Synthesis method of cediranib
A synthetic method, the technology of cediranib, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of difficult product purification, large solid residue, and difficult post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
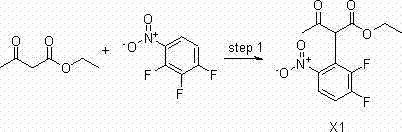
[0086] 1) Chemical synthesis of 2-(2,3-difluoro-6-nitrophenyl)-3-acetoacetate (X1)
[0087] 68 g (1 mol) of sodium ethoxide was dissolved in 300 ml of anhydrous tetrahydrofuran, cooled to 10 °C with mechanical stirring, 130 g (1 mol) of ethyl acetoacetate was added, and the addition was completed in about 1 hour, and the temperature was controlled below 35 °C. 88.5 g (0.5 mol) of 1,2,3-trifluoro-4-nitrobenzene was dissolved in 200 ml of THF, and added dropwise into the above reaction solution under ice bath conditions. The reaction was naturally raised to room temperature and stirred for 5 h, and the reaction was completed by TLC monitoring.
[0088] Pour into 500ml of 1N aqueous hydrochloric acid for quenching, extract with ethyl acetate (1L*3), combine the organic phases, wash the organic phases with saturated brine, spin dry to obtain 241g of product, yield: 84%.
[0089] The structure of the product was confirmed by NMR:
[0090] 1 H NMR Spectrum (CDCl 3 ): δppm 13.21(s,...
Embodiment 2
[0146] As described in Example 1, the difference is that sodium hydride is used in step (1), the molar ratio of the reaction materials is the same, and 125 g of the product is obtained by the reaction, and the yield is 44%.
Embodiment 3
[0148] As described in Example 1, the difference is that potassium tert-butoxide is used in step (1), the molar ratio of the reaction materials is the same, and 153 g of the product is obtained after the reaction, and the yield is 54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com