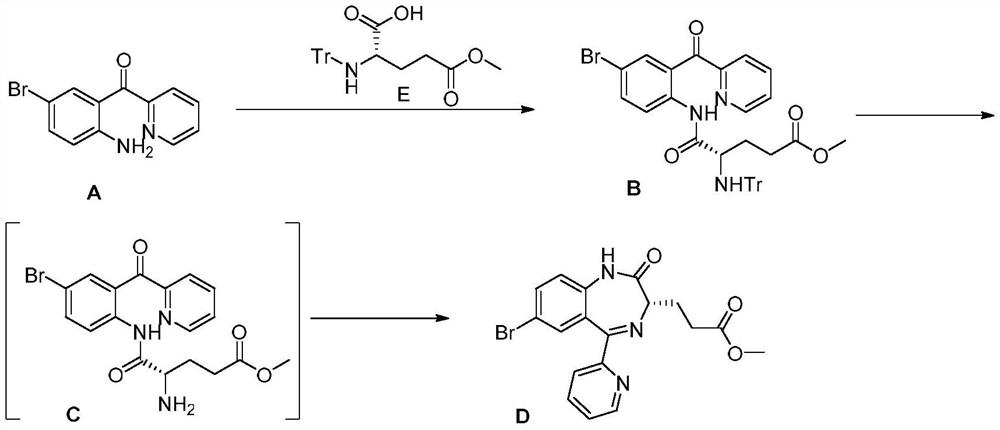
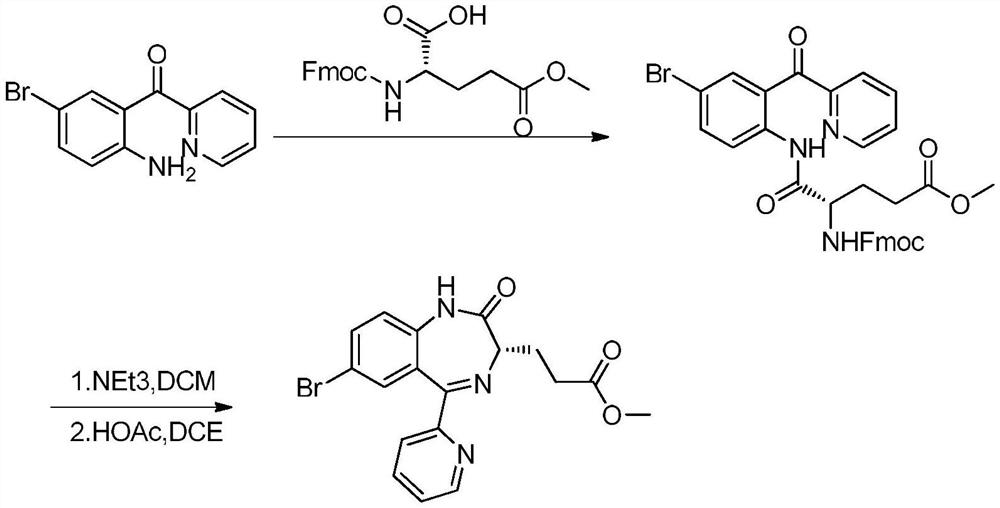
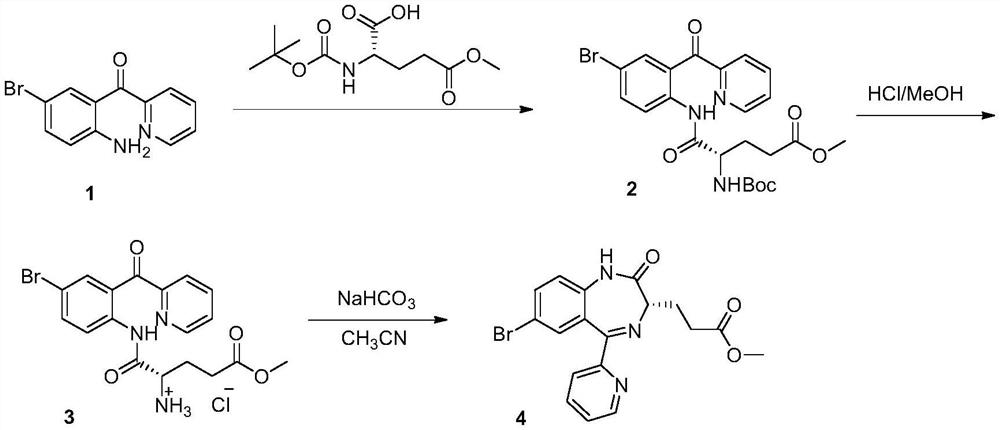
Method for preparing remazolam key intermediate
A technology of remimazolam and intermediates, which is applied in the field of preparation of key intermediates, can solve the problems of large yield loss, limited means of improving chiral purity, etc., and achieve the effect of easy removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] first step:
[0033] 2-(2-Amino-5-bromo-benzoyl)pyridine (38.6g, 139mmol) and N-Cbz glutamate-5-methyl ester (45.2g, 153mmol) were added into dichloromethane ( 200 mL), then the solution was cooled to -10°C. A solution of N,N'-dicyclohexylcarbodiimide (32.2g, 156mmol) in dichloromethane (65mL) was slowly added to the above solution at -10°C, and the reaction was stirred at -10°C for 48 hours, and the reaction solution Heat up to 15°C and filter. The filtrate was distilled under reduced pressure below 25°C, then 250mL of methyl tert-butyl ether was added, the solution was heated to 50°C and then slowly cooled to 25°C, filtered and dried at 50°C to obtain a light yellow solid (72.3g, yield: 93.6% ).
[0034] Step two:
[0035] Add intermediate B (35g, 63mmol) into glacial acetic acid (70mL), slowly add 33% hydrogen bromide / glacial acetic acid solution (45.7mL, 253mmol) into the above reaction solution at 10-12°C, and raise the temperature after adding to 20°C and sti...
Embodiment 2
[0037] first step:
[0038] N-Tr-glutamate-5-methyl ester (0.58g, 3.61mmol), 2-(2-amino-5-bromo-benzoyl)pyridine A (1.0g, 3.61mmol) and dioxane Mix 8mL / cyclohexane 25mL and stir evenly. Then add B(OCH 2 CF 3 ) 3 (0.22g, 0.72mmol) was heated and refluxed for water separation for 8 hours, and TLC detected that the reaction was complete. Concentrate under reduced pressure, add ethyl acetate and water to extract the reaction. Washed with saturated brine, and the organic layer was rotary evaporated to obtain a crude product, which was recrystallized from methanol / water to obtain 1.46 g of intermediate B with a yield of 93.0%.
[0039] Step two:
[0040] Hydrogen chloride gas was passed into a solution of B (1.46g, 2.2mmol) in tetrahydrofuran (15ml) for 15 minutes, TLC showed that after deprotection was completed, the temperature was lowered to -5°C, and then morpholine (0.96g, 11mmol) was added dropwise. After the addition, keep the reaction at low temperature for 2 hours. ...
Embodiment 3
[0042] first step:
[0043]Add N-Tr-glutamic acid-5-methyl ester E (1.2kg, 2.99mol) and 2-(2-amino-5-bromo-benzoyl)pyridine A (0.69kg, 2.49mol) into tetrahydrofuran 5L / Toluene 11L solvent, stir well. Then B(C6F5)3 (61.4g, 0.12mol) was added, and the temperature was raised to reflux for water separation and reaction for 6 hours, and TLC detected that the reaction was complete. Concentrate under reduced pressure, add ethyl acetate and water to extract the reaction. The organic layer was washed with brine, dried and spin-dried to obtain a crude product, which was recrystallized from methanol / water to obtain 1.5 kg of Intermediate B with a yield of 91.5%.
[0044] Step two:
[0045] Add intermediate B (1.5kg, 2.26mol) and acetic acid (0.2kg, 3.39mol) to dioxane (8L). After TLC shows that the protection is complete, cool down to -10°C, and then start adding triethylamine dropwise (1.14kg, 11.3mmol), keep the reaction at low temperature for 2 hours after the dropwise addition. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com