Synthesis method of cediranib
A synthesis method and technology of cediranib, applied in the field of medicinal chemistry synthesis, can solve the problems of carbonization of products, difficult purification of products, large solid residues and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
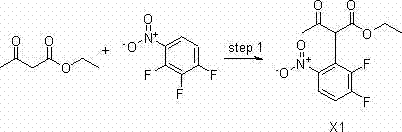
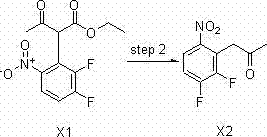
[0086] 1) Chemical synthesis of ethyl 2-(2,3-difluoro-6-nitrophenyl)-3-acetoacetate (X1)
[0087] Dissolve 68g (1mol) of sodium ethoxide in 300ml of anhydrous tetrahydrofuran, stir mechanically and cool down to 10°C, add 130g (1mol) of ethyl acetoacetate, dropwise complete within about 1 hour, and control the temperature below 35°C. Dissolve 88.5 g (0.5 mol) of 1,2,3-trifluoro-4-nitrobenzene in 200 ml THF, and add dropwise into the above reaction solution under ice-cooling conditions. The reaction was naturally raised to room temperature and stirred for 5 h, and the completion of the reaction was monitored by TLC.
[0088] Pour 500ml of 1N hydrochloric acid aqueous solution to quench, extract with ethyl acetate (1L*3), combine the organic phases, wash the organic phase with saturated brine, spin dry to obtain 241g of product, yield: 84%.
[0089] The structure of the product was confirmed by NMR:
[0090] 1 H NMR Spectrum (CDCl 3 ): δppm 13.21(s,1H),7.87(m,1H),7.30(t,1H),4.2...
Embodiment 2
[0146] As described in Example 1, the difference is that sodium hydride is used in step (1), the molar ratio of the reaction materials is the same, and 125 g of the product is obtained by the reaction, and the yield is 44%.
Embodiment 3
[0148] As described in Example 1, the difference is that potassium tert-butoxide is used in step (1), the molar ratio of the reaction materials is the same, and 153 g of the product is obtained after the reaction, and the yield is 54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com