Patents
Literature
541 results about "Metaclazepam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Metaclazepam (marketed under the brand name Talis) is a drug which is a benzodiazepine derivative. It is a relatively selective anxiolytic with less sedative or muscle relaxant properties than other benzodiazepines such as diazepam or bromazepam. It has an active metabolite N-desmethylmetaclazepam, which is the main metabolite of metaclazepam. There is no significant difference in metabolism between younger and older individuals.
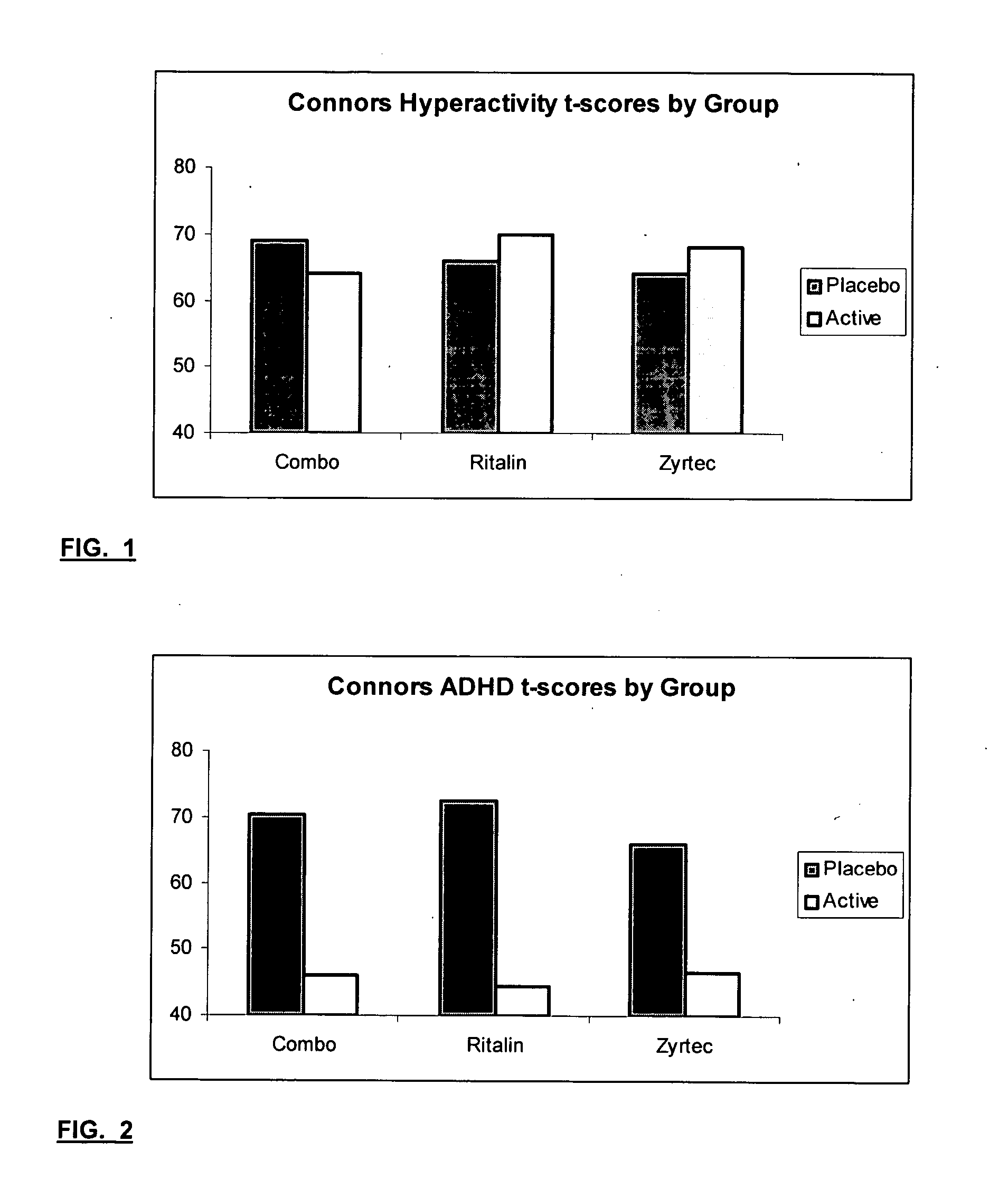
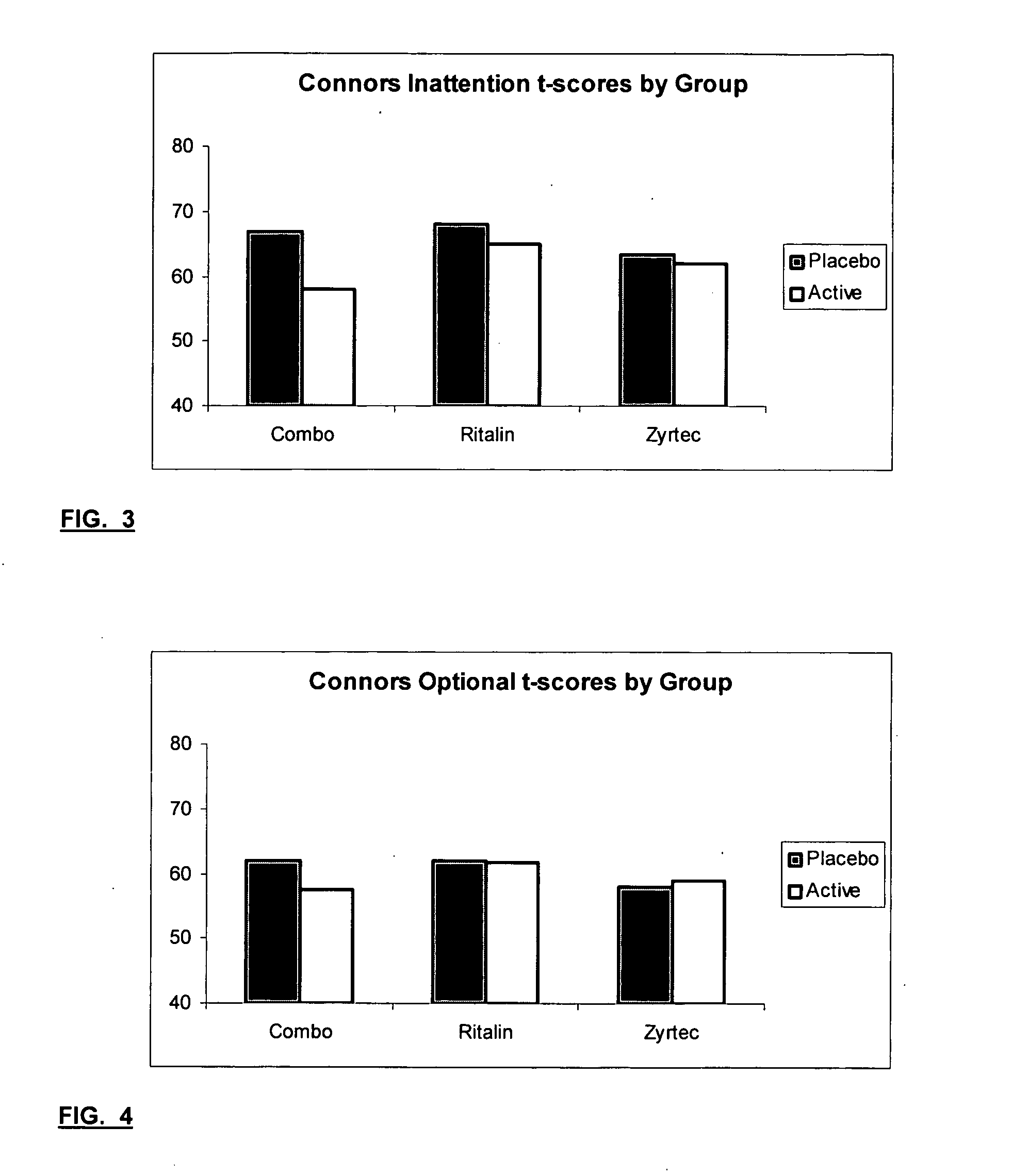
Method and synergistic composition for treating attention deficit/hyperactivity disorder
InactiveUS6541043B2Minimize side effectsBiocideHydroxy compound active ingredientsBeta-CaroteneBetaine
A composition and method for treating Attention Deficit / Hyperactivity Disorder (ADHD) is provided which can be used both with and without ethical drugs now used to treat ADHD. The composition contains dimethylaminoethanol (DMAE), omega 3-fatty acids, betaine, oligomeric proanthocyanidins (OPC), folic acid, vitamins C, E, B12, B6, B5 and beta-carotene and minerals (calcium, magnesium, zinc and selenium). Ethical drugs such as amphetamines, methylphenidate HCl and pemoline are known to control ADHD, but each has significant side effects when used in their therapeutic dose. When combining the composition with such ethical drugs, the amount of the ethical drug can be lowered below a level which causes undesirable side effects which is an important feature. Preferred compositions contain one or more of lecithin, choline, 5-hydroxytryptophan, tyrosine, Reishi Extract, Kava Extract, Gingko, Ginseng and St. John's Wort.
Owner:PHILIP C LANG
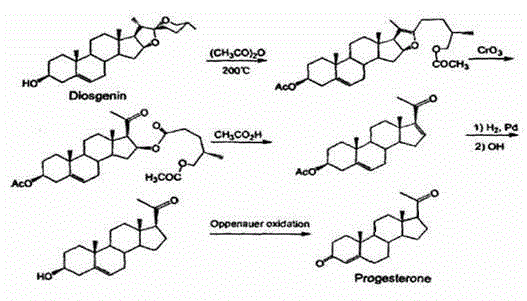
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Rapidly expanding composition for gastric retention and controlled release of therapeutic agents, and dosage forms including the composition
InactiveUS20040234608A1Improved gastric retentionHigh retention ratePowder deliveryOrganic active ingredientsGastric fluidAttention deficits
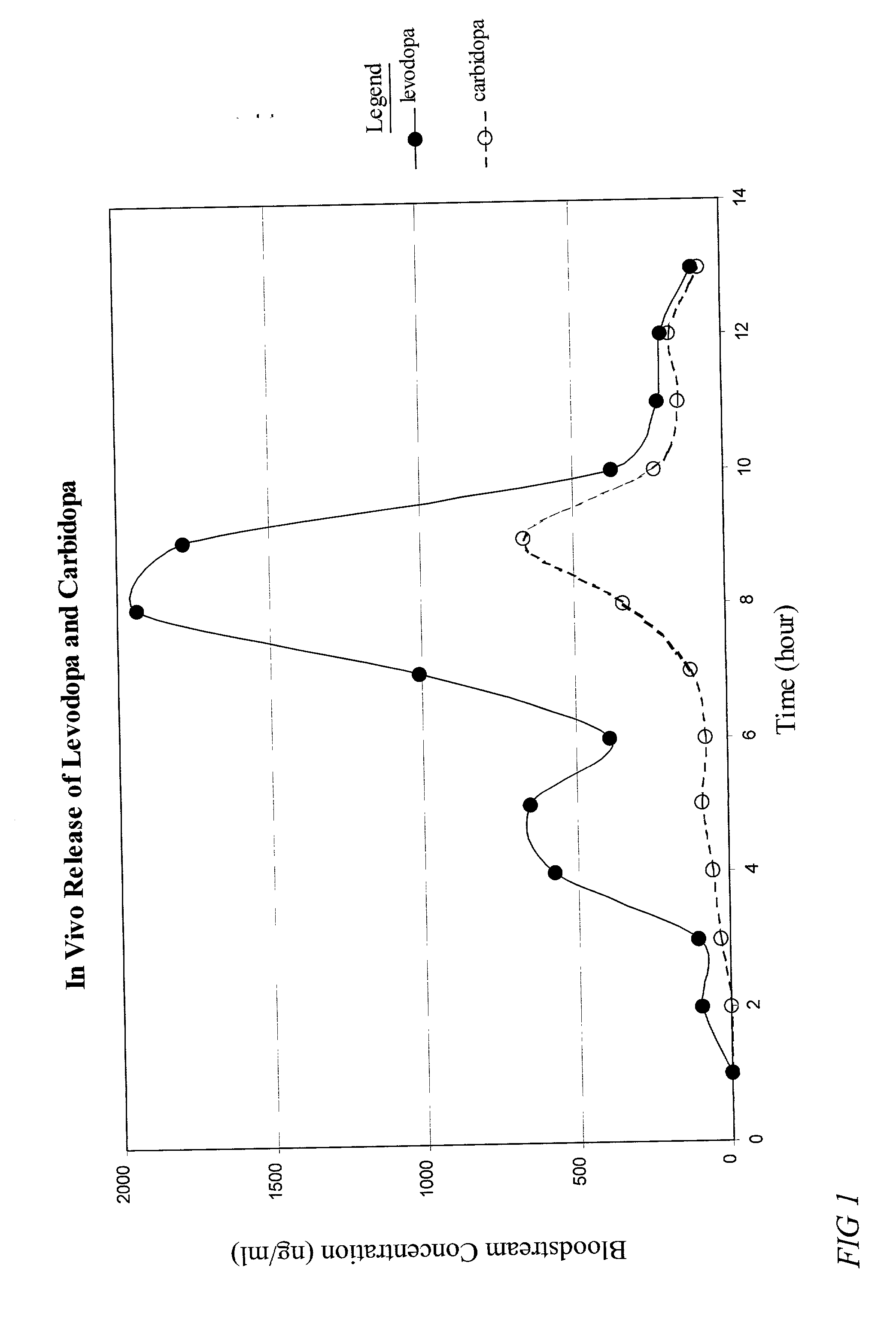
The present invention provides a pharmaceutical composition for use in a dosage form for oral administration to a patient. The composition expands upon contact with gastric fluid and promotes retention of the dosage form in the patient's stomach for a prolonged period of time. The present invention further provides pharmaceutical dosage forms containing an active ingredient, and the pharmaceutical composition. The forms are adapted for immediate or controlled release of the active ingredient. The dosage forms may be used advantageously in the treatment of Parkinson's disease with levodopa and hyperactivity and attention deficit disorder with methylphenidate.
Owner:TEVA PHARM USA INC
Oral Care Compositions With Improved Flavor
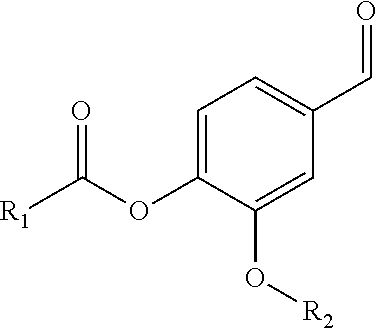
Oral care compositions having improved taste, said compositions comprising: a carrier material; from about 0.001 to about 10%, by weight of the composition, of an oral care component selected from metal salts, antimicrobial agents, bad breath reduction agents, bleaching agents, surfactants, or a combination thereof; and from about 0.0001 to about 1%, by weight of the composition, of a TRPA1 agonist selected from vanillin esters; benzoate esters; hydroxybenzoate derivatives; methoxy benzoate derivatives; hydroxybutanedioate derivatives; benzamidobenzoate derivatives; methylpropanoate derivatives; phenyl acetate derivatives; hex-3-enoate derivatives; 2-(furan-2-ylmethylsulfanyl)-3-methylpyrazine; phenylmethoxymethylbenzene; (2R)-2-azaniumyl-3-[(2R)-2-azaniumyl-3-oxido-3-oxopropyl]disulfanylpropanoate; (3E)-2-hydroxy-4,8-dimethylnona-3,7-dienal; (2R)-2-azaniumyl-3-[(2S)-2-azaniumyl-3-oxido-3-oxopropyl]disulfanylpropanoate; (3Z)-3-butylidene-2-benzofuran-1-one; 3-methyl-N-(3-methylbutyl)butan-1-imine; 2-(furan-2-ylmethyldisulfanylmethyl)furan; and combinations thereof. Uses thereof and methods of improving the taste of an oral care composition.
Owner:THE PROCTER & GAMBLE COMPANY
Synthetic technology for pyraclostrobin
ActiveCN104211641AFormation reaction is easy to controlSmooth responseOrganic chemistryMethylanilineChlorobenzene
The invention concretely relates to a synthetic technology for pyraclostrobin. The synthetic technology comprises: firstly performing cyclization to obtain 1-(4-chlorophenyl)-pyrazol-3-one, oxidizing the pyrazol ring under the effect of an oxidant to generate 1-(4-chlorophenyl)-3-hydroxypyrazole, then using 2-nitrobenzyl bromide to performing etherification to generate 1-(4-chlorophenyl)-3-[2-(nitrophenyl)methoxy]-1H-pyrazole, then using a reducing agent to perform nitro reducing, so as to generate N-hydroxyl-2-[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]aniline, then using ClCO2CH3 to perform N-acylation reaction to generate methyl N-hydroxyl-N-2-{[N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl]phenyl}formate, and finally performing hydroxyl methylation under an alkaline condition to generate pyraclostrobin. The technology enables all operations in the pyraclostrobin preparation process to be relatively controllable, helps to improve the stability of the preparation process and improve the product yield, successfully employs low-cost reagents and substantially reduces production cost, and also the employed reagents are relatively small in toxicity, is relatively beneficial for environment protection, and has no corrosivity on plastic pipes, so that the production safety is improved.
Owner:SHANDONG KANGQIAO BIO TECH CO LTD
Novel acetyloxymethyl esters and methods for using the same
Novel acetyloxymethyl esters are disclosed. Methods of treating an illness, including cancer, hemological disorders and inherited metabolic disorders, and treating or ameliorating other conditions using these compounds are also disclosed. The compounds are effective in the inhibition of histone deacetylase.
Owner:ERRANT GENE THERAPEUTICS
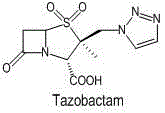
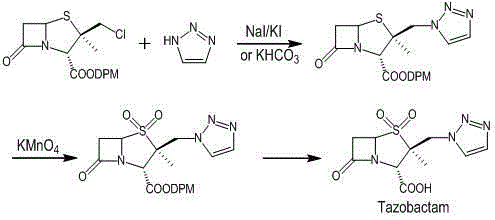
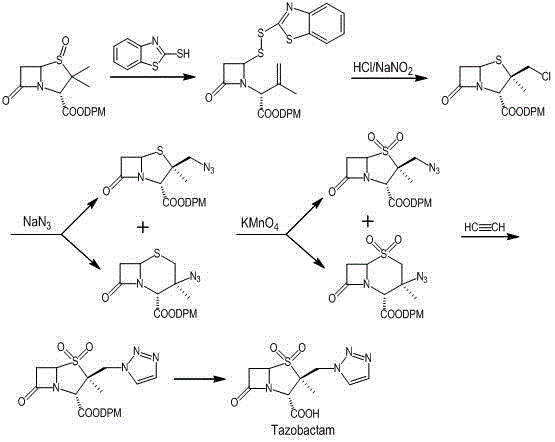
Preparation method for tazobactam
InactiveCN104031065AImprove stabilityInhibition of ring expansion reactionOrganic chemistryBulk chemical productionCatalytic oxidationM-Cresol
The invention discloses a preparation method for tazobactam. The preparation method comprises the following steps: with benzhydryl s-oxopenicillanate as a raw material, successively carrying out thermal cracking, bromination, catalytic oxidation and a reaction with 1H-1,2,3-triazole under the action of an anion resin carrier so as to obtain an important intermediate 2beta-(1H-1,2,3-triazolyl)-2alpha-methyl-benzhydryl penicillanate-1beta-oxide; and carrying out potassium permanganate oxidation and then protective group removal under the action of meta-cresol so as to obtain the target product tazobactam. The invention is characterized in that a sulfur atom is subjected to monooxidation so as to improve compound stability, then a nucleophilic substitution reaction with 1H-1,2,3-triazole is carried out so as to effectively control the possibility of ring enlargement during introduction of a triazole ring, and total yield is increased to 68%. The preparation method has the advantages of stable process, simple and convenient operation, easy separation and purification of the reaction product, a small amount of waste gas, waste water and industrial residues, high yield and suitability for clean, industrial and large-scale production.
Owner:JIANGXI HUABANG PHARMA
Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION
Disclosed herein are mixed metal-organic frameworks, Zn3(BDC)3[Cu(SalPycy)] and Zn3(CDC)3[Cu(SalPycy)], wherein BDC is 1,4-benzenedicarboxylate, CDC is 1,4-cyclohexanedicarboxylate, and SalPyCy is a ligand of the formula:These are useful for applications such as selective gas storage, selective molecular separations, and selective detection of molecules, including enantioselective applications thereof.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
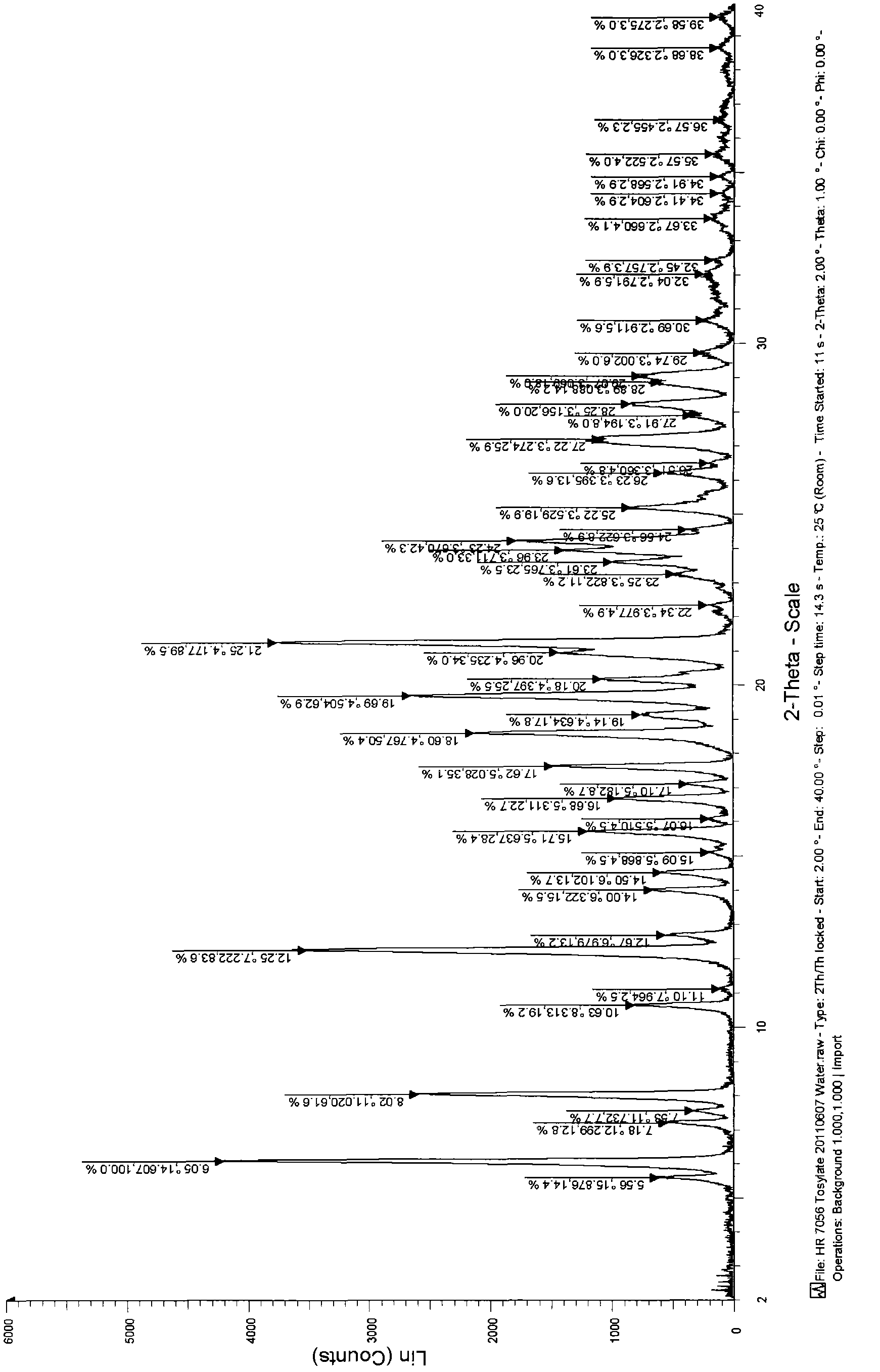
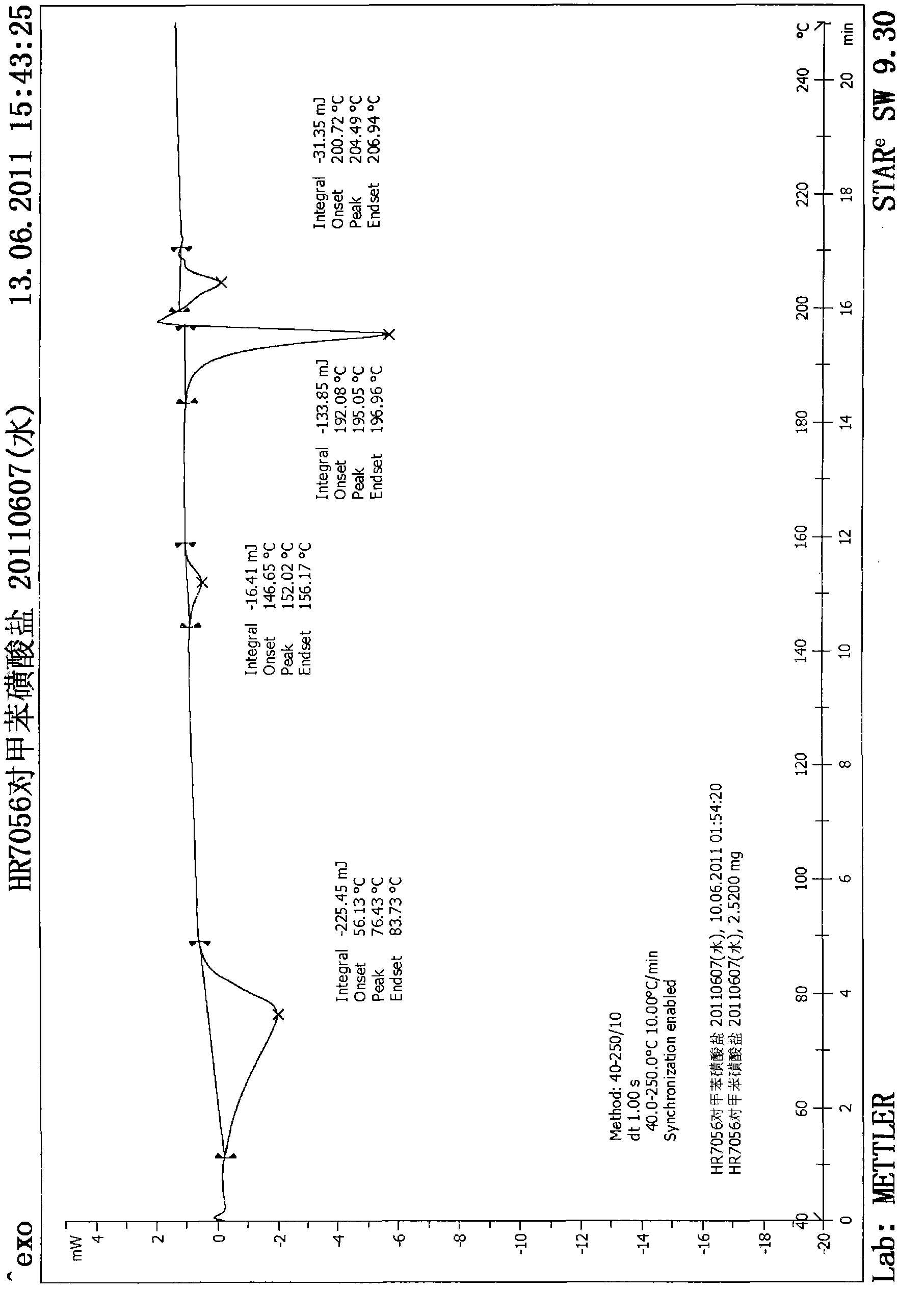
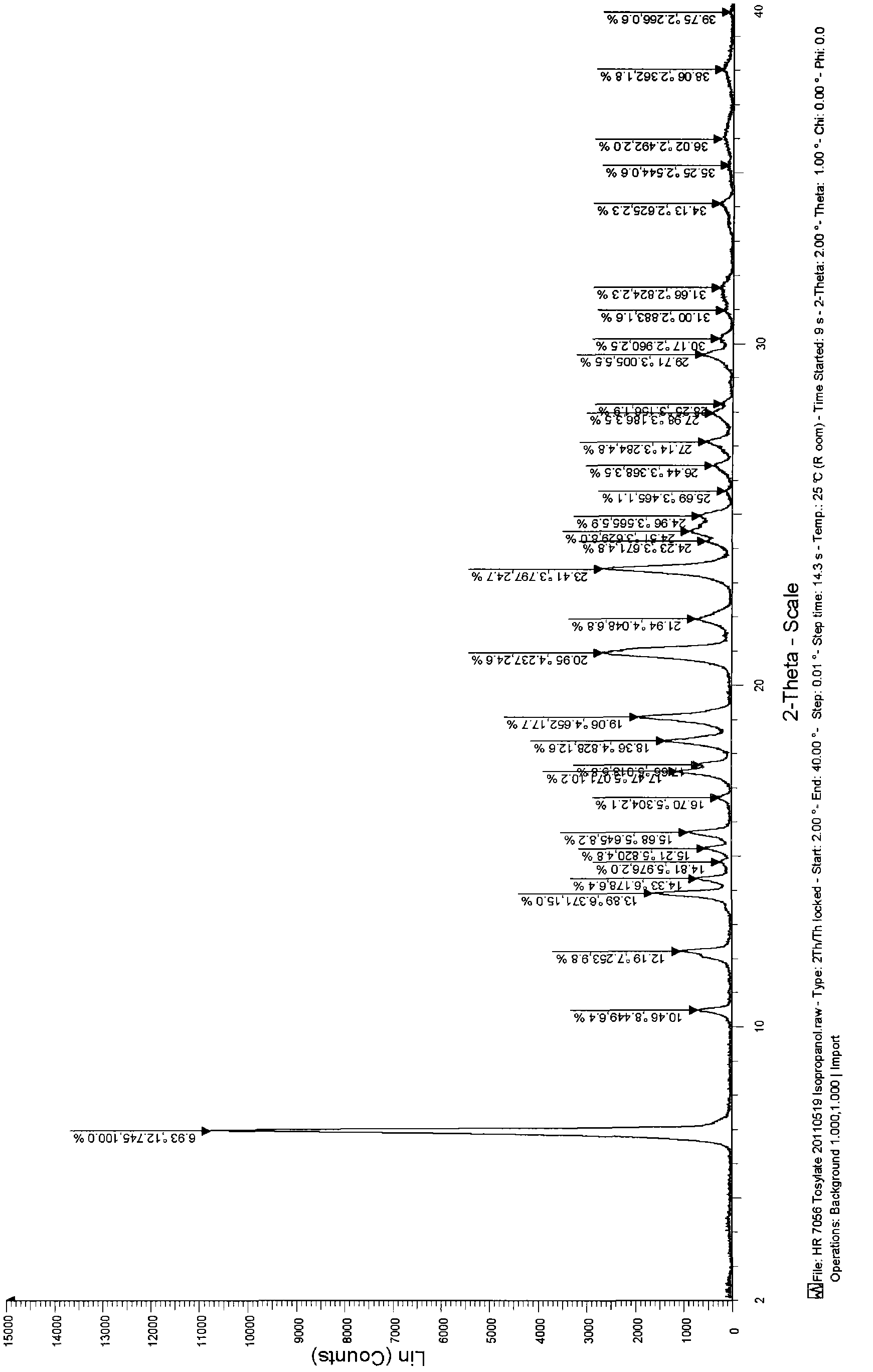
Tosilate of benzodiazepine derivative, its crystal forms, their preparation method and application
InactiveCN102964349AMeet the limit requirementsOrganic active ingredientsNervous disorderBenzodiazepineSolvent
The invention relates to a tosilate of a benzodiazepine derivative, its crystal forms, their preparation method and application, especially to the crystal forms of a 3-[(4S)-8-bromo-1-methyl-6-(2-pyridyl)-4H-imidazole[1, 2-a][1, 4]benzodiazepine-4-yl)methyl propionate (I) tosilate, their preparation method and application. The obtained crystals of the tosilate of a compound as shown in formula (I) has low solvent residual and good stability, and can be better used in clinical treatment.
Owner:JIANGSU HENGRUI MEDICINE CO LTD
Process for preparation of amisulpride
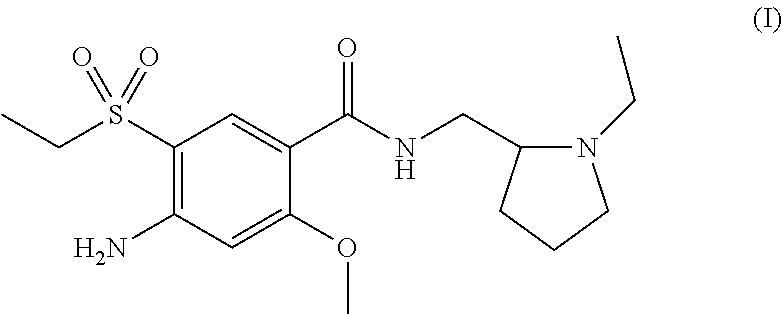
The present invention is related to a novel process for the preparation of amisulpride (I) which involves: methylation of 4-amino-salicylic-acid (VI) with dimethyl sulphate and base, optionally in presence of TBAB to obtain 4-amino-2-methoxy methyl benzoate (VII) and (ii) oxidation of 4-amino-2-methoxy-5-ethyl thio benzoic acid (IX) or 4-amino-2-methoxy-5-ethyl thio methyl benzoate (X) with oxidizing agent in the presence of sodium tungstate or ammonium molybdate to give 2-methoxy-4-amino-5-ethyl-sulfonyl benzoic acid (IV) or 2-methoxy-4-amino-5-ethyl-sulfonyl methyl benzoate (XI) respectively.
Owner:LUPIN LTD
Treatment of behavioral disorders
InactiveUS20050192290A1Ameliorate behavioral disorderSufficient amountBiocideNervous disorderTherapeutic ACTHFexofenadine
The present invention relates to a method for treating a behavior disorder comprising the administration of a therapeutically effective amount of antihistamine, such as ceterizine, fexofenadine; loratadine, and desloratadine. The behavioral disorders may include ADHD, anxieity, depression, and autism. The method may include the administration of the antihistamine in combination with a stimulant medication, such as methylphenidate, thereby to achieve a synergistic effect. In any event, the amount of antihistamine and / or stimulant is effective to downregulate neurotrophic factors such as nerve growth factor or CD40. The invention is also directed to a method of preventing the onset of behavior disorders in patients presenting with symptoms of allergic rhinitis.
Owner:MELAMED ISAAC
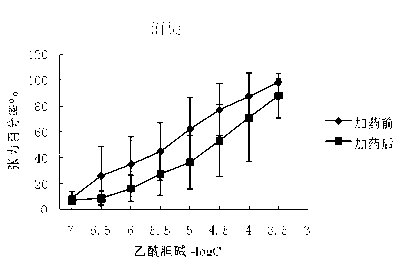
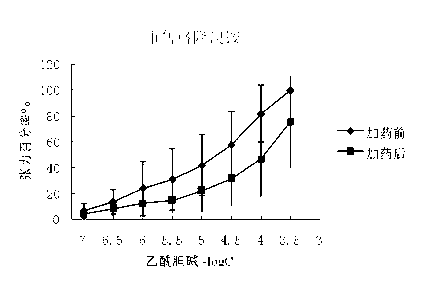
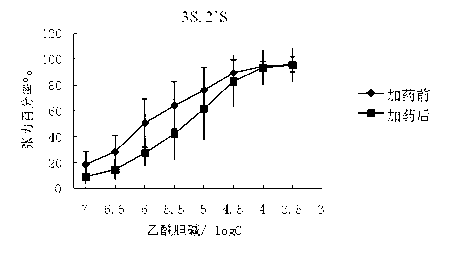
Preparation method and application of glycopyrronium bromide chiral antipode
The invention belongs to the technical field of medicine, and discloses a preparation method of (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) four type chiral monomers of muscarine receptor antagonist racemic medicine glycopyrronium bromide. The method comprises the following steps: resolving racemic alpha-cyclopentylmandelic acid by a chemical resolution method by using L-Tyrosine methyl ester and (R)-alpha-phenylethylamine as resolution reagents to respectively prepare (S)-alpha-cyclopentylmandelic acid and (R)-alpha-cyclopentylmandelic acid; and carrying out esterification reaction to respectively obtain chiral intermediates (S) / (R)-alpha-cyclopentylmethyl mandelate. L / D-malic acid used as the raw material is subjected to four reaction steps, including condensation, carbonyl reduction, catalytic hydrogenation or transfer hydrogenation reduction debenzylation, and reduction alkylation or alkylogen alkylation, in a chiral synthesis mode to obtain another important chiral intermediate (S) / (R)-N-methyl-3-hydroxypyrrolidine. The chiral intermediate is subjected to ester exchange and quaterisation to respectively obtain the four (3S,2'S), (3S,2'R), (3R,2'R) and (3R,2'S) type glycopyrronium bromide chiral monomers. The result indicates that the (3R,2'S)-glycopyrronium bromide has the strongest cholinergic antagonistic action.
Owner:SHENYANG PHARMA UNIVERSITY +1
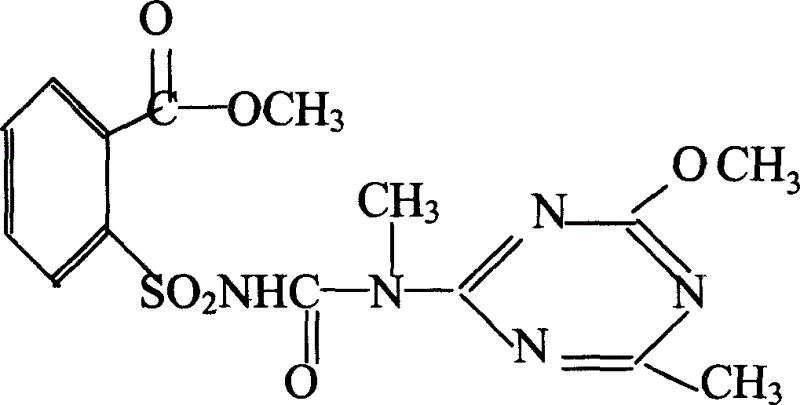
Chemical crossbred agent composition, its using method and application
InactiveCN1524417AEfficient inductionImprove sterilityBiocideAnimal repellantsBiotechnologyBenzoic acid
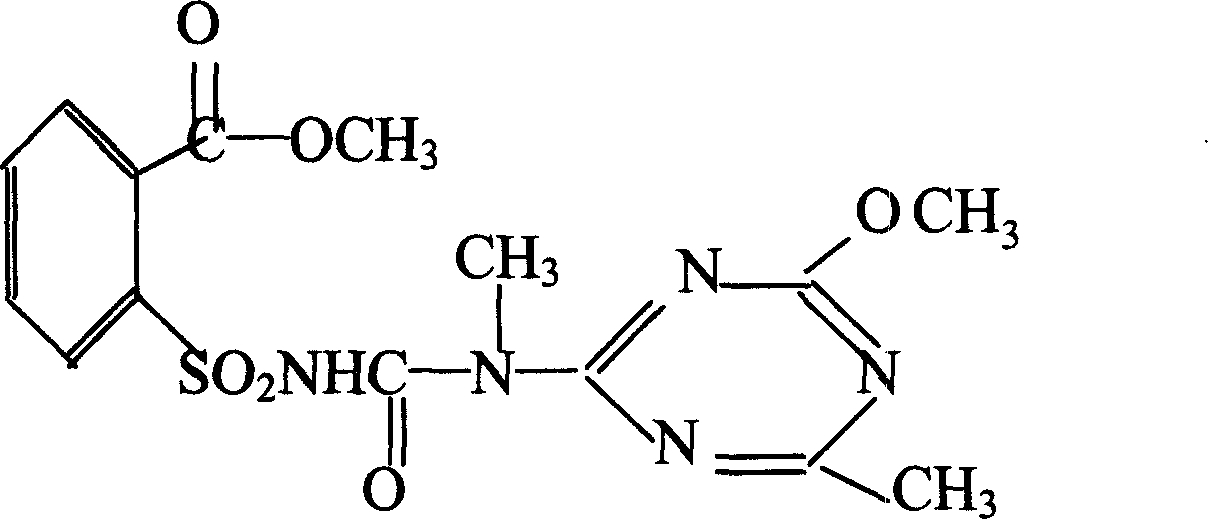
The invention relates to a rape chemical hybridization agent composition and uses thereof, wherein the composition (SX-1) contains active constituent of 2-[3-(4- methoxy-6- methyl-1,3,5- triazine-2-group)-3- methyl carbamido sulfonyl] methyl benzoate, aerosol, emulsifying agent, thickening agent, crumbling agent and soluble starch. The invention also relates to the method of use and application in rape chemical hybridization of the composition.
Owner:陕西省杂交油菜研究中心
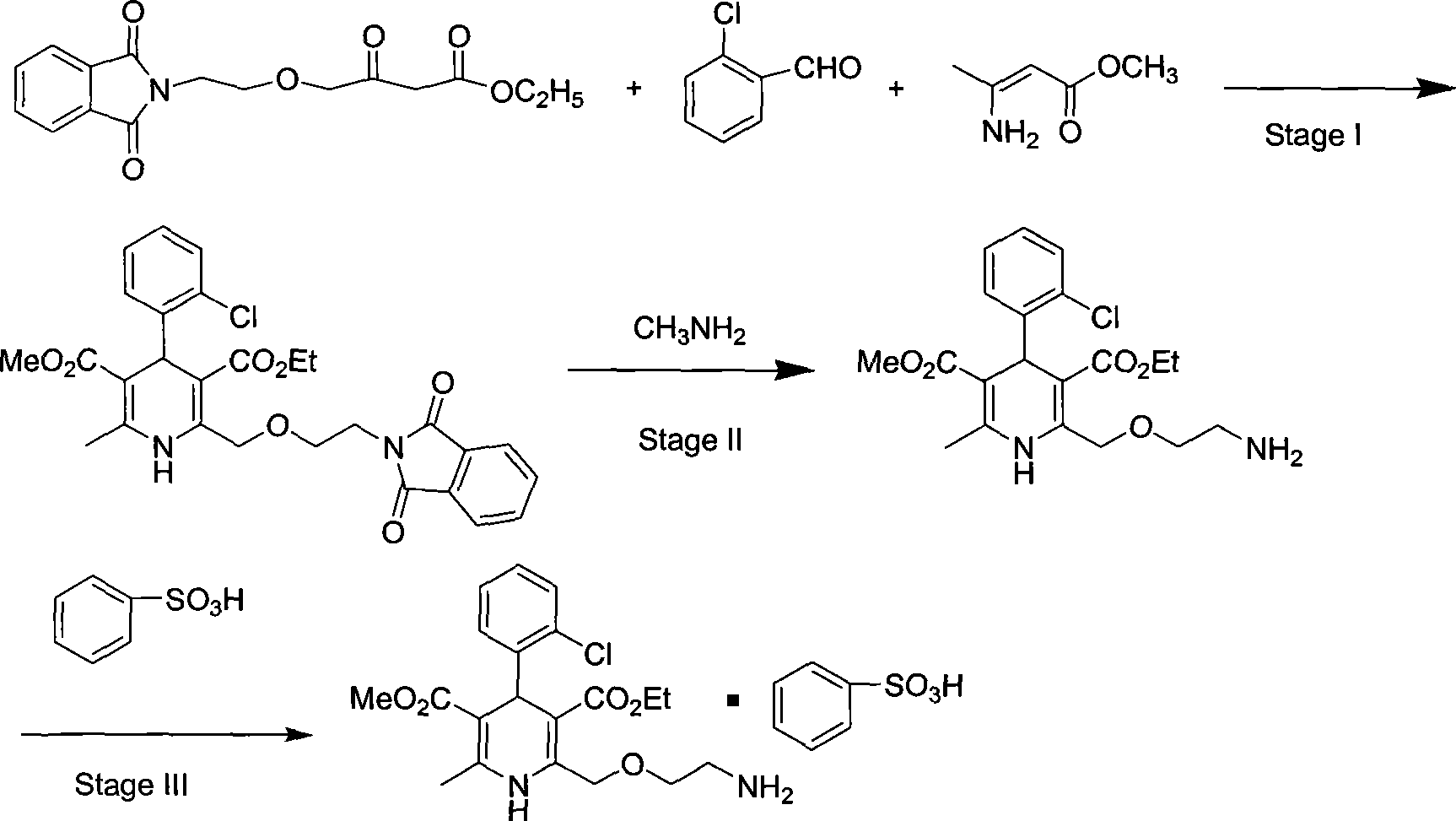
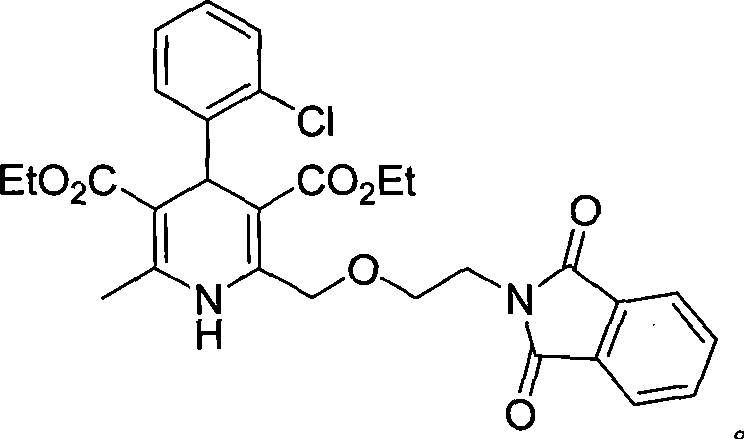
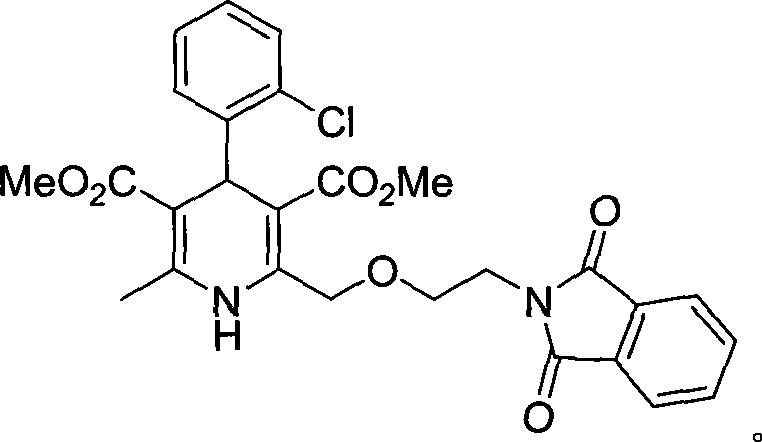
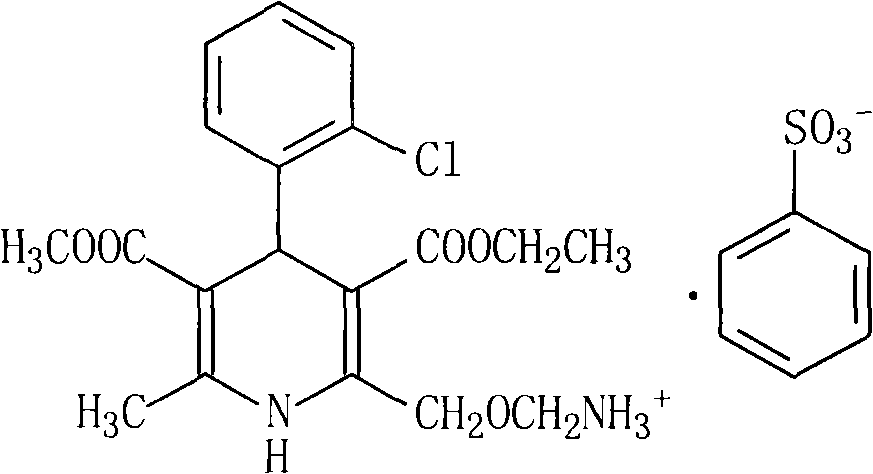
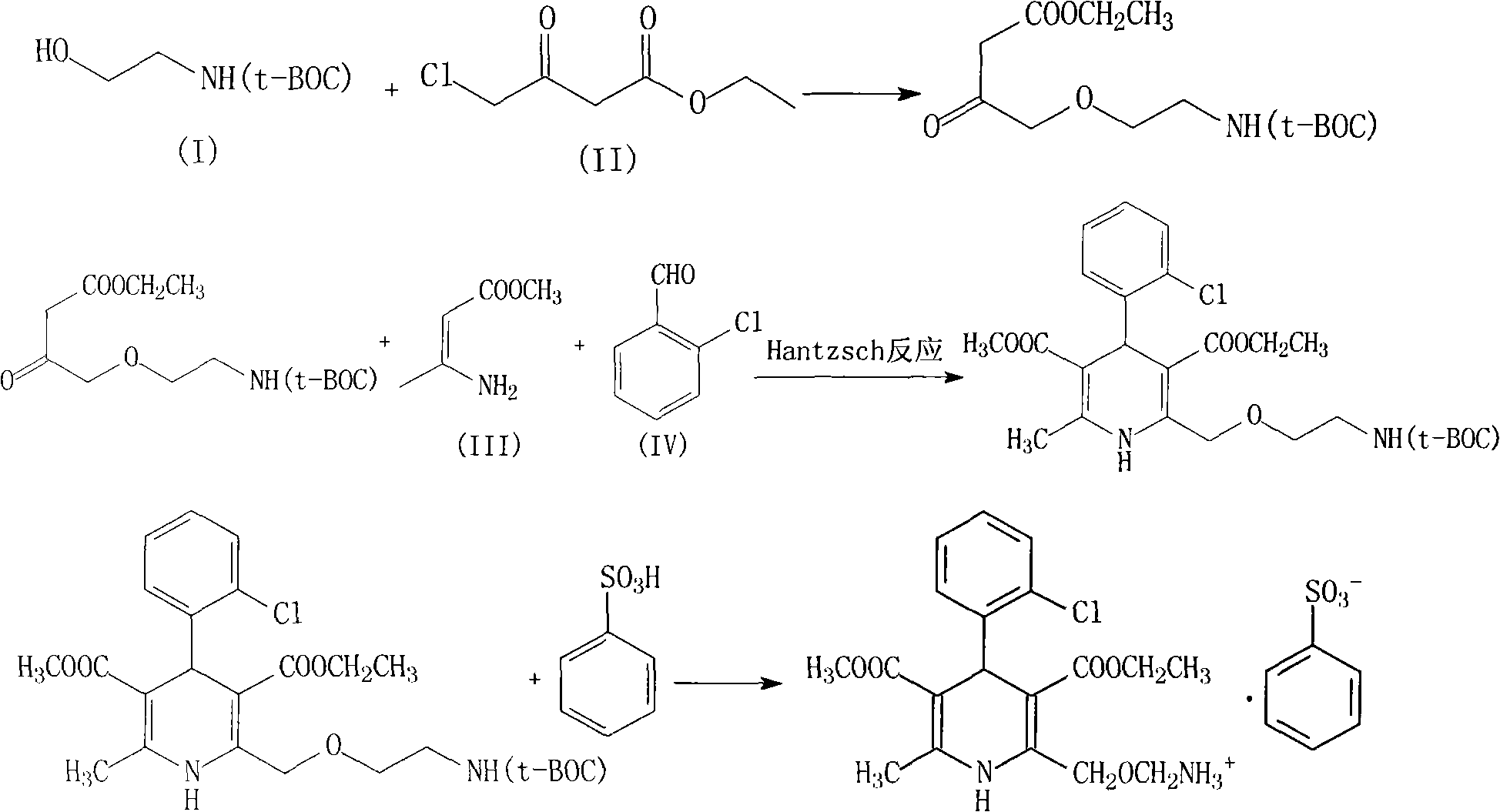
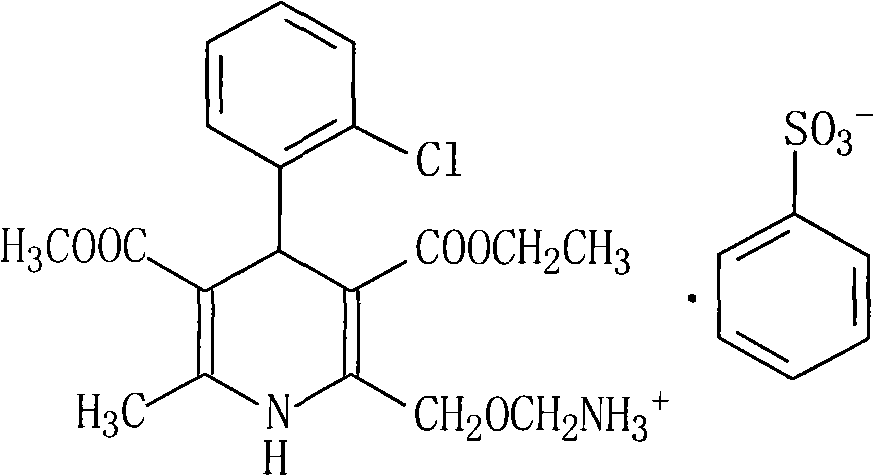
Synthesis of high-purity amlodipine besylate
The present invention relates to a synthesis method of high-purity amlodipine besylate. In particular, o-chlorobenzaldehyde, 4-(2-phthalyl imino radical ethoxy) acetoacetic ester and 3-amino methyl cro-tonate are used as raw materials for ring closure in an alcohol solvent, and when the amount of the 3-amino methyl cro-tonate is three times, the intermediate for ring closure can be prepared; the intermediate is refined by toluene / glacial acetic acid, dissolved in methylamine to form a salt in the aqueous solution, and the high-purity amlodipine besylate can be acquired after recrystallization with ethyl alcohol.
Owner:CHINA RESOURCES SAIKE PHARMA
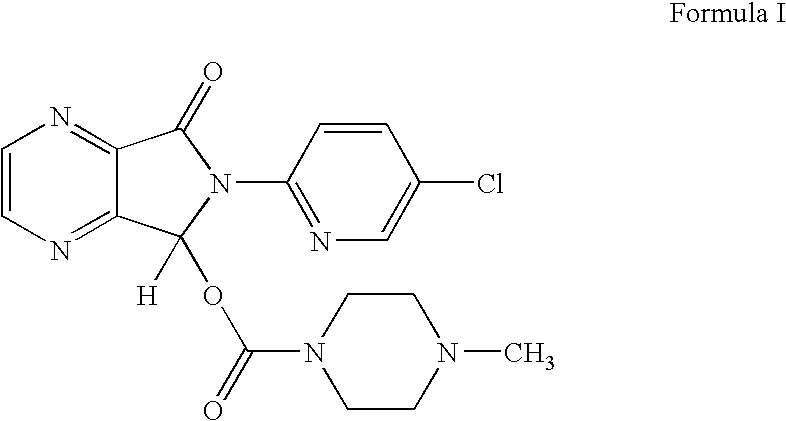
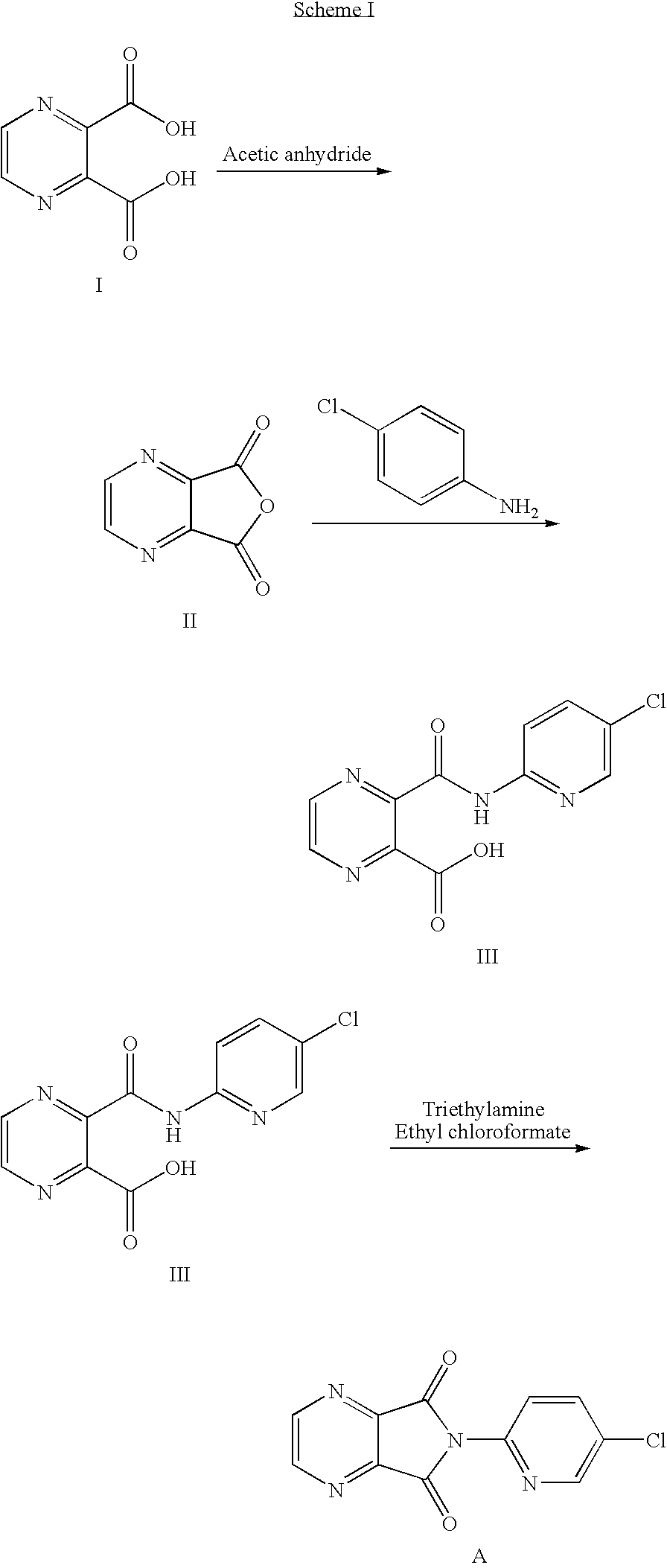
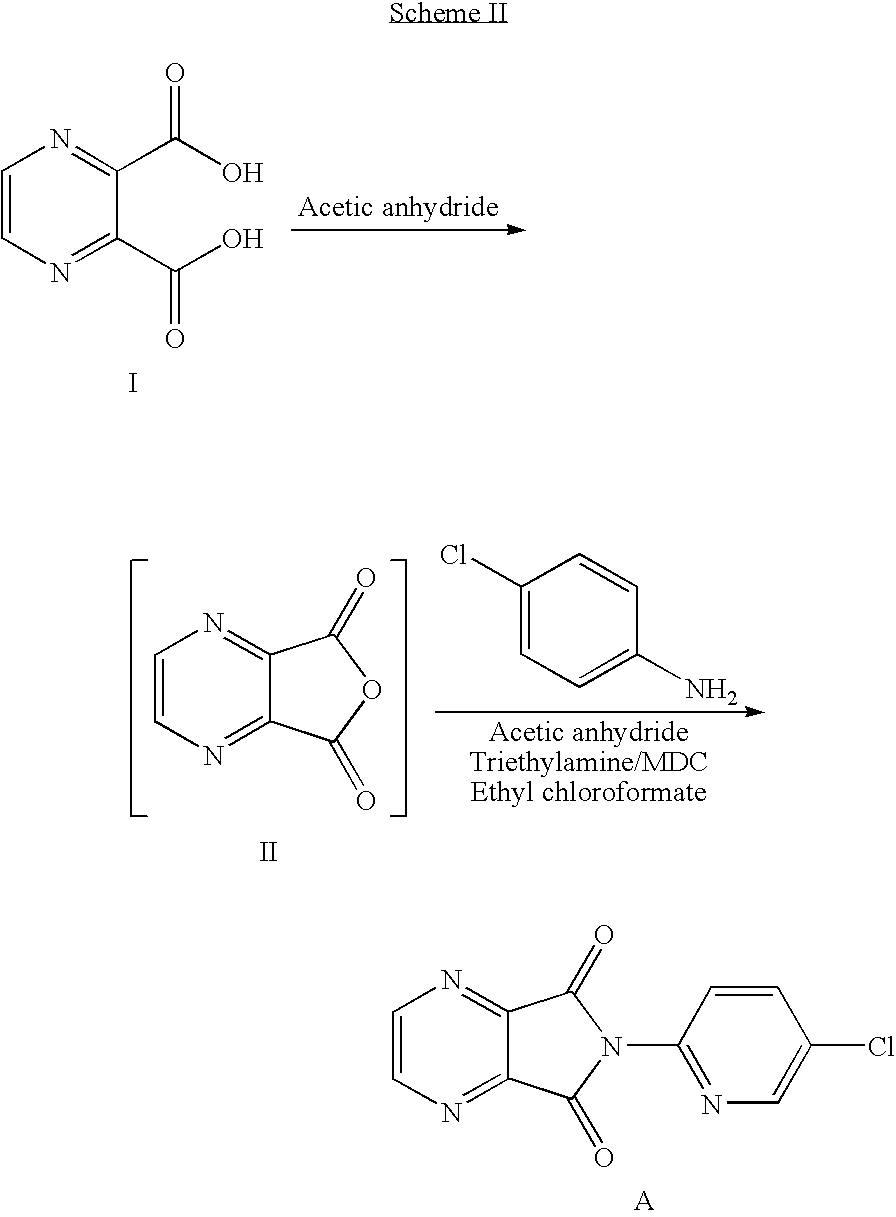
Process for the preparation of eszopiclone
InactiveUS20080146800A1Minimizes problemEfficient and cost-effective processOrganic chemistryMetaclazepamPyrazine
The invention relates to a process for making of 6-(5-chloro-2-pyridinyl)-6,7-dihydro-7-oxo-5H-pyrrolo-[3,4-b] pyrazin-5-yl-4-methyl piperazine-1-carboxylate, also known as zopiclone. The invention further describes an effective method for resolving of zopiclone into its enantiomers (eszopiclone and (R)-zopiclone) and also provides a method of recycling of (R)-zopiclone.
Owner:CENTAUR CHEM PVT +1
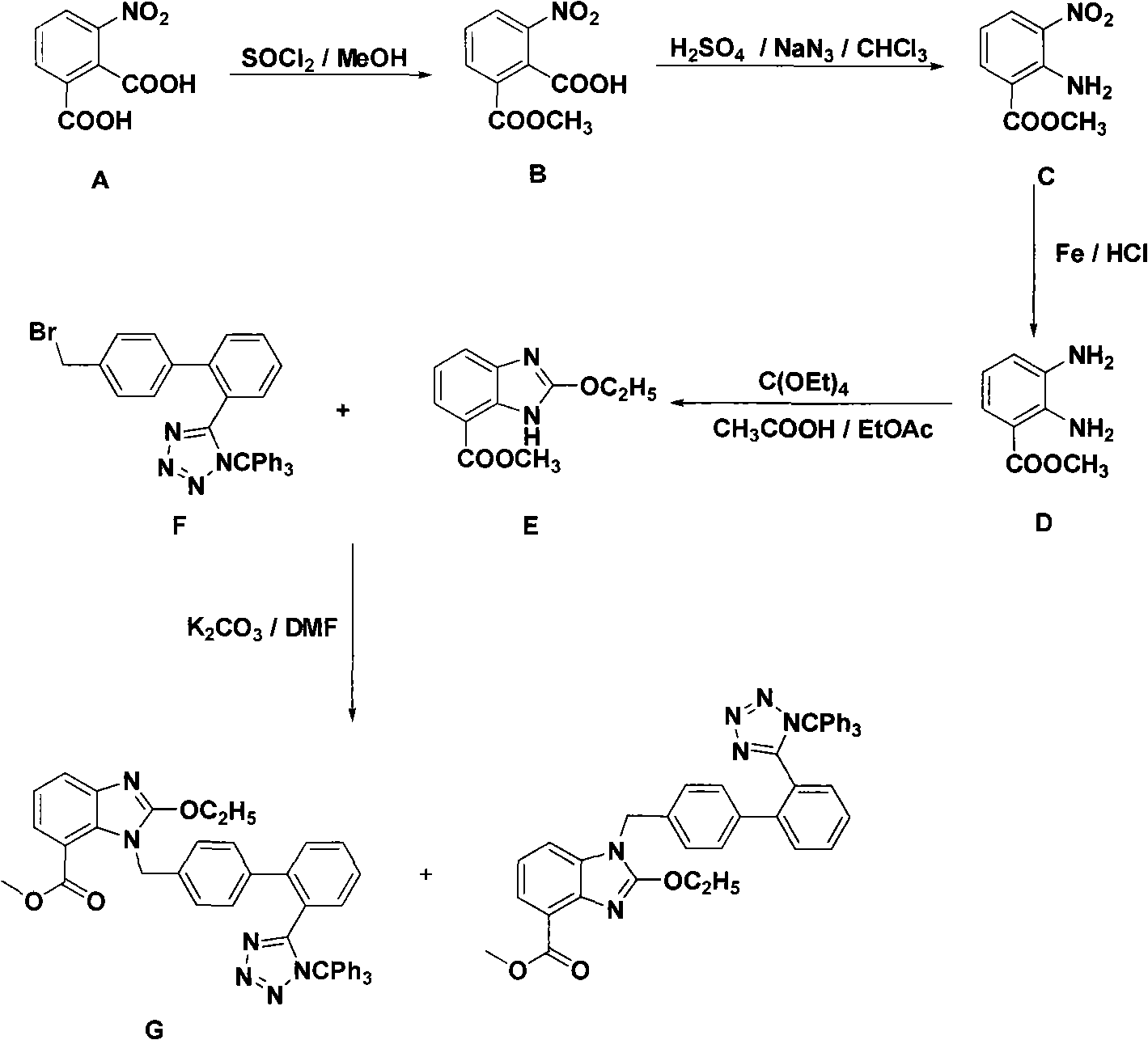
Novel preparation of trityl group candesartan cilexetil intermediate
ActiveCN101323610AThe synthesis process is simpleLow investment costOrganic chemistryBenzoic acidCandesartan
The invention discloses a novel technology for synthesizing an intermediate of trityl candesartan; the synthesis steps thereof comprise: (1) a preparation method of 2-menthyl formate-6-nitryl-benzoic acid; (2) a preparation method of 2-amido-3-nitryl-methyl benzoate; (3) a preparation method of 2, 3-diaminobenzene menthyl formate; (4) a preparation method of 2-oxethyl-4-menthyl formate-3-H-benzimidazole; and (5) the preparation method of the intermediate of trityl candesartan.
Owner:APELOA PHARM CO LTD +1
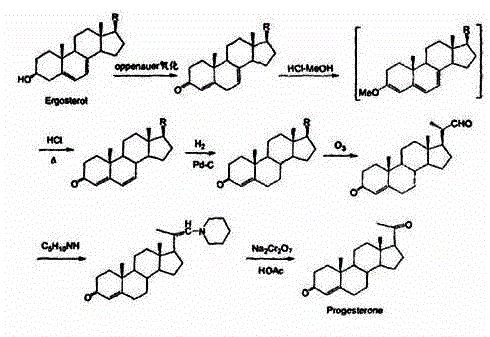
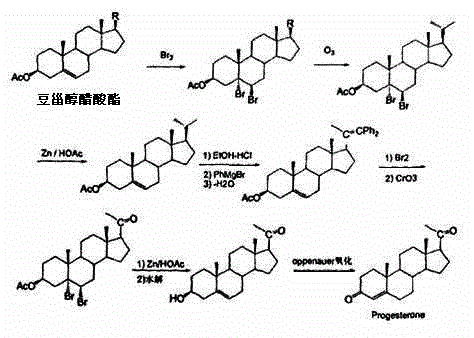
Method for preparing progesterone by taking 1,4-androstenedione as raw material
The invention discloses a method for preparing progesterone by taking 1,4-androstenedione as a raw material, which comprises the following steps: 1) dissolving 1,4-androstenedione into an organic solvent, adding the acid of trimethyl orthoformate or triethyl orthoformate, and introducing nitrogen to protect the 1,4-androstenedione to synthesize the enol ether of 1,4-androstenedione, namely 3-methoxy-androstane 3,5-diene-20-ketone; and 2) dispersing (1-methoxy ethyl)-triphenylphosphine salt in a reaction medium, an organic solvent, adding alkali at low temperature, performing a Wittig reaction of the 3-methoxy-androstane 3,5-diene-20-ketone synthesized in the step 1), and purifying and crystallizing to obtain progesterone. By adopting the 1,4-androstenedione as the raw material, the method solves the problem that of lack in raw materials for synthesizing steroid drugs such as progesterone, and improves the utilization rate of 1,4-androstenedione and the yield of progesterone; the preparation process is simple.
Owner:HUNAN KEYUAN BIO PRODS
Substituted azoles, antiviral active component, pharmaceutical composition, method for preparation and use thereof
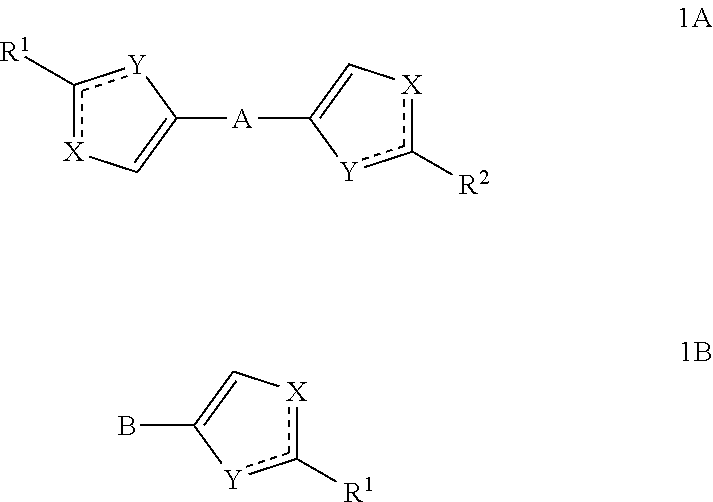
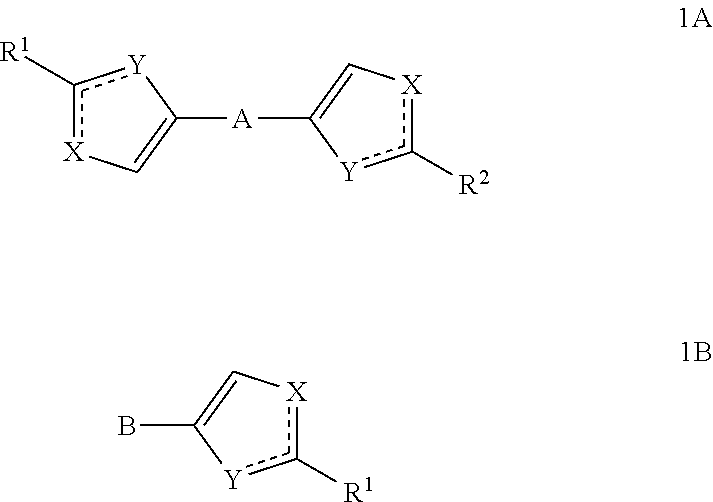
The present invention relates to novel azoles, novel antiviral active components of the general formulas 1A and 1B, pharmaceutical composition, antiviral medicament, method for prophylaxis and treatment of viral diseases, particularly caused by hepatitis C viruses (HCV). In general formulas 1A and 1Bwherein: solid lines with accompanying dotted lines () represent ordinary bond or double bond, provided that one of them is an ordinary bond, the other one is double bond; X and Y accept various meanings, one of them is—nitrogen, the other—oxygen, sulfur or NH group; R1 and R2—optionally the same radicals selected from 2-(R)- and (S)-substituted N-acyl pyrrolidine derivatives; N-methyl-N-[2-(R) and (S)-substituted 2,2-disubstituted acetamides; methyl[2-(R) and (S)-substituted ((methyl)amino)-(1-oxobutan-2-yl)-2-(R)-] and (S)-iso-propyl)-carbamates. A represents aliphatic C2-C8 biradical; dioxane, cyclo- and bicycloaliphatic, alkyloxyalkyl, alkyloxyalkylenoxyalkyl, alkenyloxyalkyl, alkynyloxyalkyl biradicals and their thioanaloges; aryl and thiophene alkynylcycloalkyl, alkynyldioxane, alkynylaryl, alkylthiophene, alkenylthiophene and alkynylthiophene, alkyloxyaryl, alkenyloxyaryl, alkynyloxyaryl, alkylthioaryl, alkenylthioaryl, alkynylthioaryl, cycloalkylthiophene, aryldioxane and thiophenyldioxane biradicals. B represents: aliphatic C2-C8 radical, including 1, 2 or 3 triple C≡C bonds; aryl and thiophene, alkynylcycloalkyl, alkynyldioxane, alkynylaryl, alkylthiophene, alkenylthiophene and alkynylthiophene, cycloalkylbenzene, 4-cycloalkylbiphenyl, bicycloalkylbenzene, 4-bicycloalkylbiphenyl, cycloalkylthiophene, aryldioxane and thiophenyldioxane radicals.
Owner:IVACHTCHENKO ALEXANDRE VASILIEVICH DR +3
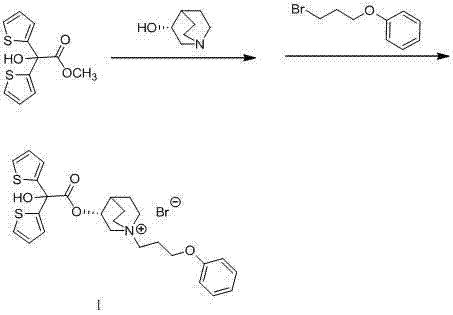
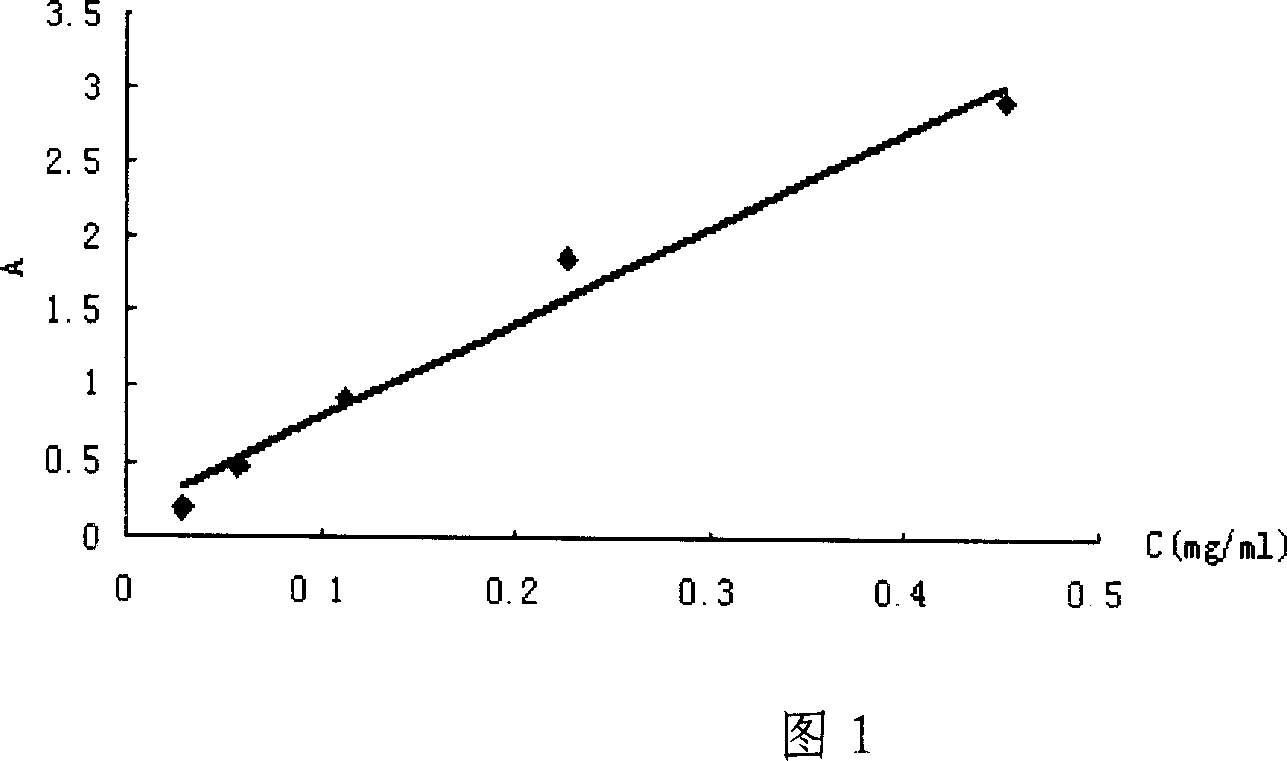
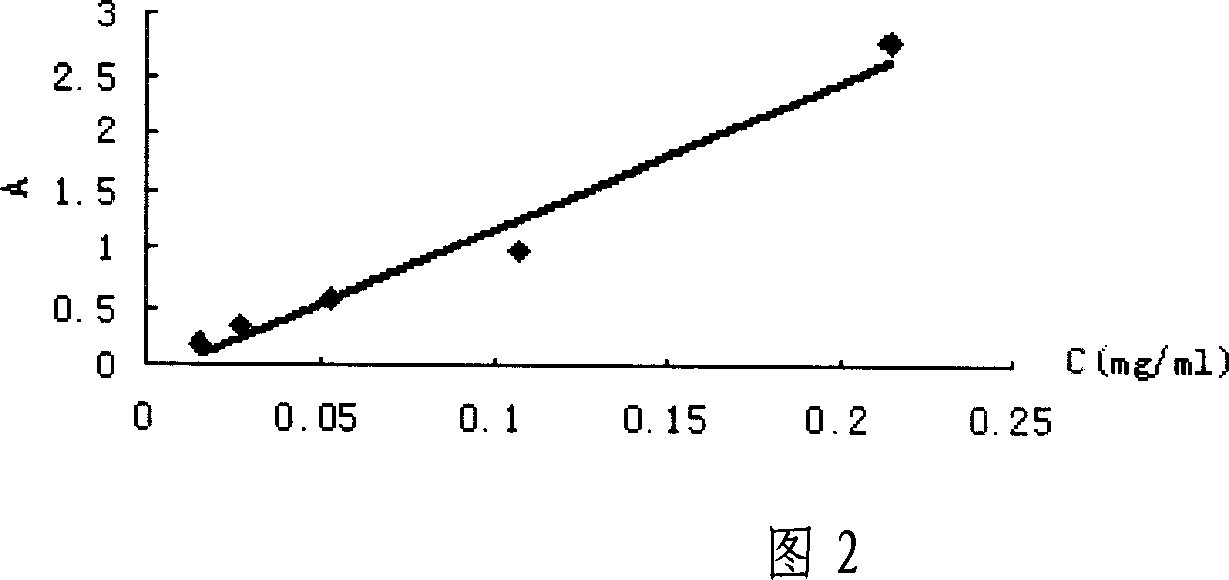
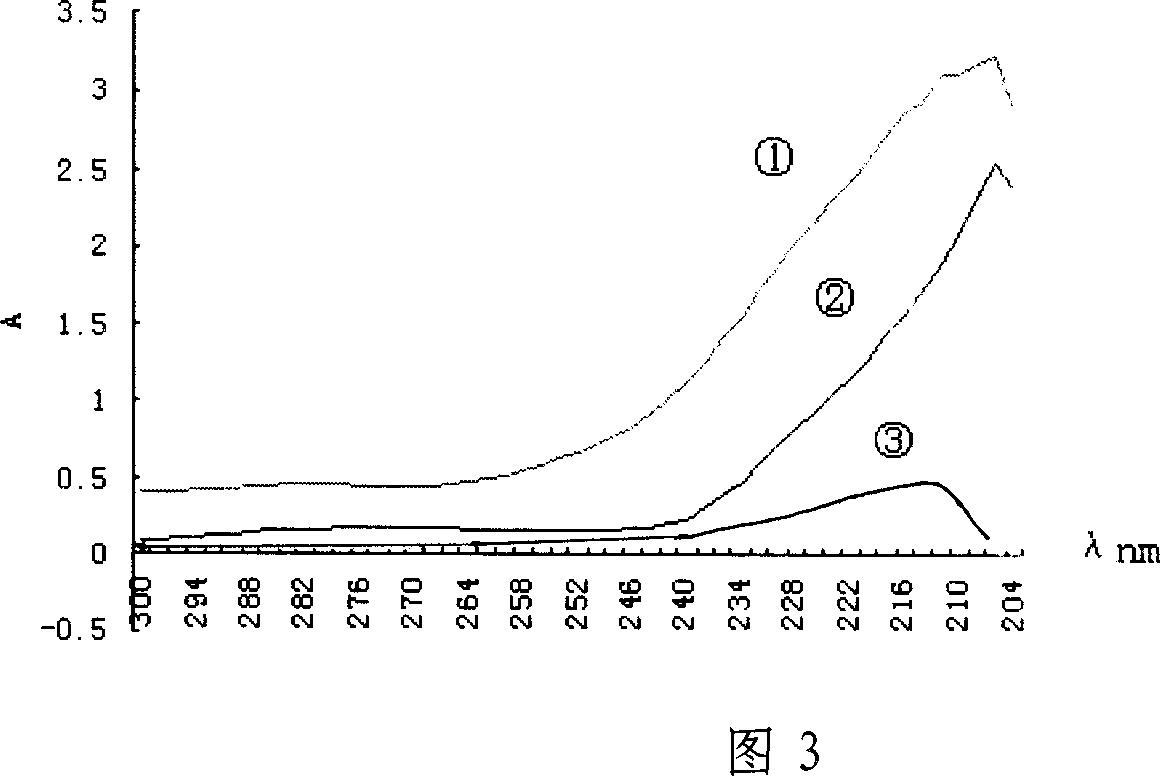
Technology for preparing aclidinium bromide employing one-pot process
The invention belongs to the field of medicinal chemistry, and discloses a technology for preparing aclidinium bromide. By adopting the technology, (R)-3-quinuclidinol, (C1)methyl-2,2-dithienyl glycolate and 3-phenoxy propyl bromide are taken as raw materials, and the aclidinium bromide is prepared by adopting a one-pot process. The method is simple to operate, and convenient for post-treatment, and has good application value.
Owner:AVENTIS PHARMA HAINAN
Synthesizing porcess for artificial antigen of cyanobromide chrysanthemum ester and assaying process thereof
InactiveCN1948964AMeet immunization requirementsSuitable for commercializationOrganic chemistryBiological testingBenzaldehydePhenyl group
The invention discloses Dehamethrin artificial antigen synthetic method. It includes the synthesis for (1R, 3R)-3-(2, 2-dibrom vinyl)-2, 2-dimethyl cyclopropyl formyl chloride, 3-(4-nitro phenoxy) benzaldehyde, [3-(4- nitro phenoxy) phenyl] cyan methanol, (1R, 3R)-3-(2, 2-dibrom vinyl)-2, 2-dimethyl cyclopropyl formic acid[3-(4-nitro phenoxy)phenyl] cyan methyl ester, artificial half antigen (1R, 3R)-3-(2, 2-dibrom vinyl)-2, 2-dimethyl cyclopropyl formic acid[3-(4-aminophenoxy) phenyl] cyan methyl ester, artificial antigen synthetic steps and its measuring.
Owner:BEIJING UNIV OF AGRI
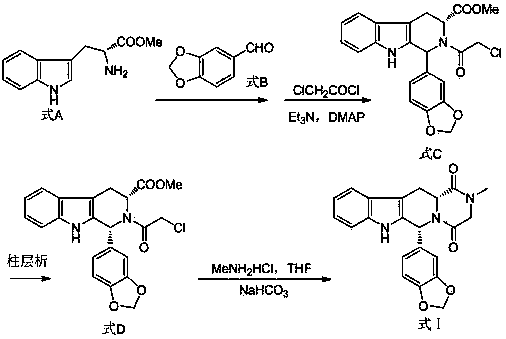
Improved tadalafil preparation method
The invention belongs to the field of preparation of chemical raw medicaments, and more in particular relates to an improved preparation method for a phosphodiesterase 5 inhibitor tadalafil. A specific synthesis route is shown in the specification. The method comprises the following steps of performing Pictet-Spengler cyclization reaction and chloroacetyl chloride acylation on starting reactants (D-tryptophan methyl ester hydrochloride and piperonal) to obtain an intermediate product, directly performing subsequent reaction on the intermediate product without purification, preparing an intermediate 1-(1,3-benzodioxol-5-yl)-2-(chloracetyl)-2,3,4,9-tetrahydro-1H-pyridino-[3,4,-B]indol-3-thiophenate methyl by using a one-pot reaction method, performing column chromatography purification to obtain a single cis-isomer, and finally reacting the single cis-isomer with methylamine hydrochloride in the presence of an inorganic base to obtain the tadalafil.
Owner:ANHUI WANBANG MEDICAL TECH
Synthesis method of cediranib
InactiveCN102603718AChange and optimize synthetic methodsReduce pollutionOrganic chemistryMetaclazepamBiochemical engineering
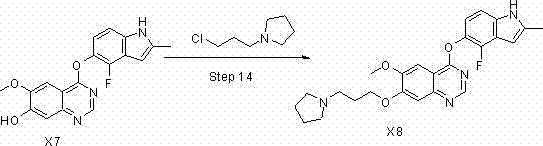
The invention discloses a preparation method of cediranib, which comprises the following steps of using trifluoro-nitrobenzene as a raw material and performing acetyl methyl adding, substitution, cyclization and protection to obtain a segment 1; and then using methyl vanillate as the raw material, performing benzyl bromine protection, nitro adding, reduction, unique cyclization and chlorination to obtain a segment 2; and performing nucleophilic substitution and deprotection on the two segments to obtain the final product cediranib. Compared with other methods, the preparation method of the cediranib has the advantages of mild reaction condition, high yield and scale amplification, and raw materials can be obtained easily.
Owner:CHEMPROSPECT PHARMTECH
Preparation method of anti-new crown drug Paxlovid intermediate
ActiveCN114133350AAvoid reactionAvoid Yield ProblemsOrganic chemistryBulk chemical productionMetaclazepamPharmaceutical Substances
The invention discloses a preparation method of an intermediate of an anti-new crown drug Paxlovid. According to the preparation method, cheap and easily available N-Boc-trans-4-hydroxy-L-proline methyl ester (compound II) is taken as an initial raw material, the Paxlovid intermediate can be obtained through a plurality of steps of reactions, and the structural formula of the anti-new crown drug Paxlovid intermediate is as shown in the formula I. The method has the advantages of simple process, low production cost, easiness in industrial production and the like.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Amlodipine besylate compound and novel preparation method thereof
InactiveCN101812014ALow priceThe reaction steps are simpleOrganic chemistryBulk chemical productionCarbonic acidDi-tert-butyl dicarbonate
The invention relates to an amlodipine besylate compound and a novel preparation method thereof, wherein the method comprises the following steps that: a moderate amino-protecting reagent of di-tert-butyl dicarbonate is adopted so that the introduction of a Boc protecting group is realized, through the reaction of intermediums of 3-amino-crotonic acid methyl ester and 2-chlorobenzaldehyde, the obtained amlodipine besylate compound is directly carried out BOC protecting group removal reaction with benzene sulfonic acid, and the final product is obtained. Consequently, the invention is dispensed with the special deprotection step, simplifies the reaction steps, is more suitable for industrialized production and has high total reaction yield.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
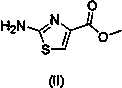
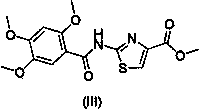
One-pot method for preparing acotiamide hydrochloride
ActiveCN104045606AReduce pollutionOvercome the shortcomings of cumbersome industrial operations and not suitable for industrial productionOrganic chemistryThiazoleSolvent
The invention relates to a one-pot method for preparing an acotiamide hydrochloride (a compound V). The method comprises the following steps: by taking 2,3,5-trimethoxybenzoic acid as shown in a formula (I) and 2-aminothiazole-4-methyl formate as shown in a formula (II) as raw materials, carrying out a condensation reaction to obtain a compound III; selecting an appropriate solvent and controlling a reaction condition, directly carrying out an aminating reaction between an intermediate III which is not separated and purified and N,N-diisoprylamino ethylamine as shown in a formula (IV), and finally obtaining the acotiamide hydrochloride (the compound V) by use of the one-pot method. The one-pot method for preparing the acotiamide hydrochloride has the advantages that the reaction steps are reduced, the operation process is simplified, and the production efficiency is improved; besides, the method is safe and environmental friendly, and suitable for industrial production. The formulas (I, II, III, IV and V) are as shown in the specification.
Owner:HANGZHOU XINBOSI BIOMEDICAL CO LTD
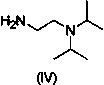
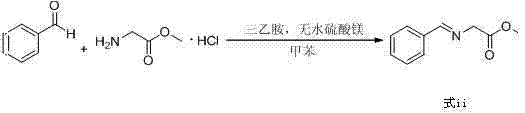
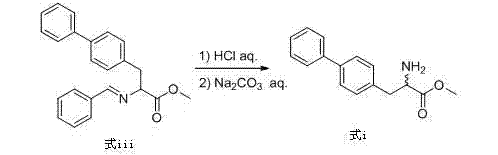
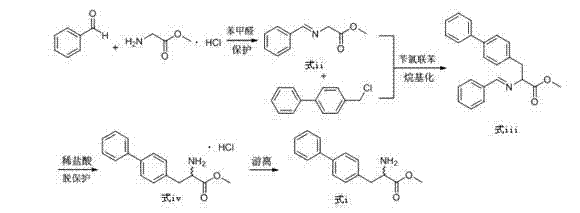
Preparation method of biphenyl alanine derivative
ActiveCN104725256ARaw materials are easy to getLow equipment requirementsOrganic compound preparationAmino-carboxyl compound preparationBenzaldehydeBenzyl chloride
The invention relates to a preparation method of a compound represented by the formula (i); and the compound represented by the formula (i) is prepared by taking glycine methyl ester or hydrochloride thereof as the starting material through benzaldehyde protection, benzyl chloride biphenyl alkylation and deprotection. According to the invention, the starting material is easy to obtain and low in cost; furthermore, an intermediate related in the reaction process is unnecessary to separate and purify; the preparation method is simple to operate and moderate in reaction condition; and therefore, the preparation method is applied to industrialized mass production.
Owner:迪嘉药业集团股份有限公司
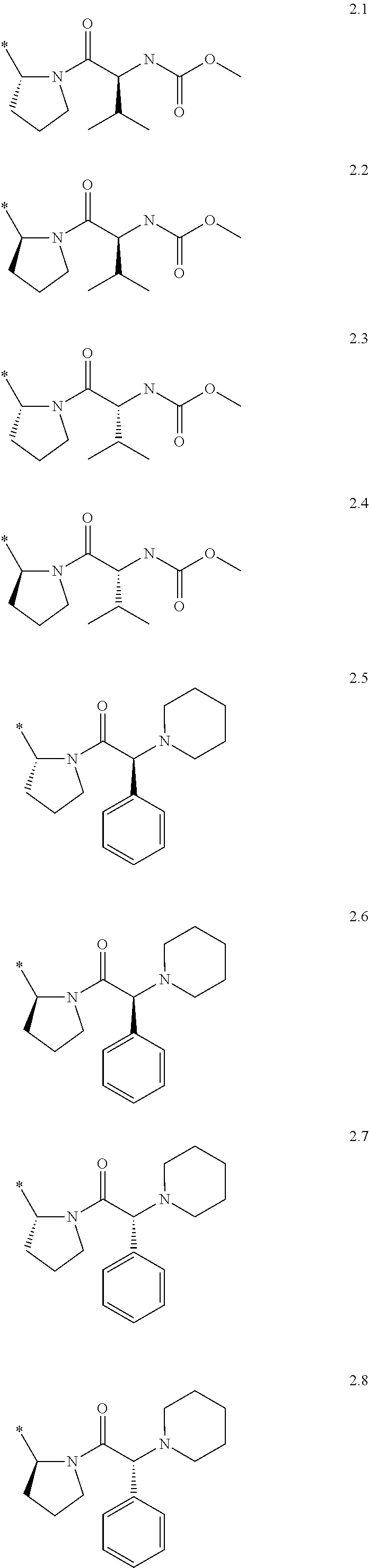
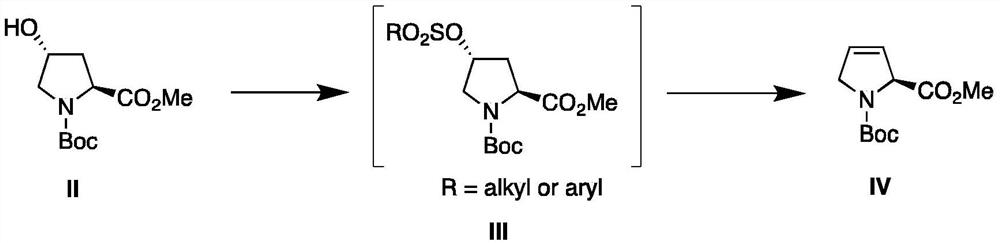
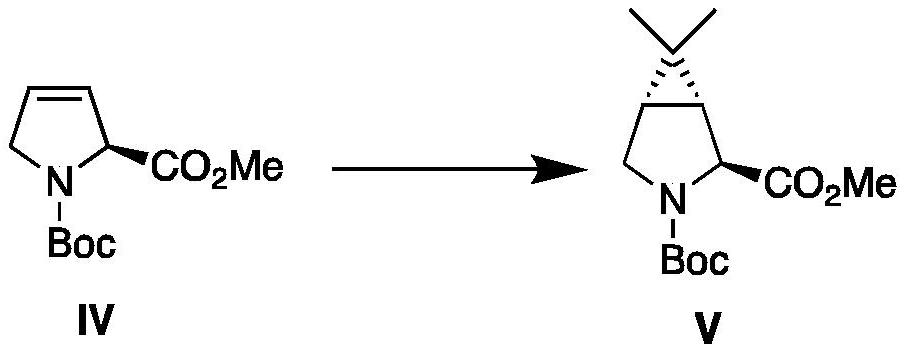
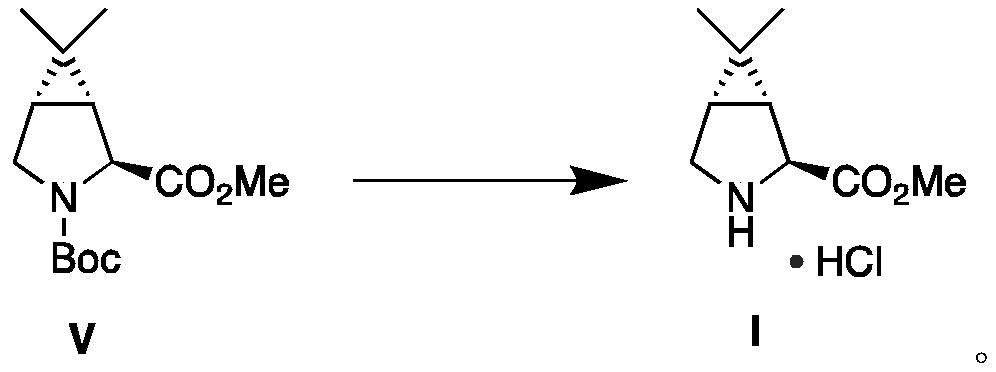
Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ...
The present invention provides for a process for preparing racemic methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate, its corresponding salt: (2S, 3R, 4S)-methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate di-p-toluoyl-D-tartaric acid (“D-DTTA”) salt or a (2R, 3S, 4R)-methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate di-p-toluoyl-L-tartaric acid salt (“L-DTTA”) in a high enantiomeric excess. This invention also provides for a process for preparing a (2S, 3R, 4S)-methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate dibenzoyl-D-tartaric acid (“D-DBTA”) salt or a (2R, 3S, 4R)-methyl 6,6-dimethyl-3-azabicyclo[3,1,0]hexane-2-carboxylate L-tartaric acid (“L-DBTA”) salt in a high enantiomeric excess. Further, this invention provides a process for preparing intermediates II, IIB, III, IV, IV salt, V, VI, and VII.
Owner:MERCK SHARP & DOHME CORP
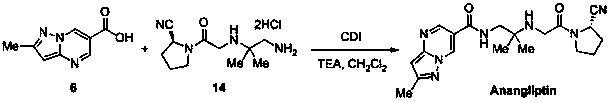
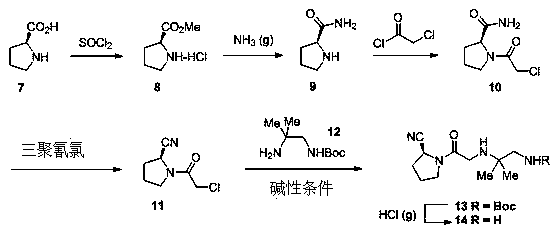
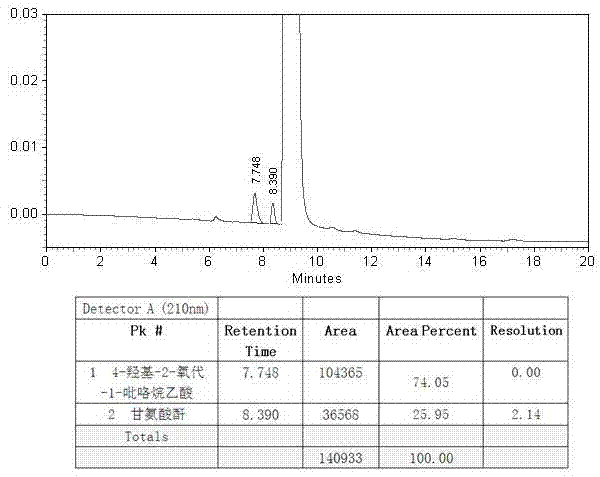
Synthesis method of anagliptin
InactiveCN105503878AHigh reaction yieldEasy post-processingOrganic chemistryBulk chemical productionDiabrezideNucleophilic substitution
The invention relates to a synthesis method of bulk drug of anagliptin for treating II type diabetes, and aims to solve the problem that at present there is no industrial synthesis method of anagliptin. The synthesis method comprises the following steps: (1) taking vinyl ethyl ether and trichloroacetic chloride as the raw materials, carrying out three-step reactions to obtain an intermediate (4) with a protected aldehyde group; carrying out dehydration condensation between the intermediate (4) and 3-amino-5-methylpyrazole to obtain pyrazolopyrimidine parent nucleus; and hydrolyzing carboxyl ethyl ester to obtain 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carboxylic acid6; (2) taking L-proline as the raw material, subjecting L-proline to methyl esterification, ammoniation, acetylation, and cyaniding reactions to obtain a chiral cyanopyrrole intermediate (11); making the chiral cyanopyrrole intermediate (11) and a diamine segment (12) carry out nucleophilic substitution reactions under an alkaline condition to obtain an intermediate (13), and finally removing the Boc protective group from the intermediate (13) in the presence of hydrochloric acid to obtain a cyanopyrrole amine intermediate (14); (3) coupling 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carboxylic acid 6 with the cyanopyrrole amine intermediate (14) under condensation conditions so as to obtain the bulk drug anagliptin.
Owner:南通佰康生物医药有限公司
Oxiracetam drug activity composition and preparation method thereof
ActiveCN102846600AComply with medicinal requirementsQuality improvementOrganic active ingredientsNervous disorderClinical efficacyEthyl acetate
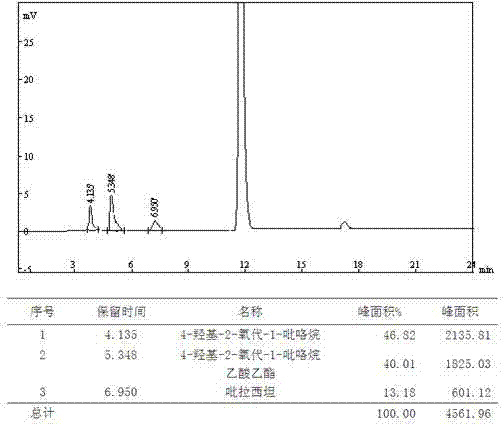
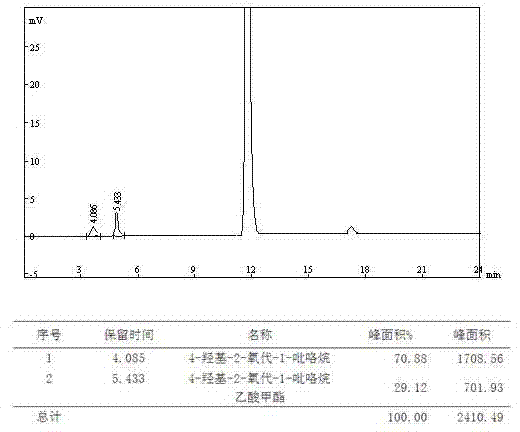
The present invention provides an oxiracetam drug activity composition, which comprises the following components: a component I, a component II and a component III, wherein the component I is oxiracetam, the component I content is more than or equal to 98.0%, the component II is glycine anhydride, the component II content is more than 0 and is less than or equal to 0.3%, the component III is one or a plurality materials selected from 4-hydroxy-2-oxo-1-pyrrolidineacetic acid, 4-hydroxy-2-oxo-1-pyrrolidine, ethyl 4-hydroxy-2-oxopyrrolidine-1-acetate, methyl 4-hydroxy-2-oxopyrrolidine-1-acetate, and piracetam, the component III content is more than 0 and is less than or equal to 1.5%, the 4-hydroxy-2-oxo-1-pyrrolidineacetic acid content is more than 0 and is less than or equal to 0.5%, and the total content of the component II and the component III is less than or equal to 1.5%. The drug activity composition of the present invention has stable quality, and can completely meet quality requirements on the drug activity composition by oxiracetam preparations. In addition, the prepared preparation has characteristics of safety, effectiveness, and controllable quality, and clinical therapy effects and medication safety of the oxiracetam preparation are ensured.
Owner:CSPC OUYI PHARM CO LTD
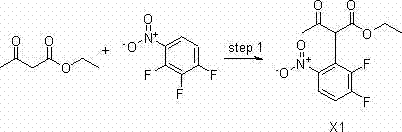
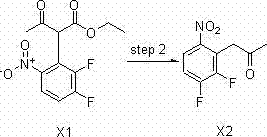
Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine
The invention relates to a synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine. The invention solves the problems such as low yield, high pollution, complicated operation, and the like of current preparation methods. The method provided by the invention comprises the steps that: methoxy methyl acetate and methyl formate are condensed under a strong-alkali condition; the condensation product is subjected to cyclization with thiourea; methylation is carried out by using chloromethane; chlorination is carried out by using phosphorus oxychloride; hydrazination is carried out by using hydrazine hydrate; the product is subjected to cyclization with cyano bromine; and under the effect of strong alkali and acrylate, a final product is obtained. With the method provided by the invention, the yield of each step is higher than 80%, and a total yield reaches 39%. The method is suitable for industrialized productions. The method provided by the invention belongs to the field of paddy rice herbicide penoxsulam intermediate preparation.
Owner:HEILONGJIANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)
![Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION](https://images-eureka.patsnap.com/patent_img/f8768c0d-5b20-4fca-b18d-c54ed6489c4c/US20130210157A1-20130815-D00001.png)
![Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION](https://images-eureka.patsnap.com/patent_img/f8768c0d-5b20-4fca-b18d-c54ed6489c4c/US20130210157A1-20130815-D00002.png)
![Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION Zn3(BDC)3[Cu(SalPycy)] AND Zn3(CDC)3[Cu(SalPycy)] - ENANTIOPURE MIXED METAL-ORGANIC FRAMEWORKS FOR SELECTIVE SEPARATIONS AND ENANTIOSELECTIVE RECOGNITION](https://images-eureka.patsnap.com/patent_img/f8768c0d-5b20-4fca-b18d-c54ed6489c4c/US20130210157A1-20130815-D00003.png)
![Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ... Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ...](https://images-eureka.patsnap.com/patent_img/7e6ad494-c2a9-401b-b49b-4c10730f2e3f/US20090240063A1-20090924-C00001.png)
![Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ... Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ...](https://images-eureka.patsnap.com/patent_img/7e6ad494-c2a9-401b-b49b-4c10730f2e3f/US20090240063A1-20090924-C00002.png)
![Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ... Process For The Preparation Of 6,6-Dimethyl-3-Azabicyclo-[3.1.0]-Hexane Compounds ...](https://images-eureka.patsnap.com/patent_img/7e6ad494-c2a9-401b-b49b-4c10730f2e3f/US20090240063A1-20090924-C00003.png)
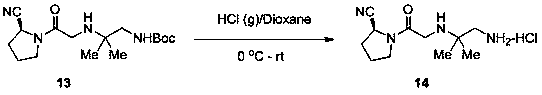
![Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine](https://images-eureka.patsnap.com/patent_img/f9d04bb3-ee16-4c50-a3dd-18ac6fa044c3/BDA00003068755500031.PNG)
![Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine Synthesizing method of 2-amino-5,8-dimethoxy[1,2,4]-triazolo[1,5-c]-pyrimidine](https://images-eureka.patsnap.com/patent_img/f9d04bb3-ee16-4c50-a3dd-18ac6fa044c3/BDA00003068755500071.PNG)