ee value of the compound II is larger than 99.0%, racemization does not occur in the reaction process, and the de value of a brivaracetam I crude product is larger than 99.0%; the brivaracetam I crude product is further purified through a crystal instead of a chirality high-pressure-liquid-phase preparing column, and the chirality purity of brivaracetam I can be further increased to be the de value of 99.80% or above; meanwhile, the content of other individual impurities of the brivaracetam I is smaller than 0.1%, and reaches the API level, and the method is suitable for industrial production. The formula is defined in the description.
Owner:上海云晟研新生物科技有限公司
Synthetic method of (S)-(+)-3-hydroxytetrahydrofuran
InactiveCN104478833AShort stepsInexpensive and easy to use raw materialsOrganic chemistrySodium borohydride3-Hydroxytetrahydrofuran
The invention provides a synthetic method of (S)-(+)-3-hydroxytetrahydrofuran. The synthetic method comprises the following steps: (1) with L-malic acid as a raw material, carrying out esterification on two carboxyls of the L-malic acid to obtain a product with a formula shown in the specification; (2) reducing a product in the first step by virtue of sodium borohydride to obtain a product with a formula shown in the specification; and (3) carrying out cyclization on a product obtained in the second step to obtain the (S)-(+)-3-hydroxytetrahydrofuran. According to the synthetic method provided by the invention, the synthetic route is short in step, the used raw materials are cheap and easy to acquire, no racemization phenomenon occurs in the reaction process, a by-product in the third step is easy to remove, the total yield is high, and the (S)-(+)-3-hydroxytetrahydrofuran is suitable for industrial production.
Owner:SUZHOU JONATHAN NEW MATERIALS TECH
Production method for cyclic dipeptide
The invention relates to a production method for cyclic dipeptide, and belongs to a natural compound synthesis technology. Cyclic (histidine-proline) dipeptide is prepared by a cooling varying-temperature synthesis and high-pressure high-temperature water phase cyclizing technology. The production method comprises the following steps: taking hydrochloride of histidine proline dipeptide methyl ester as a raw material, and synthesizing high-quality and high-purity cyclic histidine-proline dipeptide by a high-pressure high-temperature assisted method in water containing strong basic and weak acidic salt. Compared with a conventional methanol reflux method, the production method is time-saving, efficient, high in product yield and free of racemization phenomenon.
Owner:JILIN AGRICULTURAL UNIV
Method for extracting concentrated S-adenosylmethionine
ActiveCN102617681AExtended service lifeEnhanced mass transferSugar derivativesSugar derivatives preparationHollow fibre membraneIntermolecular force
The invention belongs to the field of biopharmaceuticals, and relates to a method for extracting concentrated S-adenosylmethionine. The method uses a new synchronous analytic supported liquid membrane separation technology; in a supported liquid membrane separation system, a feed phase, a first order analytic phase and a second order analytic phase are aqueous solution phases, and are connected with a water-immiscible organic liquid membrane to form an immiscible multiphase system, and all phases are connected into a whole by an interfacial chemical reaction; and an organic solution containing an extraction agent is adsorbed in a microporous support body of a hydrophobic hollow fiber membrane by a supported liquid membrane under the action of intermolecular force and capillaries, and a substance to be separated is transferred to the analytic phase through the liquid membrane phase from the feed phase by utilizing an interface coordination chemistry reaction at both sides of the liquid membrane, a facilitated transport action generated in the liquid membrane, and an analytic reaction between the liquid membrane phase and analytic phase interfaces. According to the method, emulsifying and demulsifying working procedures are eliminated, so that the separation process is simpler, more reliable and more practical, the cost is greatly reduced, and the large-scale production is easy to implement.
Owner:HUAZHONG UNIV OF SCI & TECH
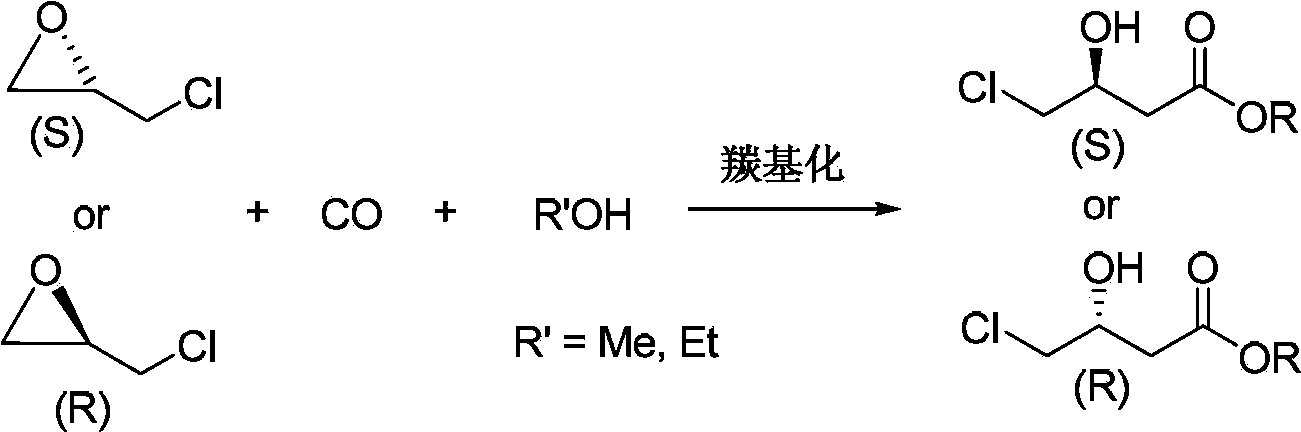
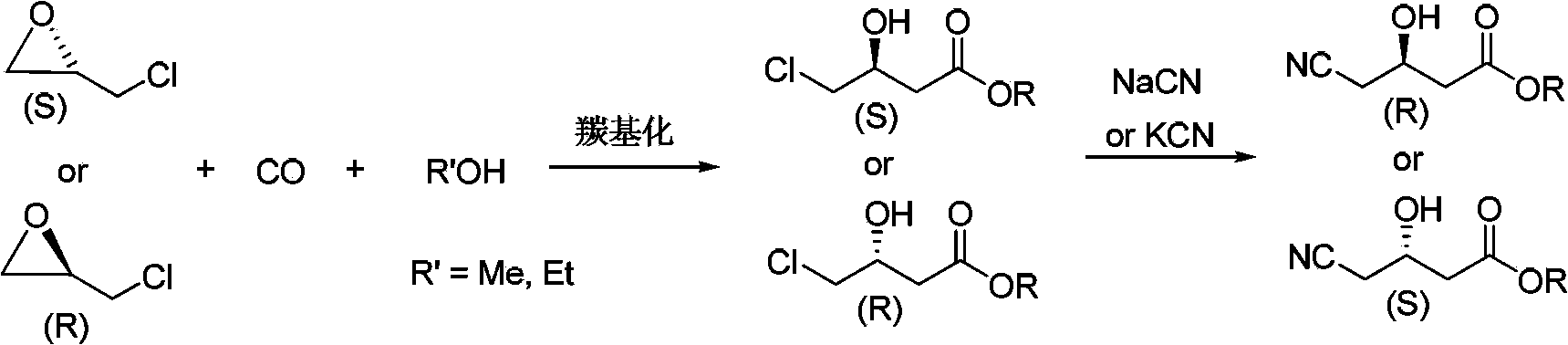
Preparation method for chiral 4-chloro-3-hydroxybutyrate
InactiveCN103420837AMeet the development requirements of fine chemical industry greenLow costPreparation by carbon monoxide or formate reactionEthyl ChlorideHigh pressure
The invention discloses a preparation method for chiral 4-chloro-3-hydroxybutyrate. The preparation method specifically comprises: adding chiral chloropropylene oxide, an alcohol, a catalyst into a high-pressure reaction vessel, introducing carbon monoxide, heating for a reaction, and obtaining chiral 4-chloro-3-hydroxybutyrate after the reaction. The preparation method has the advantages of being cheap and easily-available in raw materials, mild in reaction conditions, high in yield, low in cost, and the like. Compared with conventional synthetic methods for chiral 4-chloro-3-hydroxybutyrate, the preparation method employs a reaction with atom economical efficiency of 100%, is environment friendly, low in 'three wastes (waste gas, waste water and industrial residue) ' and applicable to industrial production.
Owner:SUZHOU OST ADVANCED MATERIALS +1
Method for reducing amide compounds
InactiveCN103435430AMild reaction conditionsEasy to operateCarbamic acid derivatives preparationOrganic compound preparationTitanium tetrachlorideMagnesium
The invention discloses a method for reducing amide compounds, which reduces various amides through low valent tiron. Low valent tiron prepared by reducing titanium tetrachloride through magnesium powder is reacted with amide in tetrahydrofuran at 0 DEG C or normal temperature so as to obtain corresponding amine. The method has the advantages that the reaction condition is mild, reagent adopted is chip and easy to get, the method is convenient to operate, substrate has a wide applicable scope, functional group has good compatibility, the reaction is rapid, and the yield is high.
Owner:SICHUAN UNIV
Amino acid organic liquid fertilizer and preparation method thereof
InactiveCN108101674APromote growthImprove biological activityAnimal corpse fertilisersAlkali orthophosphate fertiliserSlurryDrug biological activity
The invention discloses amino acid organic liquid fertilizer and a preparation method thereof. The amino acid organic liquid fertilizer is prepared from the following components in parts by weight: 90to 110 parts of poultry feather, 4 to 6 parts of glucose, 4 to 6 parts of biogas slurry, 15 to 20 parts of macro elements, 1 to 5 parts of trace elements and 0.8 to 1.5 parts of chelating agent. Thepreparation method comprises the following steps: adding the glucose into the biogas slurry, fermenting in a closed manner to obtain a fermented solution; then adding the poultry feather into the fermented solution, fermenting in a closed manner to prepare an amino acid solution, then adding the chelating agent, macro elements and trace elements into the amino acid solution to react, finally cooling to the room temperature, thus obtaining the amino acid organic liquid fertilizer. The amino acid organic fertilizer is prepared by adopting microorganisms to ferment the poultry feather. By adopting the preparation method, no racemization effect is produced, the environment is not polluted, the production cost is low, the obtained amino acid is high in biological activity and low in content oftoxic and harmful articles; and meanwhile, the amino acid organic liquid fertilizer provided by the invention is easier to absorb by crops, so that the resource waste can be reduced.
Owner:SICHUAN AGRI UNIV
Synthesizing process of (S)-2-benzyloxy-pentan-3-one
ActiveCN103193609AAvoid the problem of hydrolysisAvoid dangerCarbonyl compound preparation by condensationOrganic layerBenzyl chloride
The invention discloses a synthesizing process of (S)-2-benzyloxy-pentan-3-one. The synthesizing process comprises the following steps: (1) mixing ethyl lactate with nafoxidine in proportion and performing a reflux reaction for 12 to 48 hours, thereby generating an amide compound; (2) mixing the amide compound with benzyl chloride, alkali and a solvent and reacting, cooling to normal temperature after the reaction, washing an organic layer by using water, concentrating the organic layer under reduced pressure and removing primary distillates, thereby obtaining benzyl-protected amide; and (3) dripping a tetrahydrofuran solution of the benzyl-protected amide into a tetrahydrofuran solution of ethylmagnesium bromide and reacting, adjusting the pH value by using an acid solution after the reaction, extracting a water layer after layering, combining the organic layers, drying, filtering and concentrating filtrate until the filtrate becomes dry, thereby obtaining (S)-2-benzyloxy-pentan-3-one. The synthesizing process is relatively simple to operate, high in safety and low in raw material cost, thereby satisfying the industrial amplification requirement.
Owner:山东格新精工有限公司
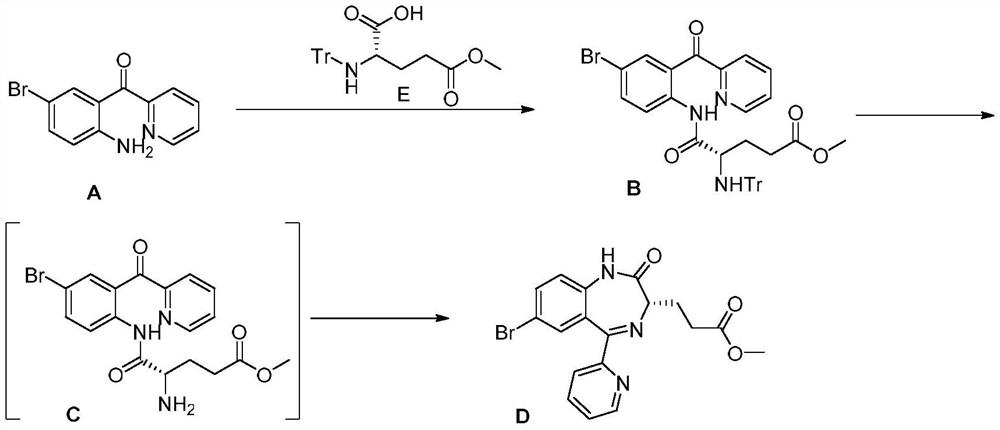
Method for preparing remazolam key intermediate
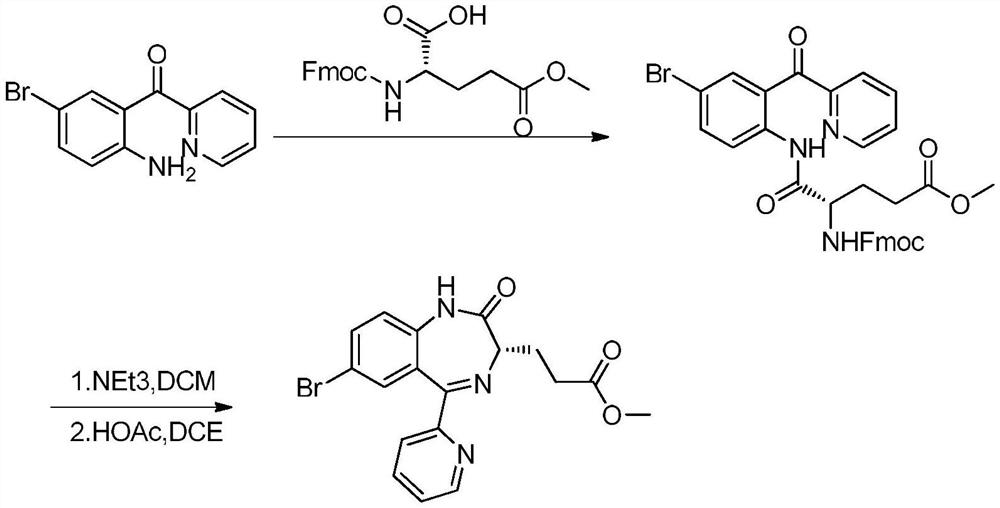
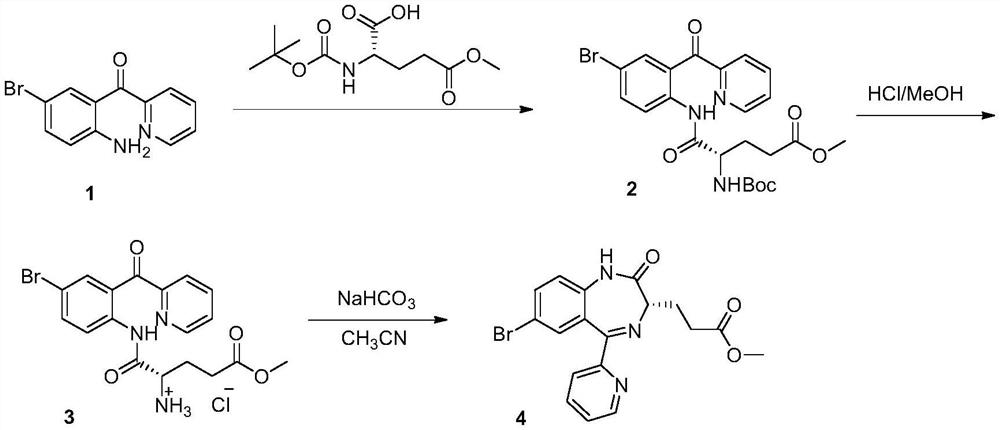
InactiveCN114014839AEasy to removeNo change in enantioselectivityOrganic chemistry methodsPropanoic acidPhenacyl
The invention discloses a preparation method of a remazolam key intermediate (3S)-7-bromo-2,3-dihydro-2-oxo-5-(2-pyridyl)-1H-1,4-benzodiazepine-3-methyl propionate, and belongs to the technical field of medical intermediates. Herein, 2-(2-amino-5-bromo-benzoyl)pyridine A and N-Tr-glutamic acid-5-methyl ester are adopted as raw materials, a condensation reaction is performed under the action of a boron-containing reagent to obtain a compound B, then deprotection is performed to obtain a compound C, and finally ring closing is performed under the alkaline low-temperature condition to obtain a compound D. The method is good in process reproducibility, simple, convenient and stable to operate, easy to separate products in each step, high in yield, environment-friendly and suitable for industrial large-scale production.
Owner:SHANGHAI ZAIQI BIO TECH
Preparation method for chiral 4-cyano-3-hydroxybutyrate
InactiveCN103420870AMeet the development requirements of fine chemical industry greenLow costPreparation by cyanide reactionAlcoholCarbonylation
The invention discloses a preparation method for chiral 4-cyano-3-hydroxybutyrate. The preparation method specifically comprises that: under the effect of a carbonylation catalyst, chiral chloropropylene oxide is taken as an initial raw material, and reacted with an alcohol and carbon monoxide for a carbonylation reaction, and corresponding chiral 4-chloro-3-hydroxybutyrate with maintained configuration is obtained and then subjected to cyanidation for preparation of chiral 4-cyano-3-hydroxybutyrate with high optical purity. Compared with conventional synthetic methods, the preparation method has the advantages of fewer steps, easily available and cheap raw materials, fewer 'three wastes (waste gas, waste water and industrial residue) ', simple post treatment, low equipment requirements, and applicability to industrial production.
Owner:SUZHOU OST ADVANCED MATERIALS +1
Normal pressure crystallization method for monosodium glutamate
InactiveCN102407030ANo racemizationReduce generationSolution crystallizationFood preparationL-Pyroglutamic AcidCrystallization temperature
The invention provides a normal pressure crystallization method for monosodium glutamate, aiming to solve the problems that a great amount of pyroglutamic acid and racemized glutamic acid can be generated because temperatures of upper and lower parts of a sodium glutamate solution are different in the current monosodium glutamate crystallization method. The invention has the keypoint that: the crystallization process is finished in normal pressure crystallization equipment, a heating and evaporating process is finished in negative pressure heating and evaporating equipment, the normal pressure crystallization equipment and the negative pressure heating and evaporating equipment are connected end to end by a pipeline, and a pressure pump and a feed inlet are arranged on the pipeline between the normal pressure crystallization equipment and the negative pressure heating and evaporating equipment. The normal pressure crystallization method for monosodium glutamate provided by the invention has the beneficial effects that: monosodium glutamate can be crystallized under a normal pressure, the crystallization temperature is kept at 55-60 DEG C all the way, the generation of the pyroglutamic acid can be reduced furthest, and racemization of glutamic acid can be avoided.
Owner:张吉浩
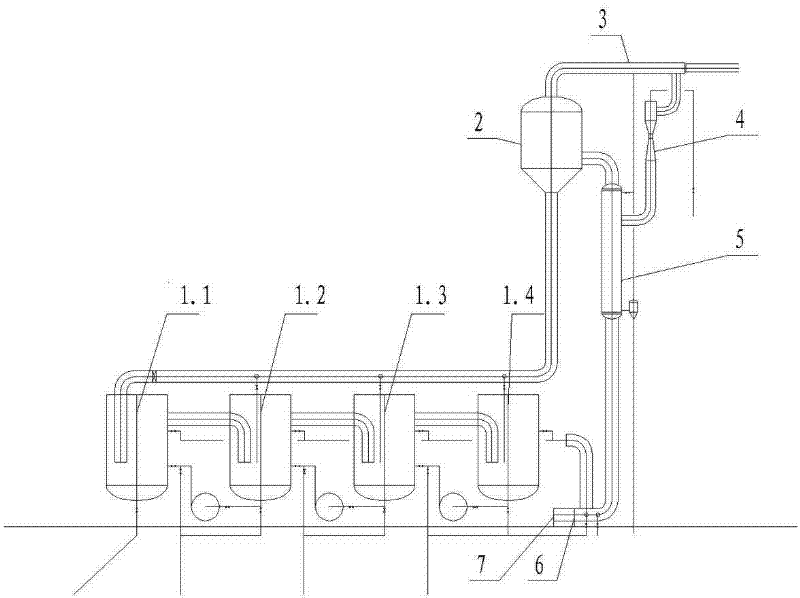
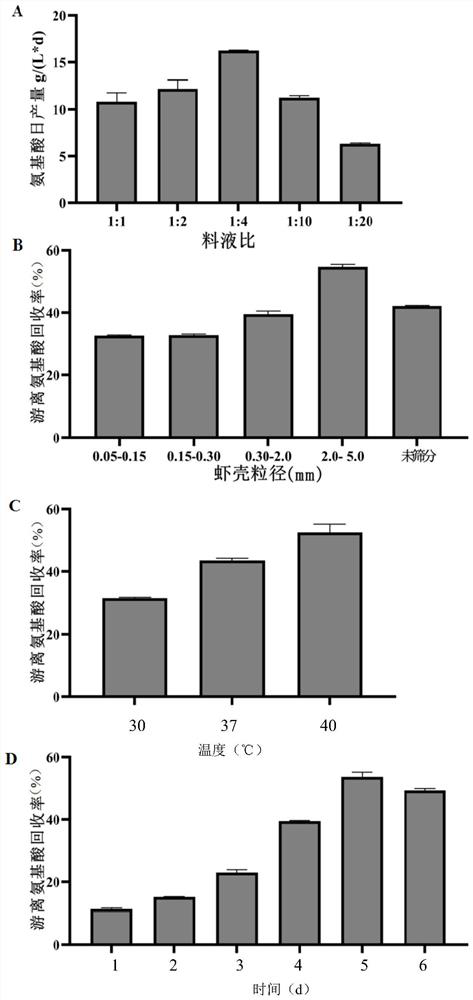
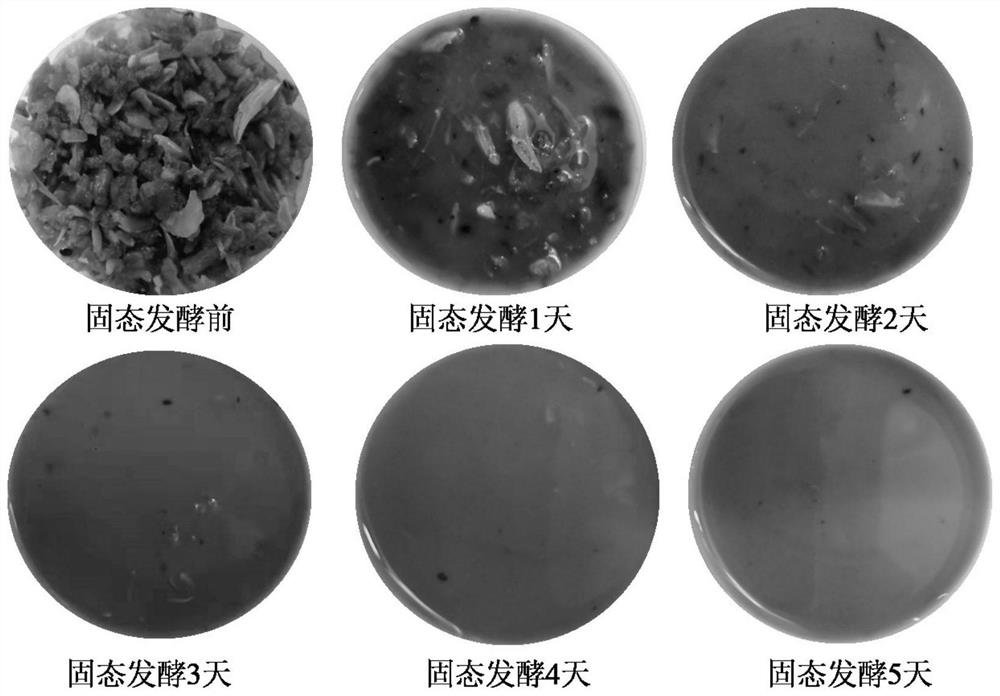
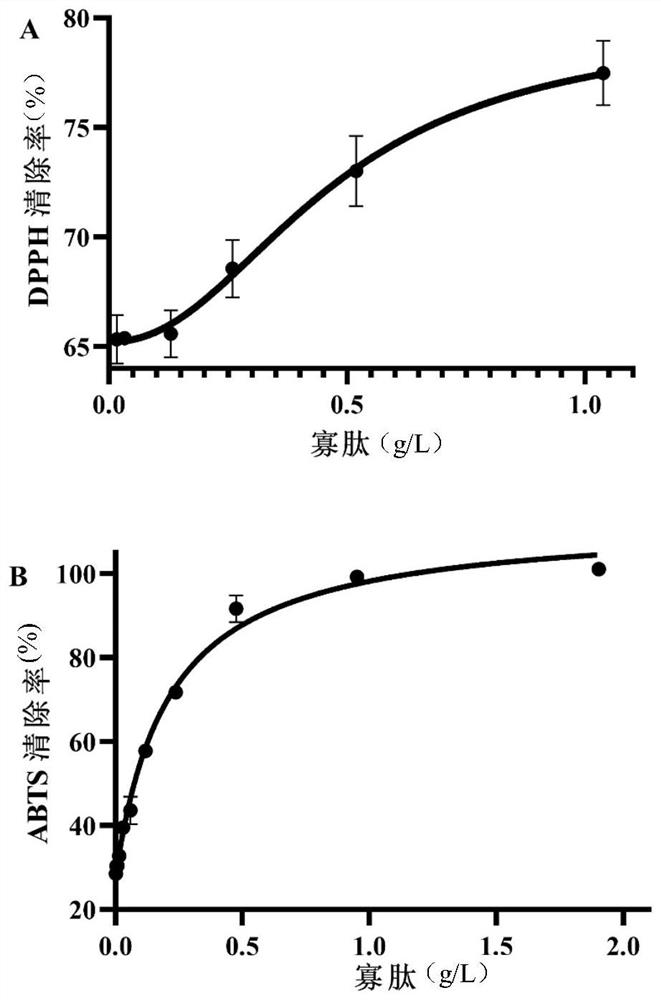
Method for producing amino acid, oligopeptide, calcium lactate and chitin by treating shrimp shell waste through solid-state fermentation of streptomyces and application of amino acid, oligopeptide, calcium lactate and chitin
ActiveCN114438144ARealize direct productionImprove cleanlinessOrganic active ingredientsPeptide/protein ingredientsNutritionCALCIUM LACTOBIONATE
The invention discloses a method for producing amino acid, oligopeptide, calcium lactate and chitin by treating shrimp shell waste through streptomyces solid state fermentation and application of the method, and belongs to the technical field of biological engineering. The invention relates to a method for producing amino acid, oligopeptide, calcium lactate and chitin by treating shrimp shell waste through streptomycete solid state fermentation, which comprises the following steps: inoculating streptomycete seed fermentation liquor into a fermentation culture medium containing the shrimp shell waste to obtain fermentation liquor, and separating to obtain first supernate and first precipitate; and preparing an amino acid product, membrane separation raffinate, an oligopeptide product, chitin and calcium lactate. According to the method, a large amount of amino acid is produced from shrimp shell waste for the first time, the recovery rate reaches 61.55%, the free amino acid concentration reaches 82.5 g / L, the amino acid productivity reaches 16.5 g / (L * d), the content of essential amino acid reaches 58.52%, and the shrimp shell waste is an excellent medicine and health care product raw material, a food nutrition additive and a feed additive.
Owner:SOUTH CHINA UNIV OF TECH
Method for extracting synephrine from ripe navel orange peels
ActiveCN107954883AAvoid racemizationHigh extraction rateOrganic compound preparationAmino-hyroxy compound preparationFreeze-dryingFiltration
The invention discloses a method for extracting synephrine from ripe navel orange peels. The method comprises the steps as follows: (1) dried navel orange peels are crushed and then mixed with distilled water, complex enzyme is added for enzymolysis, and an enzymolysis mixture is obtained; (2) the enzymolysis mixture is subjected to ultrahigh-pressure enzyme deactivation treatment, the pH is adjusted to 5.0-6.0, subcritical extraction is performed under nitrogen protection, centrifugation is performed after extraction, and a liquid supernatant is collected; (3) the liquid supernatant obtainedin the step (2) is subjected to ultrafiltration separation with an ultrafiltration membrane with the molecular interception being 5,000 u-10,000 u, and a concentrated solution is collected; (4) the concentrated solution obtained in the step (3) is mixed and stirred with ethanol, the obtained mixed solution is subjected to reduced pressure suction filtration and freeze drying, and synephrine is obtained. The method has the advantages of being high in extraction rate and extraction efficiency, mild in treatment condition, environmentally friendly and the like.
Owner:HEZHOU UNIV
Synthesis method of N-benzyl-alpha-methylbenzylamine
InactiveCN108658782AHigh optical purityConducive to loadPhysical/chemical process catalystsOrganic compound preparationNano sizeBenzaldehyde
The invention discloses a synthesis method of N-benzyl-alpha-methylbenzylamine. According to the method, phenylethylamine, benzaldehyde, KBH4, (NH4)2Mo7O24, Ni(NO3)2, 4,4'-dipyridyl, urea formaldehyderesin and H3PMo12O40 are used as main raw materials. The synthesis method is used for carrying out nucleophilic addition through phenylethylamine and benzaldehyde under the action of a catalyst whichis P-Mo2Ni / NC and then further reducing to obtain N-benzyl-alpha-methylbenzylamine. Compared with the conventional synthesis method, the synthesis method has the advantages that limited carbonizationof polyacids is capable of effectively avoiding excessive growth of metal carbide particles; nano-sized monodispersed catalyst particles can be obtained; racemization is not generated when KBH4 is used as a reducing agent for reducing intermediate products which are imine materials; and phenylethylamine with two configurations and extremely high optical purity can be easily obtained.
Owner:徐州得铸生物科技有限公司
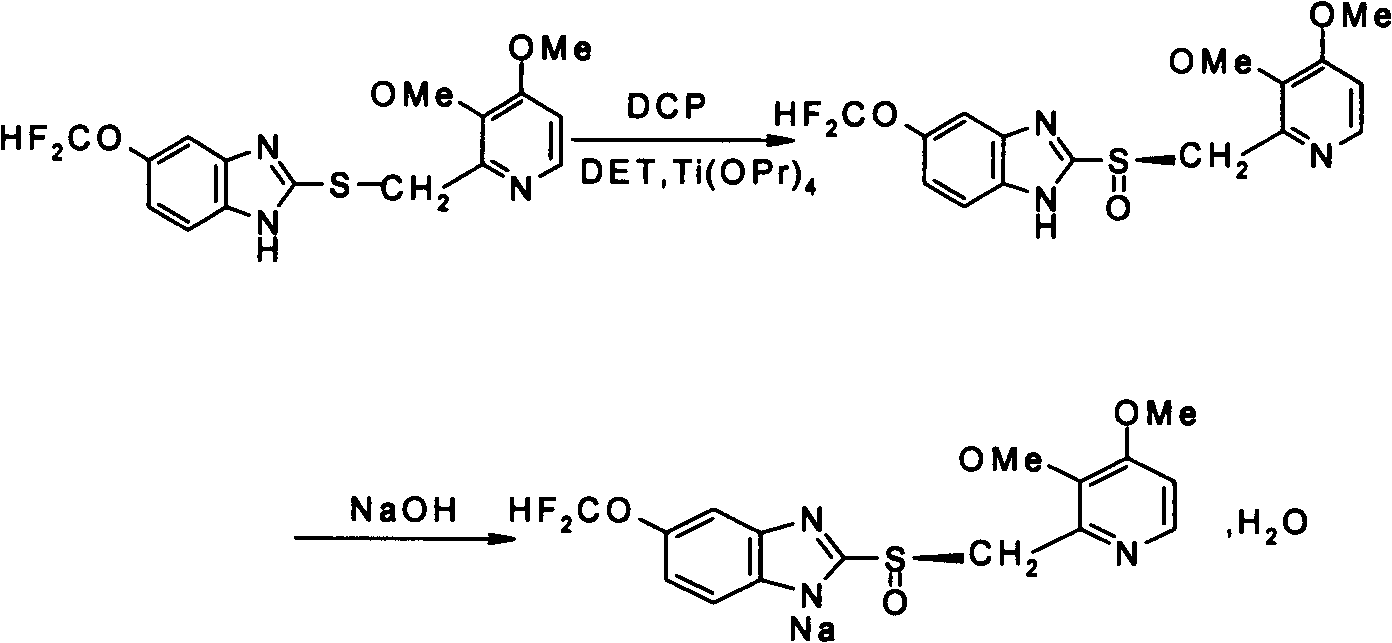
Preparation and purification method of pantoprazole optical antimer
InactiveCN103804355AShort reaction timeHigh synthesis efficiencyOrganic chemistryPurification methodsSulfur
The invention provides a preparation and purification method of a pantoprazole optical antimer. The method comprises the following steps: carrying out a reaction on 5-difloromethoxyl-2-{[(3, 4-dimethoxyl-2-pyridyl)methyl]sulfur}-1H-benzimidazole and optically active diethyl tartrate (D-(-)-diethyl tartrate or L-(+)-diethyl tartrate) according to different feeding sequences; reacting completely within 2 hours and filtering to obtain optically active pantoprazole; then, purifying to obtain a pure product S-(-)-pantoprazole or R-(+)-pantoprazole. The method provided by the invention is simple and convenient to operate, higher in product purity and high in yield, and the reaction time is shortened and the production cost is saved, so that the method is more suitable for industrialized large-scale production.
Owner:HC SYNTHETIC PHARMA CO LTD
Method for reducing amide compounds
InactiveCN103435430BMild reaction conditionsEasy to operateCarbamic acid derivatives preparationOrganic compound preparationTitanium tetrachlorideTetrahydrofuran
The invention discloses a method for reducing amide compounds, which reduces various amides through low valent tiron. Low valent tiron prepared by reducing titanium tetrachloride through magnesium powder is reacted with amide in tetrahydrofuran at 0 DEG C or normal temperature so as to obtain corresponding amine. The method has the advantages that the reaction condition is mild, reagent adopted is chip and easy to get, the method is convenient to operate, substrate has a wide applicable scope, functional group has good compatibility, the reaction is rapid, and the yield is high.
Owner:SICHUAN UNIV
4-(N, N-dimethylamino) azobenzol-4'-sulfuryl fluoride and its synthesizing method and use
InactiveCN100442051CEasy to detectEasy to purifySulfonic acids salts preparationBiological testingChromatographic separationSulfonyl fluoride
The invention discloses a novel labeling reagent 4-(N,N-dimethylamino)azobenzene-4'-sulfonyl fluoride capable of derivatization reaction with protein and amino acid and its synthesis method and the compound in protein, The application in amino acid analysis, the synthesis method of its intermediate p-acetamidobenzenesulfonyl fluoride and the application of this compound in protein and amino acid analysis. The labeling reagent uses aniline as a raw material to prepare p-acetylaminobenzenesulfonyl fluoride through acetylation, chlorosulfonation and fluorination reactions, and then hydrolyzes the p-acetylaminobenzenesulfonyl fluoride, undergoes diazotization and coupling reactions. The labeling reagent is easy to synthesize, easy to store, derivatizes with primary or secondary amino acids under mild conditions, stable derivatized products, convenient liquid phase or capillary chromatographic separation, high instrument response value, and detection sensitivity up to pmol level. Achieve the effect of chemical derivatization-instrumental detection.
Owner:李寿椿
A kind of preparation method of furanone compound
ActiveCN106588831BHigh chiral purityShort synthetic routeOrganic compound preparationPreparation from carboxylic acid esters/lactonesInorganic saltsHigh pressure
The invention discloses a method for preparing furanone compounds, and provides a method for preparing a furanone compound III. The method includes the following steps that in a solvent, in the presence of inorganic salt, a compound II and a reducing agent are subjected to a reduction reaction, and the furanone compound III is obtained; the solvent is a fatty alcohol solvent or a mixed solvent of a fatty alcohol solvent and water. The brivaracetam can be prepared with the furanone compound III only with the three steps, and the synthetic route is short; the ee value of the compound II is larger than 99.0%, racemization does not occur in the reaction process, and the de value of a brivaracetam I crude product is larger than 99.0%; the brivaracetam I crude product is further purified through a crystal instead of a chirality high-pressure-liquid-phase preparing column, and the chirality purity of brivaracetam I can be further increased to be the de value of 99.80% or above; meanwhile, the content of other individual impurities of the brivaracetam I is smaller than 0.1%, and reaches the API level, and the method is suitable for industrial production. The formula is defined in the description.
Owner:SHANGHAI BOCIMED PHARMA CO LTD
Synthetic Technology of (s)-2-Benzyloxypentan-3-one
ActiveCN103193609BWide variety of sourcesCheap sourceCarbonyl compound preparation by condensationOrganic layerBenzyl chloride
The invention discloses a synthesizing process of (S)-2-benzyloxy-pentan-3-one. The synthesizing process comprises the following steps: (1) mixing ethyl lactate with nafoxidine in proportion and performing a reflux reaction for 12 to 48 hours, thereby generating an amide compound; (2) mixing the amide compound with benzyl chloride, alkali and a solvent and reacting, cooling to normal temperature after the reaction, washing an organic layer by using water, concentrating the organic layer under reduced pressure and removing primary distillates, thereby obtaining benzyl-protected amide; and (3) dripping a tetrahydrofuran solution of the benzyl-protected amide into a tetrahydrofuran solution of ethylmagnesium bromide and reacting, adjusting the pH value by using an acid solution after the reaction, extracting a water layer after layering, combining the organic layers, drying, filtering and concentrating filtrate until the filtrate becomes dry, thereby obtaining (S)-2-benzyloxy-pentan-3-one. The synthesizing process is relatively simple to operate, high in safety and low in raw material cost, thereby satisfying the industrial amplification requirement.
Owner:山东格新精工有限公司
Water-soluble ynamide coupling reagent and preparation method and use thereof
PendingUS20220144793A1Simple structureEasy to prepareCarbamic acid derivatives preparationOrganic compound preparationAcyl groupTosylhydrazone
The present disclosure discloses a water-soluble ynamide coupling reagent and a method for using the water-soluble ynamide coupling reagent in the synthesis of amide, polypeptide, ester and thioester compound. The ynamide coupling reagent has the structure represented by the following formula (I):and in the formula (I), R is one selected from the group consisting of methylsulfonyl, benzenesulfonyl, p-toluenesulfonyl, trifluoroacetyl and other electron withdrawing groups.
Owner:XIAN EASY PEPTIDE BIOTECHNOLOGY CO LTD
A method for extracting synephrine from ripe navel orange peel
ActiveCN107954883BAvoid racemizationHigh extraction rateOrganic compound preparationAmino-hyroxy compound preparationEnzymatic hydrolysisMedicine
Owner:HEZHOU UNIV
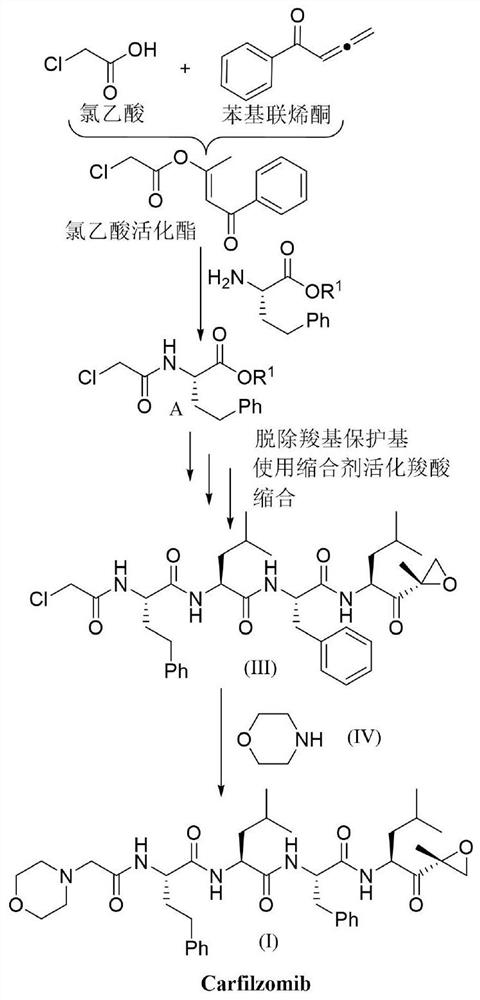
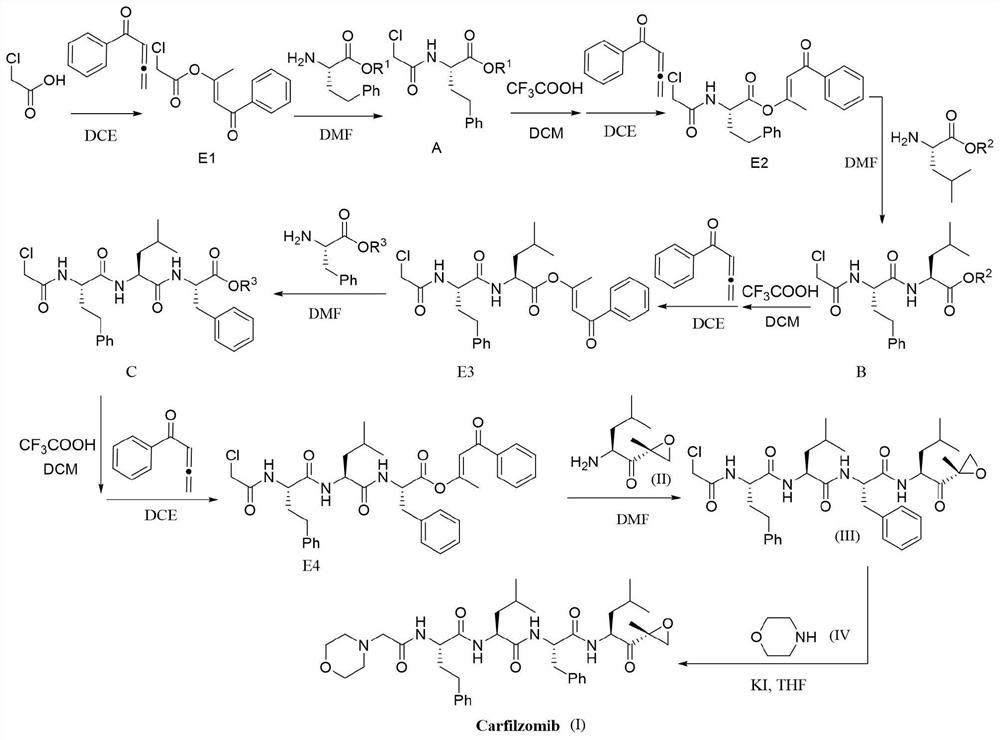
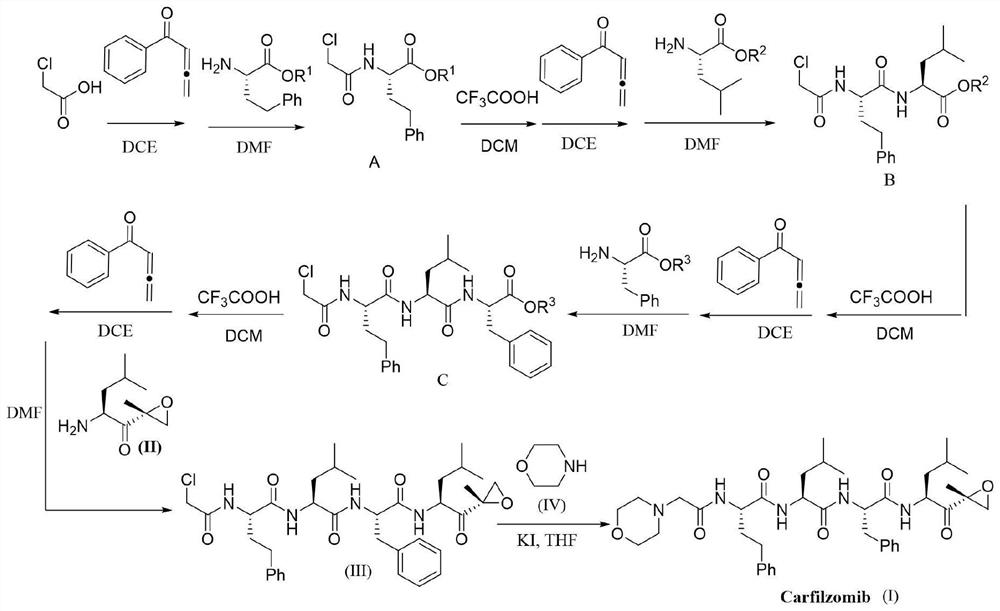
Method for efficiently preparing carfilzomib
ActiveCN112830957AMild reaction conditionsEasy to operatePeptidesBulk chemical productionCarboxylic groupPentanone
The invention provides a method for efficiently preparing carfilzomib, which comprises the following steps: by taking chloroacetic acid, homophenylalanine, phenylalanine, leucine and (2S)-2-amino-4-methyl-1-[(2R)-2-methyl oxiranyl]-1-pentanone trifluoroacetate as raw materials and phenyl allene ketone as a condensation reagent, carrying out a condensation reaction to obtain carfilzomib; carrying out step-by-step coupling condensation through the steps of carboxylic acid activation, condensation, carboxyl protecting group removal and the like to obtain an intermediate product, and then carrying out catalytic condensation reaction on the intermediate product and morpholine to obtain a target compound. The condensing agent used in the method has the advantages of simplicity in preparation, small molecular weight and no racemization when the chiral carboxylic acid is activated. Meanwhile, the method is mild in reaction condition and simple to operate, the total yield is up to 68-72%, and the method has excellent atom economy and is a novel, efficient and very practical synthesis method of carfilzomib.
Owner:JIANGXI NORMAL UNIVERSITY
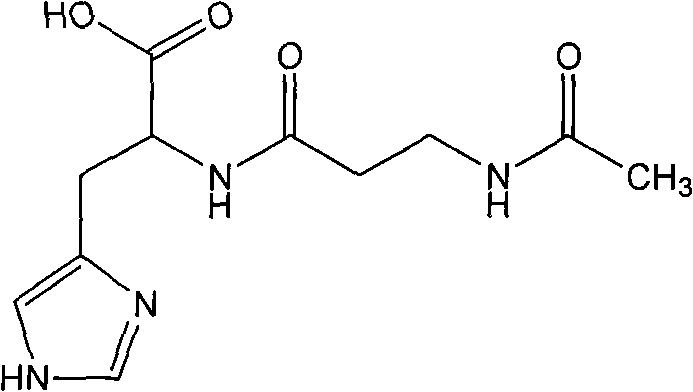
Prepartion method of N-acetyl-L-carnosine
ActiveCN101585813BNo racemizationSuitable for industrial productionSenses disorderOrganic chemistryAcetyl chlorideOrganic solvent
A prepartion method of N-acetyl-L-carnosine, relates to pharmaceutical intermediates synthesis technology field. Dissolving p-nitrophenol into organic solvent, reacting with acetyl chloride to obtain active ester solution at the presence of acid-binding agent; dissolving the L-carnosine and protection agent into water to obtain aqueous solution, then adding the avtive ester solution into the aqueous solution for reacting, and when the reaction is finished, demixing, decoloring the water layer and decompressing condensing to pulpous state, then mixing the condensed residue with acetate acid andadding into polar solvent for stirring, cold separating and drying to obtain the N-acetyl-L-carnosine. The advantages are: the molar yield is more than 90%, the chemical purity is more than 90%, therefore high yield and ideal purity is suitable for industrial production.
Owner:SUZHOU FUSHILAI PHARMA CO LTD
Method for preparing key intermediate of medicament
ActiveCN103539639BGood chemical purityHigh yieldOrganic chemistryOrganic compound preparationCombinatorial chemistryDistillation method
The invention relates to the field of medicines, and in particular relates to a method for preparing a key intermediate (II) of a medicament. The method is characterized in that the key intermediate is prepared by a reaction of a compound IV and phosphorus oxybromide. According to the preparation method, the defects that a compound II is prepared by using a column chromatography in the prior art and large-scale industrial production is difficult to realize by the prior art can be overcome, the key intermediate can be directly prepared by a re-crystallization or reduced-pressure distillation method, and the key intermediate is high in purity.
Owner:CHANGZHOU PHARMA FACTORY
A method for enzymatically preparing levodopa
ActiveCN104726513BRich sourcesReduce manufacturing costMicroorganism based processesFermentationTyrosinePseudomonas
The invention discloses a method for preparing levodopa by enzymatic method. Insert 3 to 10% of the inoculum into the fermentation medium for fermentation and culture, centrifuge after fermentation, and collect the bacterial cells; add tyrosine and bacterial cells to the buffer solution, at 18 to 30°C, pH5. Under the condition of 0-6.0, the enzymatic reaction is carried out to convert tyrosine into levodopa. In the conversion process, the present invention adopts static cell conversion technology, preferably intermittent weak ventilation technology, which improves the catalytic efficiency of the enzyme, the concentration of levodopa can reach 27g / L, and the molar conversion rate of tyrosine can reach more than 99%, and Mild conditions, high enantiomer selectivity, no racemization, more suitable for industrial production, with significant economic benefits and industrial application value.
Owner:SHANDONG YANGCHENG BIOLOGY TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com