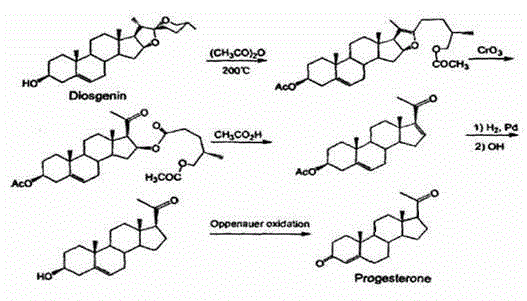
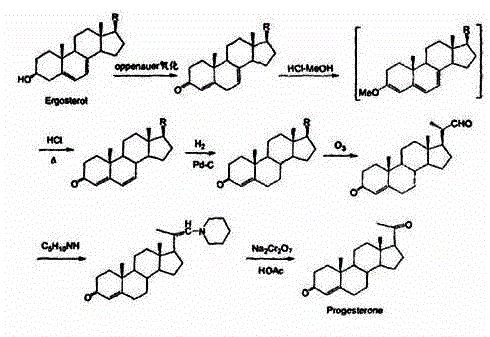
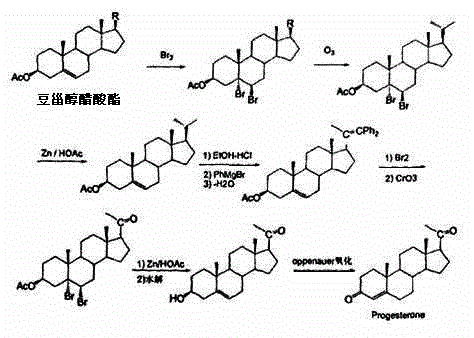
Method for preparing progesterone by taking 1,4-androstenedione as raw material
A technology of androstenedione and progesterone, which is applied in the field of preparation of progesterone, can solve the problems of large environmental pollution and restrictions, achieve low prices, low manufacturing costs, and solve the effects of resource scarcity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1) Add 10g of 1,4-androstenedione (4-AD), 10mL of absolute ethanol, 10mL of triethyl orthoformate, and 0.1g of p-toluenesulfonic acid into a dry three-necked flask, and stir , pass nitrogen protection, heat up to 40 o C, and keep warm for 3 hours; Then, neutralize the reaction solution with absolute ethanol containing pyridine, adjust the pH to 7.0, and cool to 0 o C, suction filtration; then wash the crystals with absolute ethanol containing pyridine, and dry to constant weight to obtain the enol ether of 4-AD, namely 3-ethoxy-androst 3,5-dien-20-one 10.5 g;
[0041] 2) Put 18.0g of (1-methoxyethyl)-triphenylphosphine tetrafluoroborate into another dry three-necked flask, then add 100mL of anhydrous tetrahydrofuran into it, and pass through nitrogen protection, Cool down to -50 o C, add 4.0g potassium tert-butoxide in batches to the system, and stir at this temperature for 2 hours; then, add enol ether 3-ethoxyl-androster 3, which is dissolved with 10.5g 4-AD, into ...
Embodiment 2
[0043] 1) Add 10g of 4-AD, 10mL of anhydrous methanol, 10mL of trimethyl orthoformate, and 0.05g of concentrated sulfuric acid into a dry three-necked flask in turn, stir, pass through nitrogen protection, and stir at 25°C for 3 hours; Then, neutralize with absolute ethanol containing pyridine, adjust the pH to 7.0, cool to -10 o C, suction filtration; then wash the crystals with absolute ethanol containing pyridine, and dry to constant weight to obtain 10.1 g of enol ether 3-ethoxy-androst 3,5-dien-20-one of 4-AD;
[0044] 2) Put 18.0g of (1-methoxyethyl)-triphenylphosphine tetrafluoroborate into another dry three-necked flask, then add 100mL of anhydrous tetrahydrofuran into it, pass through nitrogen protection, and cool down to -80 o C, dropwise add n-butyllithium solution containing 44 mmol to the system, and stir at this temperature for 1 hour; then slowly raise the temperature of the system to -40 o C, and add 10.5g of 4-AD enol ether 3-ethoxy-androst 3,5-dien-20-one d...
Embodiment 3
[0046] 1) Add 20g of 1,4-androstenedione (4-AD), 20mL of anhydrous tetrahydrofuran, 20mL of triethyl orthoformate, and 0.2g of p-toluenesulfonic acid into a dry three-necked flask, and stir , pass nitrogen protection, heat up to 50 o C, and keep warm for 3 hours; Then, neutralize the reaction solution with absolute ethanol containing pyridine, adjust the pH to 7.0, and cool to 0 o C, suction filtration; then wash the crystals with absolute ethanol containing pyridine, and dry to constant weight to obtain the enol ether of 4-AD, that is, 3-ethoxy-androst 3,5-dien-20-one 21.8 g;
[0047] 2) Put 36.0g of (1-methoxyethyl)-triphenylphosphine tetrafluoroborate into another dry three-necked flask, then add 200mL of anhydrous tetrahydrofuran into it, pass through nitrogen protection, and cool down to -50 o C. Add 88 mmol of sodium methoxide solution in batches to the system, and stir at this temperature for 2 hours; then, add 21.8 g of 4-AD enol ether 3-ethoxy-androster 3,5 - 40 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com