Preparation method of biphenyl alanine derivative
A technology for biphenylalanine and derivatives, which is applied in the field of preparation of biphenylalanine derivatives, can solve the problems of low atom utilization, high price, and high cost, and achieve low requirements for equipment and low price in the process , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
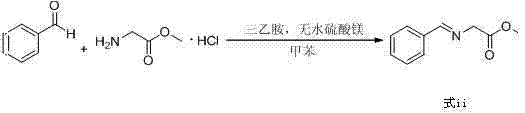
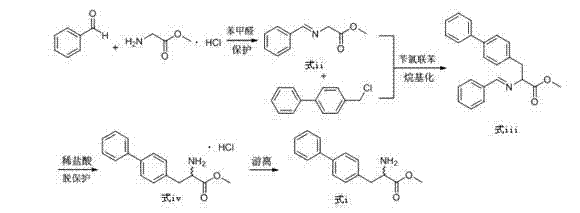
[0042] Toluene (300mL), glycine methyl ester hydrochloride (50g, 0.40mol), triethylamine (44.5g, 0.44mol), anhydrous magnesium sulfate (48.1g, 0.40mol), benzaldehyde (38.2g, 0.36mol ) into a 1000mL three-neck flask in turn, react at 30°C for 2h, filter out insoluble matter, add 300mL of water to the organic layer, stir for 10min, and separate the layers to obtain a toluene solution of compound (formula iii).
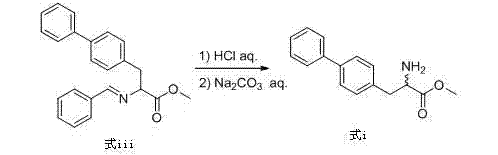
[0043] Add potassium hydroxide (44.9g, 0.8mol) and 4-chloromethylbiphenyl (77.04g, 0.38mol) to the toluene solution of compound (formula iii), react at 30°C for 1h, add water 300mL, stir for 10min, Separate the layers, add 200mL 6M dilute hydrochloric acid to several layers, stir at room temperature for 1h, separate the layers, and wash the aqueous layer with saturated Na 2 CO 3 The pH of the solution was adjusted to 7~8, a large amount of white solid was precipitated, and 79.6 g of biphenylalanine methyl ester (formula i) was obtained by filtration, with a purity (HPLC...
Embodiment 2
[0045] Toluene (300mL), glycine methyl ester hydrochloride (50g, 0.40mol), triethylamine (44.5g, 0.44mol), anhydrous magnesium sulfate (48.1g, 0.40mol), benzaldehyde (40.32g, 0.38mol ) into a 1000mL three-neck flask in turn, react at 30°C for 4h, filter out insoluble matter, add 300mL of water to the organic layer, stir for 10min, and separate the layers to obtain a toluene solution of compound (formula iii).
[0046] Add potassium hydroxide (44.9g, 0.8mol) and 4-chloromethylbiphenyl (81.1g, 0.4mol) to the toluene solution of compound (formula iii), react at 30°C for 1h, add water 300mL, stir for 10min, Separate the layers, add 200mL 6M dilute hydrochloric acid to several layers, stir at room temperature for 1h, separate the layers, and wash the aqueous layer with saturated Na 2 CO 3 The pH of the solution was adjusted to 7~8, a large amount of white solid was precipitated, and 83.8 g of biphenylalanine methyl ester (formula i) was obtained by filtration, with a purity (HPLC)...
Embodiment 3
[0048] Toluene (300mL), glycine methyl ester hydrochloride (50g, 0.40mol), triethylamine (44.5g, 0.44mol), anhydrous magnesium sulfate (48.1g, 0.40mol), benzaldehyde (46.7g, 0.44mol ) into a 1000mL three-neck flask in turn, react at 30°C for 6h, filter out insoluble matter, add 300mL of water to the organic layer, stir for 10min, and separate the layers to obtain a toluene solution of compound (formula iii).
[0049] Add potassium hydroxide (44.9g, 0.8mol) and 4-chloromethylbiphenyl (89.21g, 0.44mol) to the toluene solution of compound (formula iii), react at 35°C for 1h, add water 300mL, stir for 10min, Separate the layers, add 200mL 6M dilute hydrochloric acid to several layers, stir at room temperature for 1h, separate the layers, and wash the aqueous layer with saturated Na 2 CO 3 The pH of the solution was adjusted to 7~8, a large amount of white solid was precipitated, and 84.0 g of biphenylalanine methyl ester (formula i) was obtained by filtration, with a purity (HPLC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com