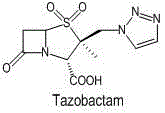
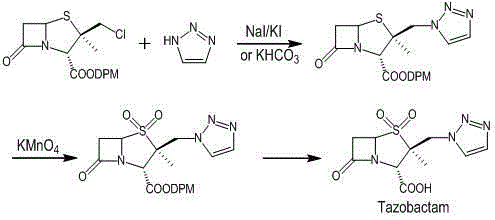
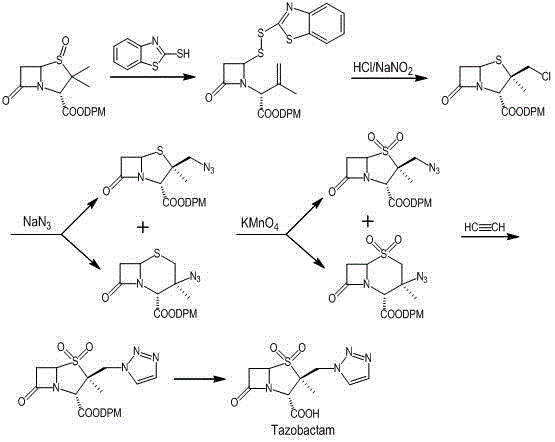
Preparation method for tazobactam
A tazobactam and triazole-based technology, which is applied in the field of preparation of tazobactam, can solve the problems of high probability of six-membered ring by-products, restrictions on industrial scale production, difficulty in nucleophilic substitution, etc., and achieve process stability , easy operation and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0040] Example 1: Preparation of 3-methyl-[2-oxo-4-(2-benzothiazoledithio)-1-azetidinyl]-3-butene diphenylmethyl ester (compound 3)
[0041] Using diphenylmethyl penicillanic acid sulfoxide (compound 2) as the raw material, referring to the preparation method in the literature (Synthesis, 2005, 3, 442-446), crystallized with acetone to obtain a light yellow solid powder with a yield of 95%.
example 2
[0042]Example 2: Preparation of 2β-bromomethyl-2α-methyl-penicillanic acid benzhydryl ester (compound 4)
[0043] The solid matter (compound 3) 26g (0.05mol) that obtains in the example 1 is dissolved in the 300mL methylene chloride, is cooled to below 0 ℃, adds 33.5g (0.075mol) copper bromide anhydrous, after adding, in 0 ~ Stir and react at 5°C for 10-12 hours, take a sample TLC to detect the disappearance of the raw material point, filter, rinse the filter cake with 50mL dichloromethane, and wash the filtrate with 200mL water, 200mL saturated sodium bicarbonate, and 200mL water respectively to obtain 2β-bromomethyl The dichloromethane solution of 2α-methyl-penicillanic acid diphenylmethyl ester (compound 4) was directly used in the next reaction.
example 3
[0044] Example 3: Preparation of 2β-bromomethyl-2α-methyl-penicillanic acid benzhydryl ester-1β-oxide (compound 5)
[0045] Add 30 mL of methanol to the 2β-bromomethyl-2α-methyl-penicillanic acid diphenylmethyl ester (compound 4) dichloromethane solution obtained in Example 2, cool down to below -5°C, add dropwise 30 mL of 50% hydrogen peroxide / The sodium tungstate mixture was dropped in about 30 minutes, and the temperature was controlled at 0~5°C for 4 hours, and then the temperature was raised to 10~15°C for 4~6 hours, and the raw material (compound 4) disappeared by sampling TLC. Add 200mL water, stirred for 5 minutes, allowed to stand and separate layers, and the dichloromethane liquid layer was washed with 200mL 5% sodium bicarbonate aqueous solution to obtain 2β-bromomethyl-2α-methyl-penicillanic acid diphenylmethyl ester- The dichloromethane solution of 1β-oxide (compound 5) was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com