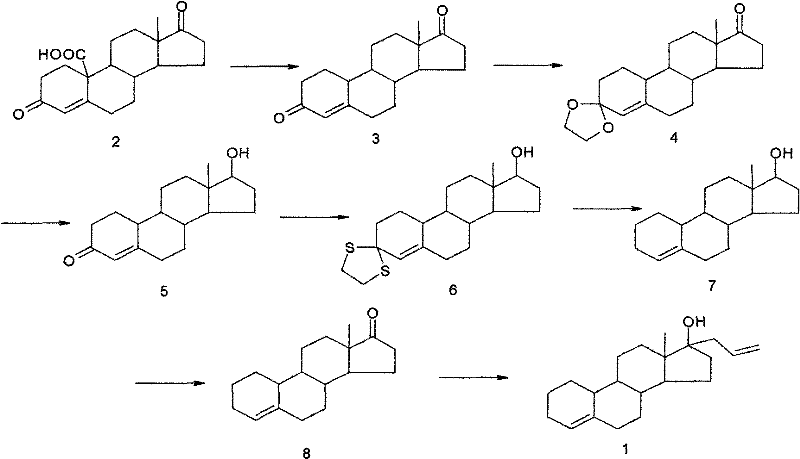
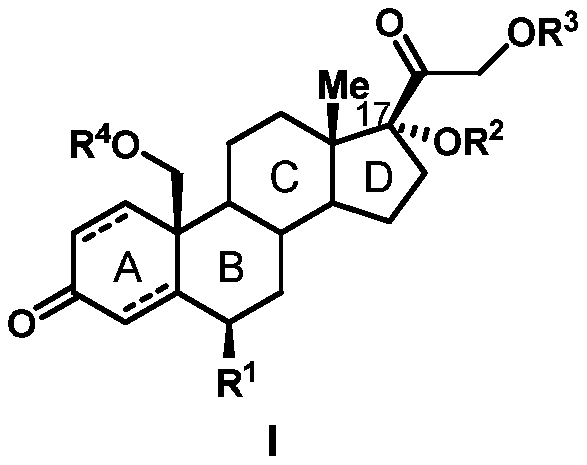
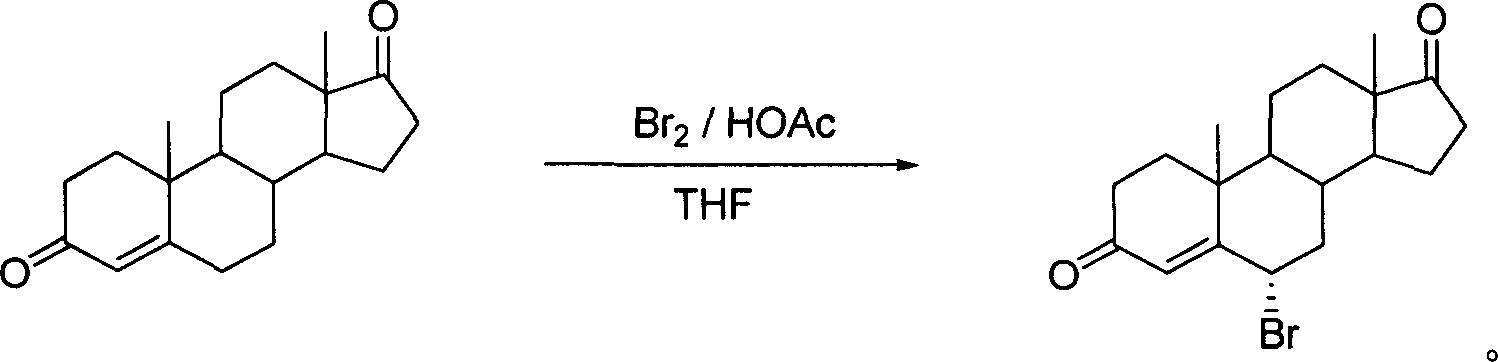Patents
Literature
36 results about "Enediynone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Process for fermentation of phytosterols to androstadienedione
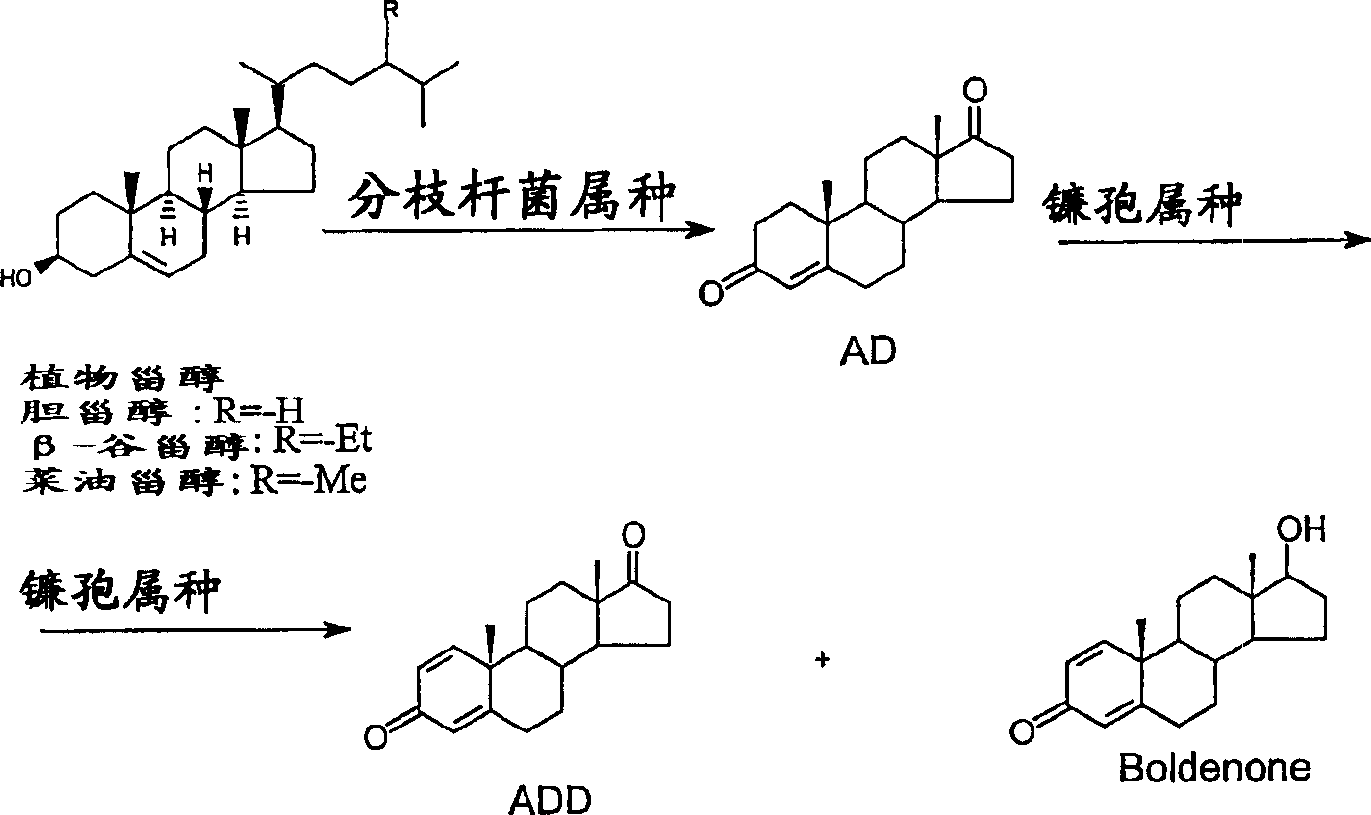
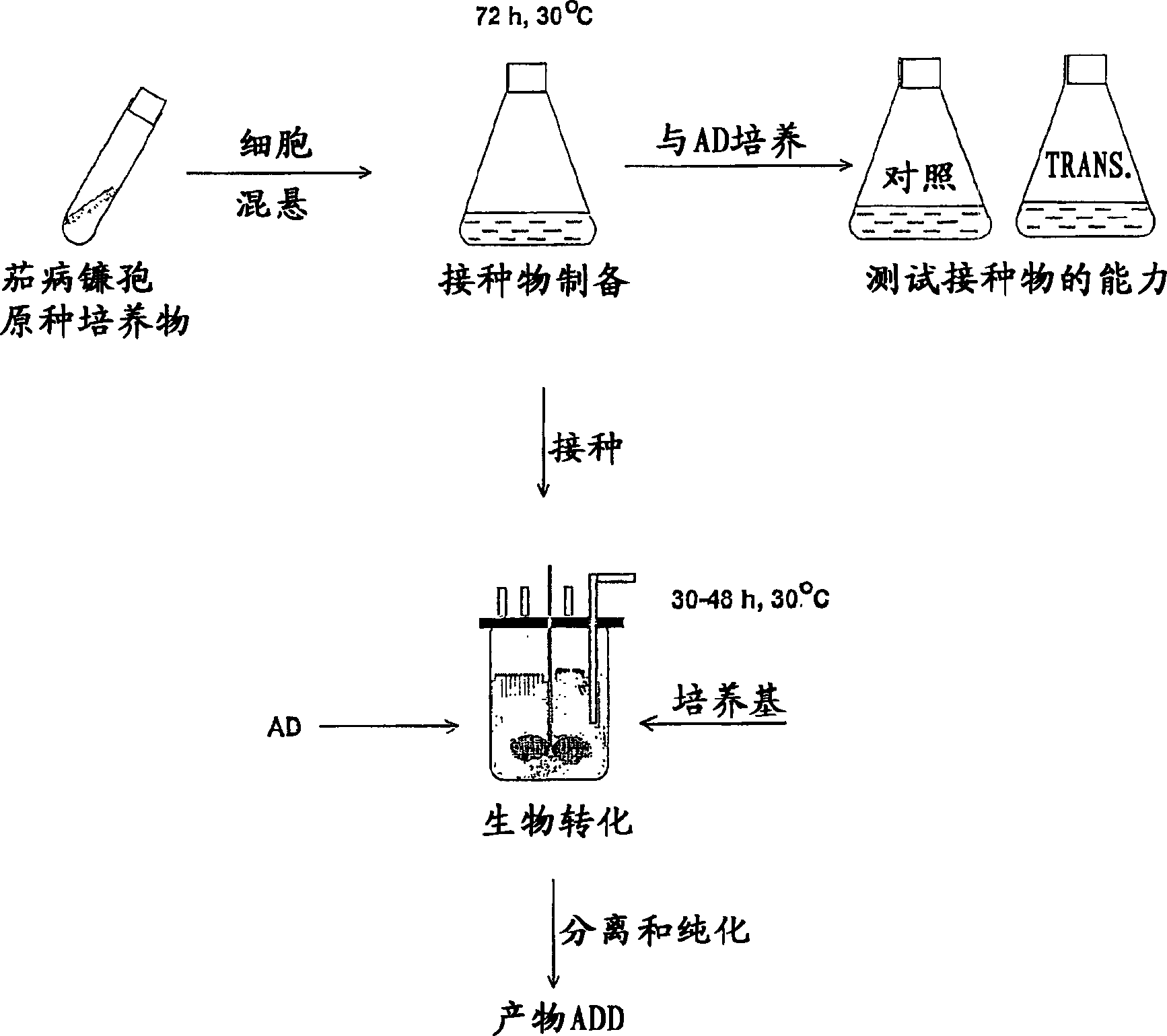
A process of fermenting a phytosterol composition to produce androstenedione (androst-4-ene-3,17-dione) and subsequently androstadienedione (and rosta-1,4-diene,3,17-dione) comprises propagating a microbial culture of the genus Mycobacterium in a first nutrient medium, placing the microbial culture and the phytosterol composition in a bioreactor for a sufficient time to transform the composition substantially to androstenedione (the "AD solution"), propagating a fungal culture of the genus Fusarium in a second nutrient medium, and then injecting one or more of 1) Fusarium sp, or 2) the fungal culture medium into the bioreactor for a sufficient time to transform the AD solution to androstadienedione.
Owner:NV ORGANON
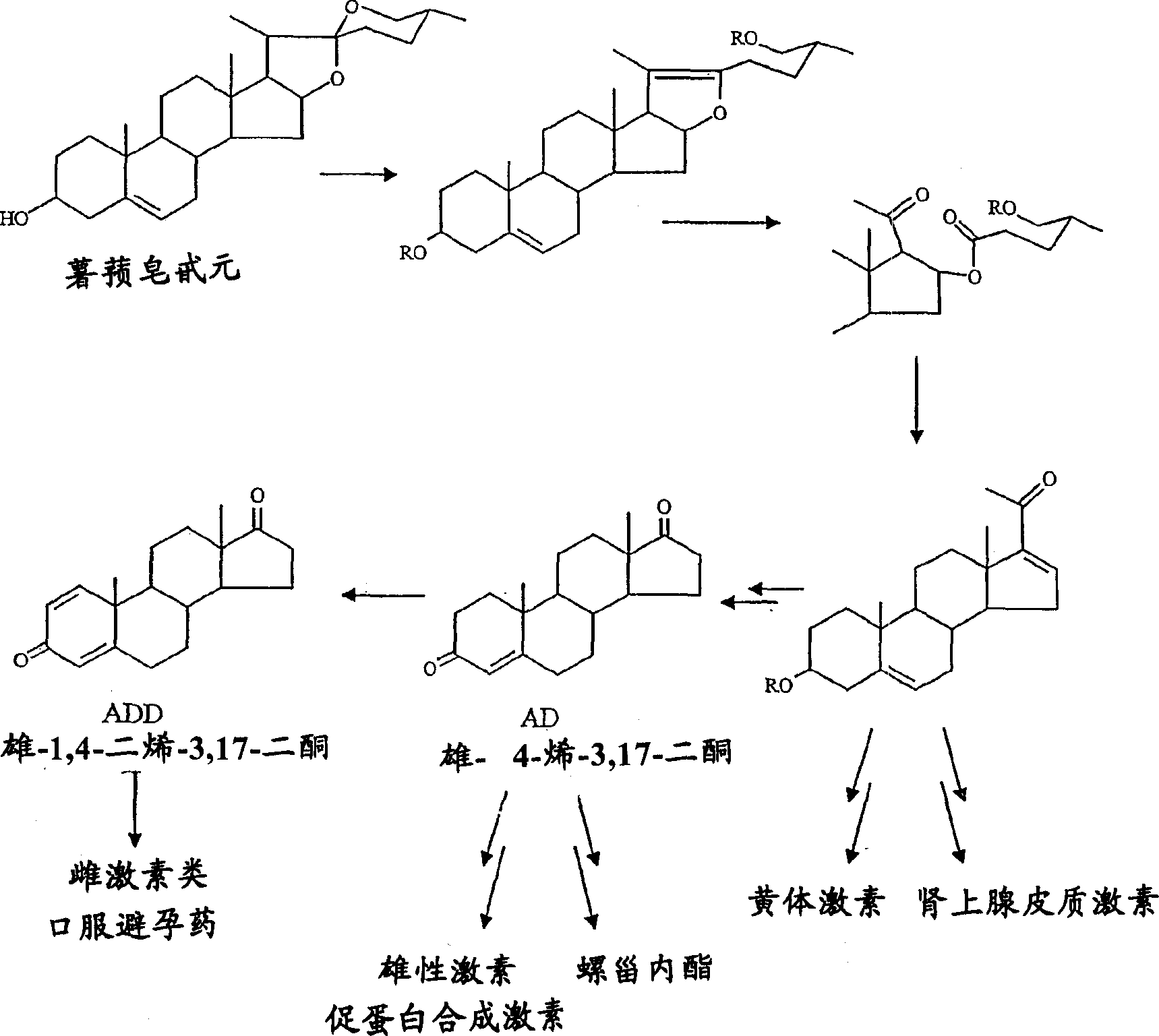
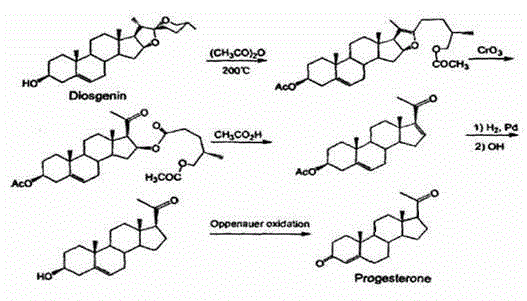
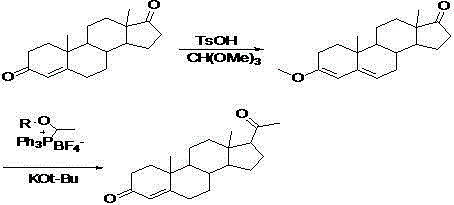
Method for preparing progesterone by taking 1,4-androstenedione as raw material
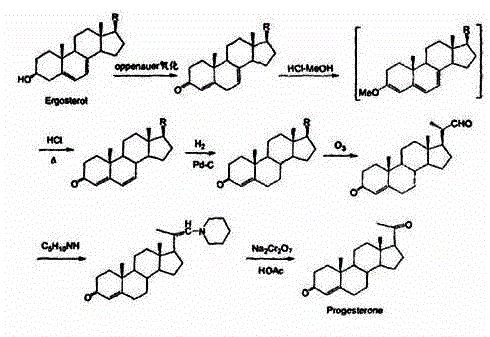
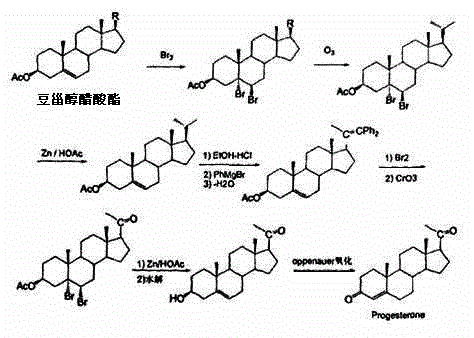
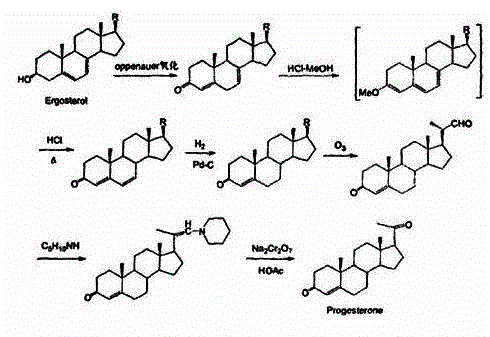
The invention discloses a method for preparing progesterone by taking 1,4-androstenedione as a raw material, which comprises the following steps: 1) dissolving 1,4-androstenedione into an organic solvent, adding the acid of trimethyl orthoformate or triethyl orthoformate, and introducing nitrogen to protect the 1,4-androstenedione to synthesize the enol ether of 1,4-androstenedione, namely 3-methoxy-androstane 3,5-diene-20-ketone; and 2) dispersing (1-methoxy ethyl)-triphenylphosphine salt in a reaction medium, an organic solvent, adding alkali at low temperature, performing a Wittig reaction of the 3-methoxy-androstane 3,5-diene-20-ketone synthesized in the step 1), and purifying and crystallizing to obtain progesterone. By adopting the 1,4-androstenedione as the raw material, the method solves the problem that of lack in raw materials for synthesizing steroid drugs such as progesterone, and improves the utilization rate of 1,4-androstenedione and the yield of progesterone; the preparation process is simple.
Owner:HUNAN KEYUAN BIO PRODS
Preparation method of important intermediate of cortisone acetate
Owner:JIANGSU YUANDA XIANLE PHARMA
Compositions and methods for making androstenediones
The invention provides compositions and methods for producing androstenedione (4-androstenedione), of improved purity and for modulating its production, for example by deletion or inactivation of ksdA, cxgA, cxgB, cxgC, or cxgD. The invention also provides methods and compositions, including nucleic acids that encode enzymes, for producing 1,4-androstadiene-3,17-dione (ADD) and related pathway compounds, including 20-(hydroxymethyl) pregna-4-en-3-one and 20-(hydroxymethyl)pregna-1,4-dien-3-one. The compositions of the invention include nucleic acids, probes, vectors, cells, transgenic plants and seeds, transgenic animals, kits and arrays.
Owner:VERENIUM CORP (US)
Method and kit for detecting various related substance of CAH at same time
InactiveCN108088934AStrong specificityHigh sensitivityComponent separation11-DesoxycortisolChemistry
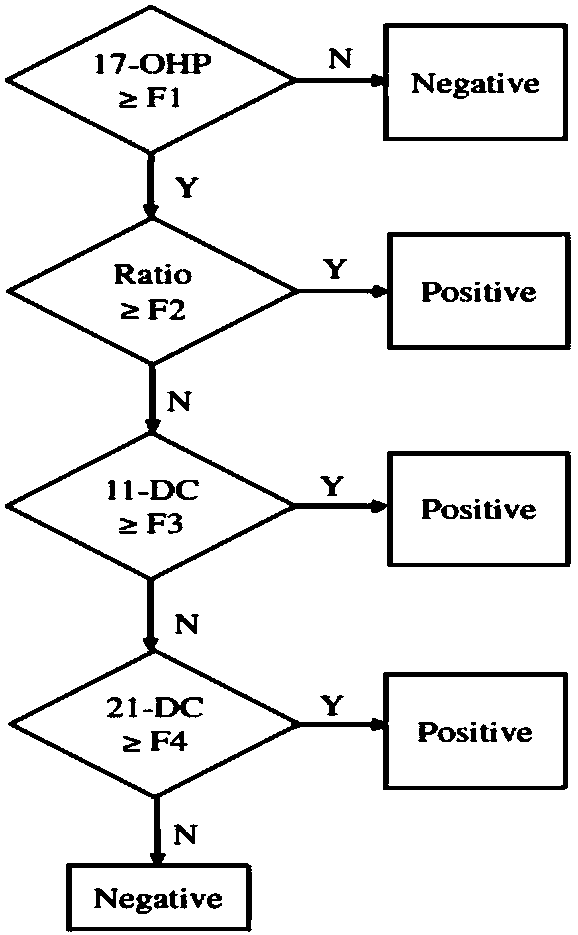
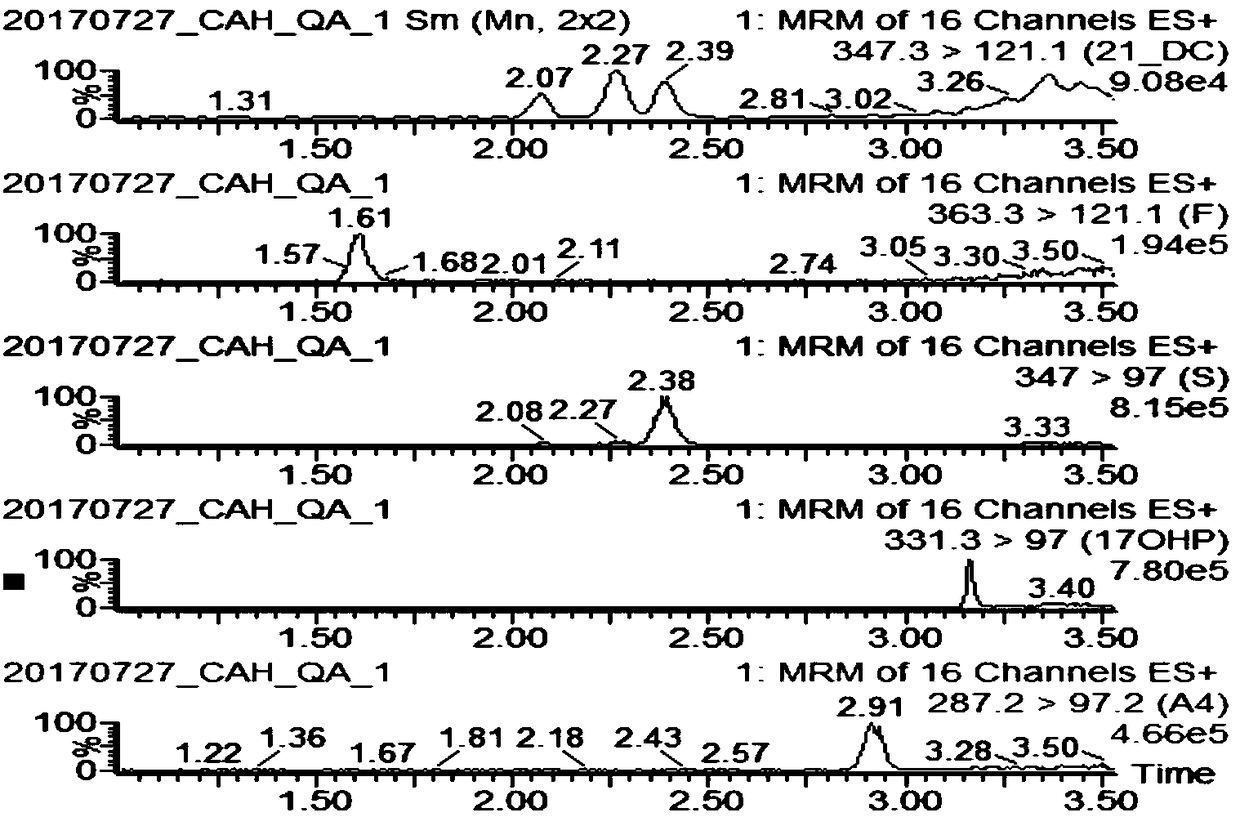
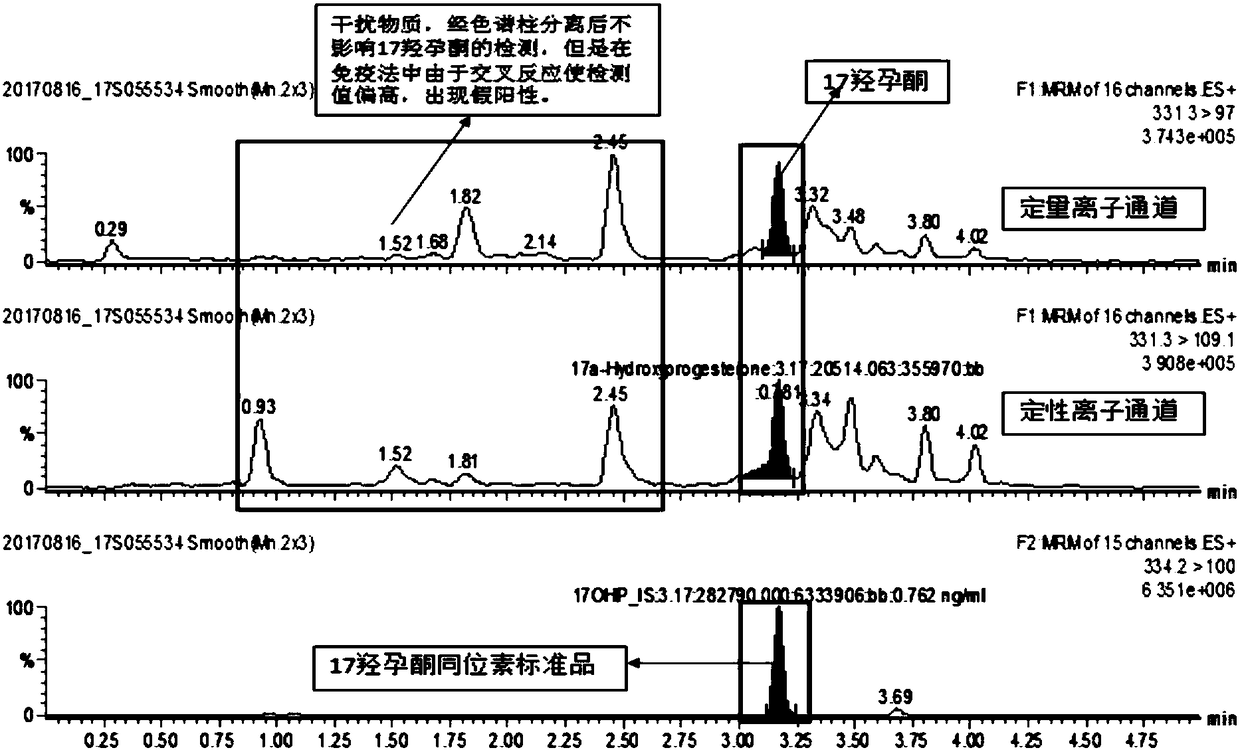
The invention discloses a method and a kit for detecting various related substance of CAH at the same time. The method is used for detecting 17 alpha progesterone, androstenedione, 11-deoxycortisol, 21-deoxycortisol and cortisol at the same time. The method includes: (1), adding an organic solvent into a mixed internal standard product to prepare a stock solution, and diluting to obtain extractionworking liquid; (2), using the extraction working liquid to incubate a to-be-detected blood spot sample; (3), centrifuging, taking supernate, concentrating, and using 45% methanol for re-dissolving;(4), using LC-MS / MS to detect a re-dissolving product, wherein the mixed internal standard product includes isotope internal standard products of 17 alpha progesterone, androstenedione, 11-deoxycortisol, 21-deoxycortisol and cortisol. The method can quantify five hormones in one time, can provide specific, sensitive, low-false positive and high-positive-prediction-value CAH screening and can screen CAH of 11-OHD and 21-OHD at the same time, thereby improving screening efficiency and quality.
Owner:SHENZHEN HUADA GENE INST
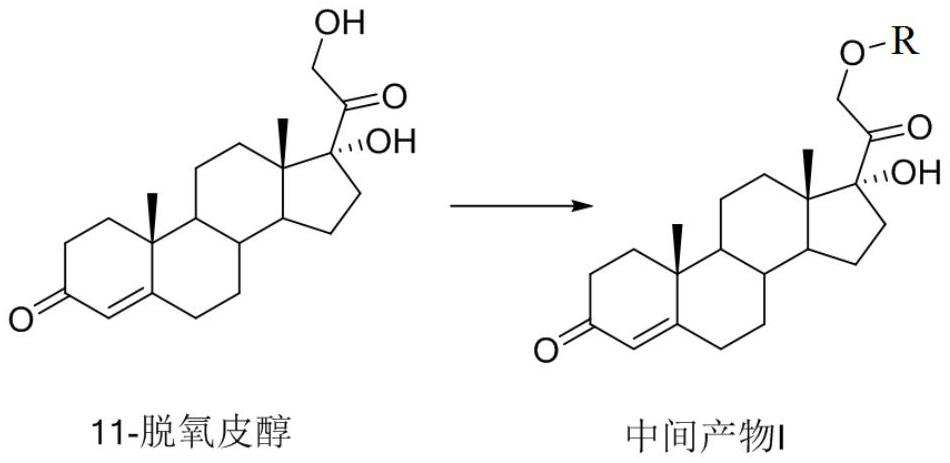
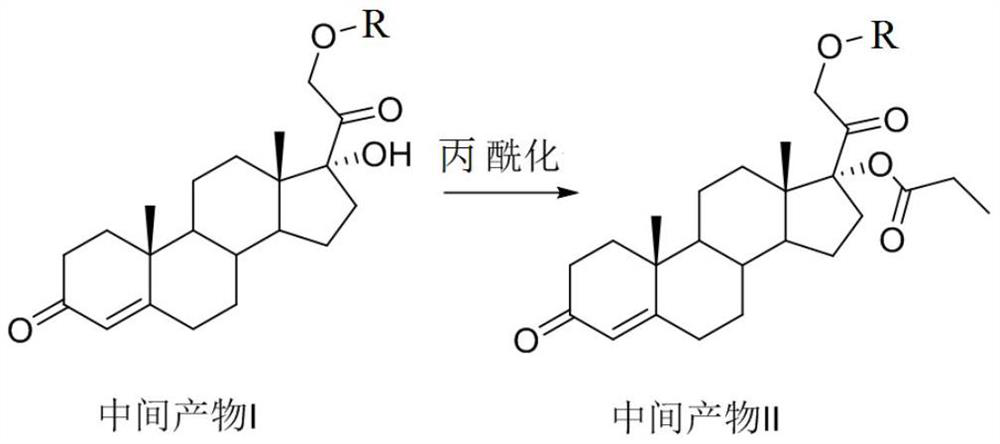
Method for synthesizing 21-hydroxy-17-(1-oxopropoxy)pregna-4-ene-3,20-dione
InactiveCN112028956AEasy to prepareEasy to synthesizeSteroidsBulk chemical productionChemical synthesisDiketone
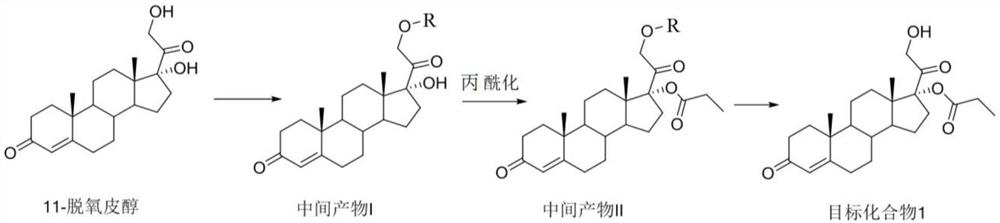
The invention relates to a method for synthesizing 21-hydroxy-17-(1-oxopropoxy)pregna-4-ene-3,20-dione, which comprises the steps of using 11-deoxypicanol as a raw material, selectively esterifying 17alpha-site secondary hydroxyl of 11-deoxypicanol by a chemical synthesis protective group means, and simultaneously retaining 21st-site primary hydroxyl of 11-deoxypicanol without esterification, thereby obtaining 21-hydroxy-17-(1-oxopropoxy)pregna-4-ene-3,20-dione. The method provided by the invention is a one-kettle reaction intermediate product without separation and purification, is more suitable for large-batch industrial preparation, and has the advantages of mild reaction conditions, high yield, simple post-treatment and the like.
Owner:那路新 +1
Preparation method of allylestrenol
The invention provides a preparation method of allylestrenol. The allylestrenol is prepared by a five-step reaction based on estr-4-ene-3,17-dione as a starting material. The method comprises the following steps: 1) carrying out dithioketal protection on 3-keto so as to obtain a compound (3); 2) carrying out dihydric alcohol ketal protection on 17-keto so as to obtain a compound (4); 3) removing 3-thioaketal through a Birch reduction reaction so as to obtain a compound (5); 4) hydrolyzing for 17-position deprotection so as to obtain a compound (6); and 5) carrying out Grignard reaction so as to obtain a target compound (1). Through a reaction route in the invention, the high-purity product is obtained in high yield and high selectivity; and operation is simple, synthesis route is short, and purification is convenient, thereby greatly reducing production cost. The method is suitable for industrial production.
Owner:北京市科益丰生物技术发展有限公司
3-sterone-1, 2-dehydrogenase as well as gene sequence and application thereof
ActiveCN111500600AImprove solubilityHas high enzymatic activityOxidoreductasesFermentationEscherichia coliAndrostane
The invention provides 3-sterone-1, 2-dehydrogenase as well as a gene sequence and application thereof. The invention discloses a gene sequence for expressing 3-sterone-1, 2-dehydrogenase, the gene sequence is shown as SEQ ID. 4, and the gene sequence is used for expression in escherichia coli. The 3-ketosterone-1, 2-dehydrogenase disclosed by the invention can be used for efficiently catalyzing androstane-4-ene-3, 17-dione (4-AD) reaction to generate androstane-1, 4-diene-3, 17-dione (ADD), and has high enzymatic activity. In addition, the soluble 3-sterone-1, 2-dehydrogenase is obtained, andthe solubility of the enzyme is improved.
Owner:武汉艾默佳华生物科技有限公司
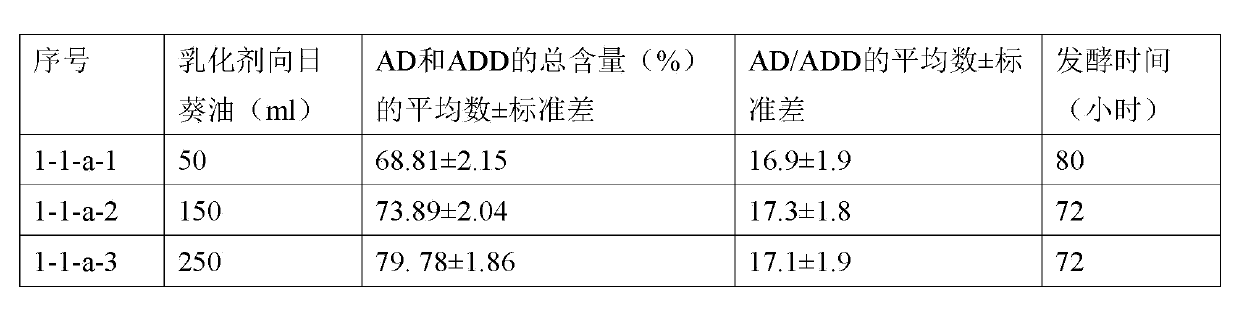
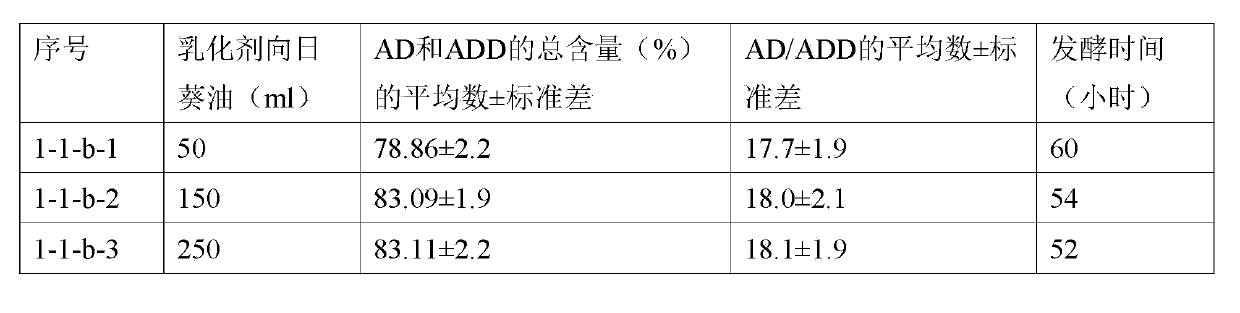
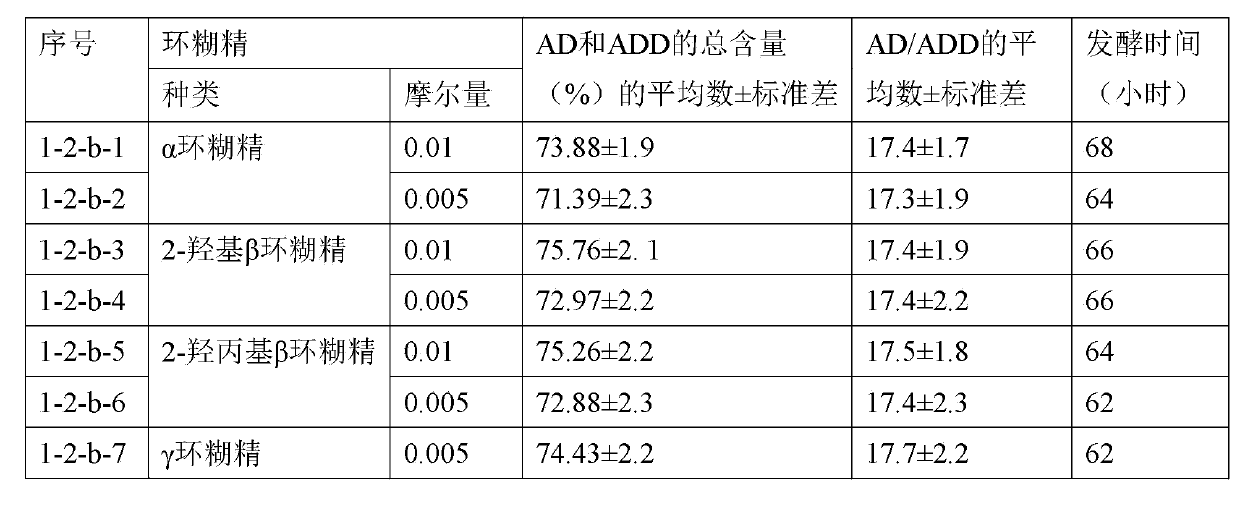
Method utilizing cyclodextrin to assist plant sterol composition to prepare androstane-4-ene-3,17-diketone
The invention relates to a method utilizing cyclodextrin to assist a plant sterol composition to prepare androstane-4-ene-3,17-diketone, and provides a preparation method utilizing nocardia or mycobacterium to ferment a plant sterol composition so as to obtain androstane-4-ene-3,17-diketone and / or androstane-1,4-diene-3,17-diketone, wherein cyclodextrin is added during the fermentation process, and the mole ratio of the cyclodextrin to the plant sterol composition is 1:10 to 1:2.
Owner:TIANJIN JINYAO GRP
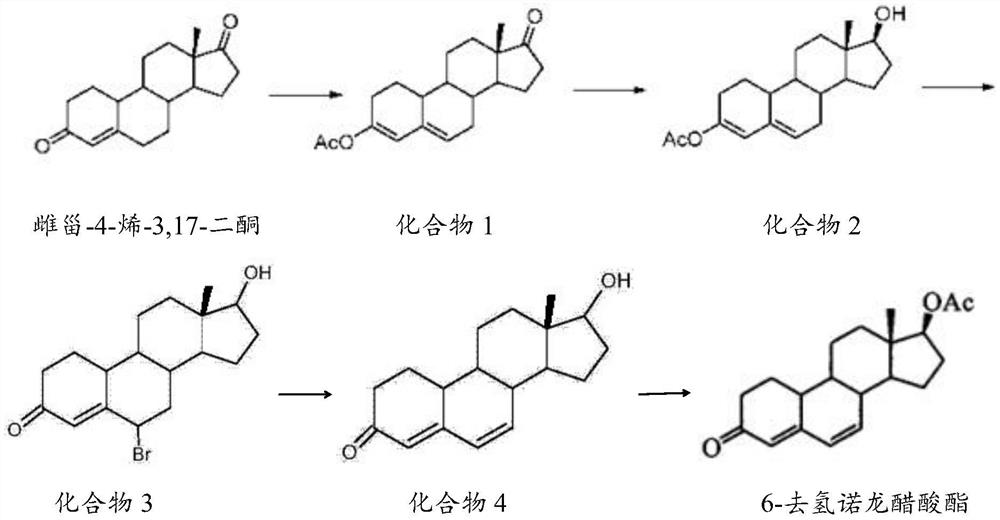
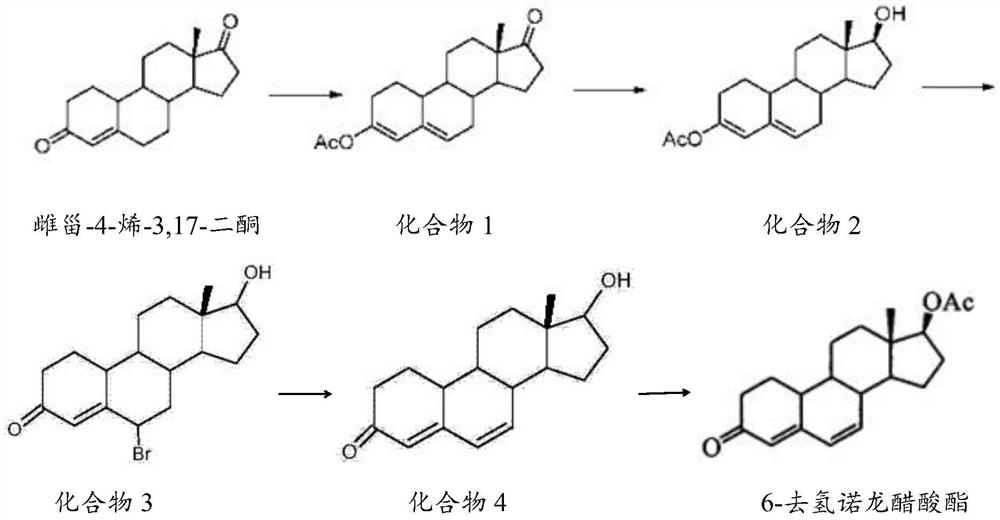
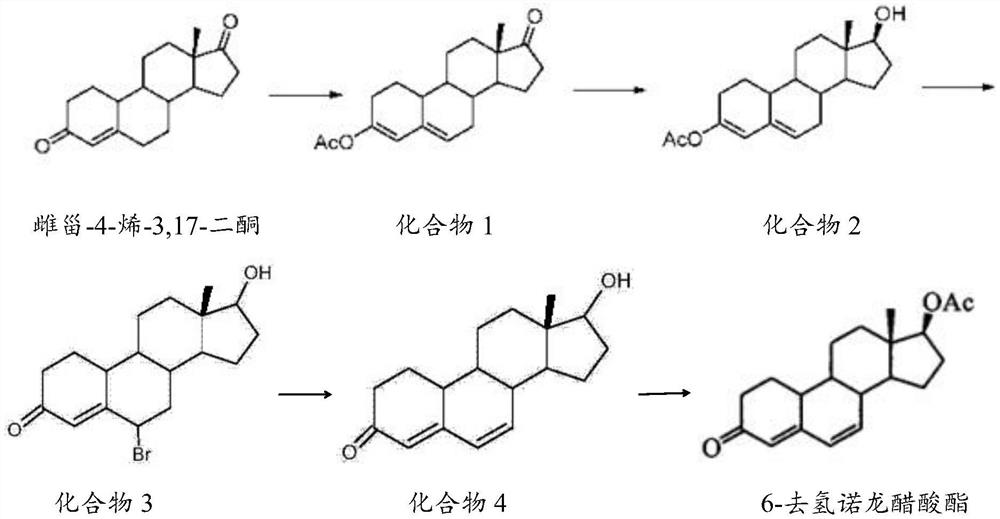
Preparation method of 6-dehydronandrolone acetate
The invention belongs to the technical field of steroid drug intermediates, and provides a preparation method of 6-dehydronandrolone acetate. The preparation method comprises the following steps: carrying out a catalytic reaction on estra-4-ene-3,17-dione, acetic anhydride and p-toluenesulfonic acid to obtain a compound 1; carrying out a reduction reaction on the compound 1, hydroboron and aluminum trichloride to obtain a compound 2; subjecting the compound 2 and N-bromosuccinimide to reacting with DMF to obtain a solution of a compound 3; carrying out an addition reaction on the solution of the compound 3 and alkali to obtain a compound 4; and subjecting the compound 4, acetic anhydride, triethylamine and dichloromethane to a catalytic reaction to obtain 6-dehydronandrolone acetate. By adding borohydride and the aluminum trichloride in a reasonable ratio, hydrolysis of a 3-site ester group is effectively avoided, and side reactions are few; and meanwhile, the yield and the purity of a target product are remarkably improved by reasonably setting a synthesis route and controlling a reaction temperature.
Owner:YICHENG GOTO PHARMA
Preparation method of important intermediate of cortisone acetate
Owner:JIANGSU YUANDA XIANLE PHARMA
Impurity of ciclesonide and preparation method thereof
The invention relates to a impurity compound ((11beta,16alpha)-16,17-[[(R)-cyclohexyl methylene]bis(oxy)]-11-hydroxy-21-(2-methyl-1-carbonyl propoxy)-1,5-cyclopregnane-3-ene-2,20-dione) of glucocorticoids drug ciclesonide, a preparation method thereof, and an application of the impurity compound as a reference in quality control of the ciclesonide.
Owner:CHONGQING PHARMA RES INST
3-sterone-1,2-dehydrogenase and application thereof
The invention provides 3-sterone-1,2-dehydrogenase and an application thereof. An amino acid sequence of the 3-sterone-1,2-dehydrogenase is as shown in SEQ ID.2. The KsdD211 of the invention can catalyze a reaction of androsta-4-ene-3,17-dione (4-AD) to produce androst-1,4-diene-3,17-dione (ADD, 1,4-androstenedione), and the TLC analysis verifies the high enzymatic activity of the KsdD211. The HPLC analysis of the KsdD211catalytic reaction shows that the KsdD211 completely converts 4-AD into ADD within 18 h.
Owner:HUBEI UNIV OF TECH
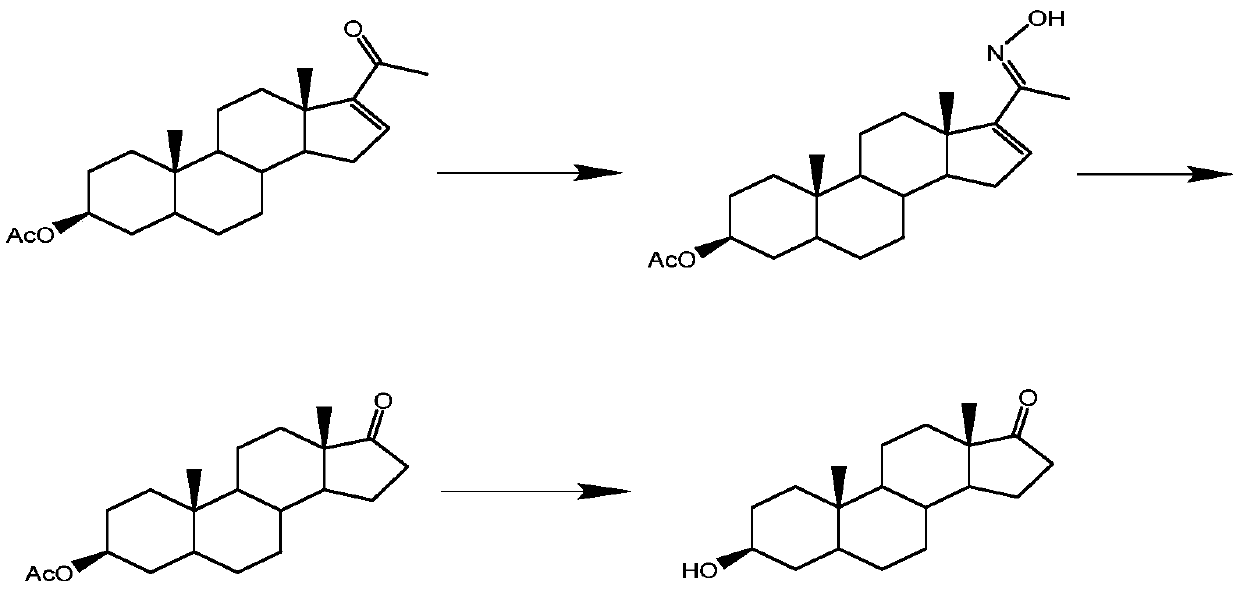
A chemical-enzymatic method for preparing dehydroepiandrosterone
The invention discloses a method for preparing dehydroepiandrosterone through a chemical-enzyme method. The method comprises that dehydroepiandrosterone is prepared from 4-androstenedione as an initial substrate orderly through a chemical method and a biological method. In preparation, the two reaction processes are optimized. In the chemical method-based 5-androstenedione preparation process, a reaction solution is added into an aqueous solution with sodium ascorbate and acetic acid so that reaction conditions are mild. In the second biological method-based dehydroepiandrosterone preparation process, a ketoreductase is used as a catalyst so that the product yield and purity are improved. In the whole reaction processes, use amounts of a coenzyme and potassium tert-butoxide are low and a high practical value is obtained.
Owner:ENZYMEWORKS
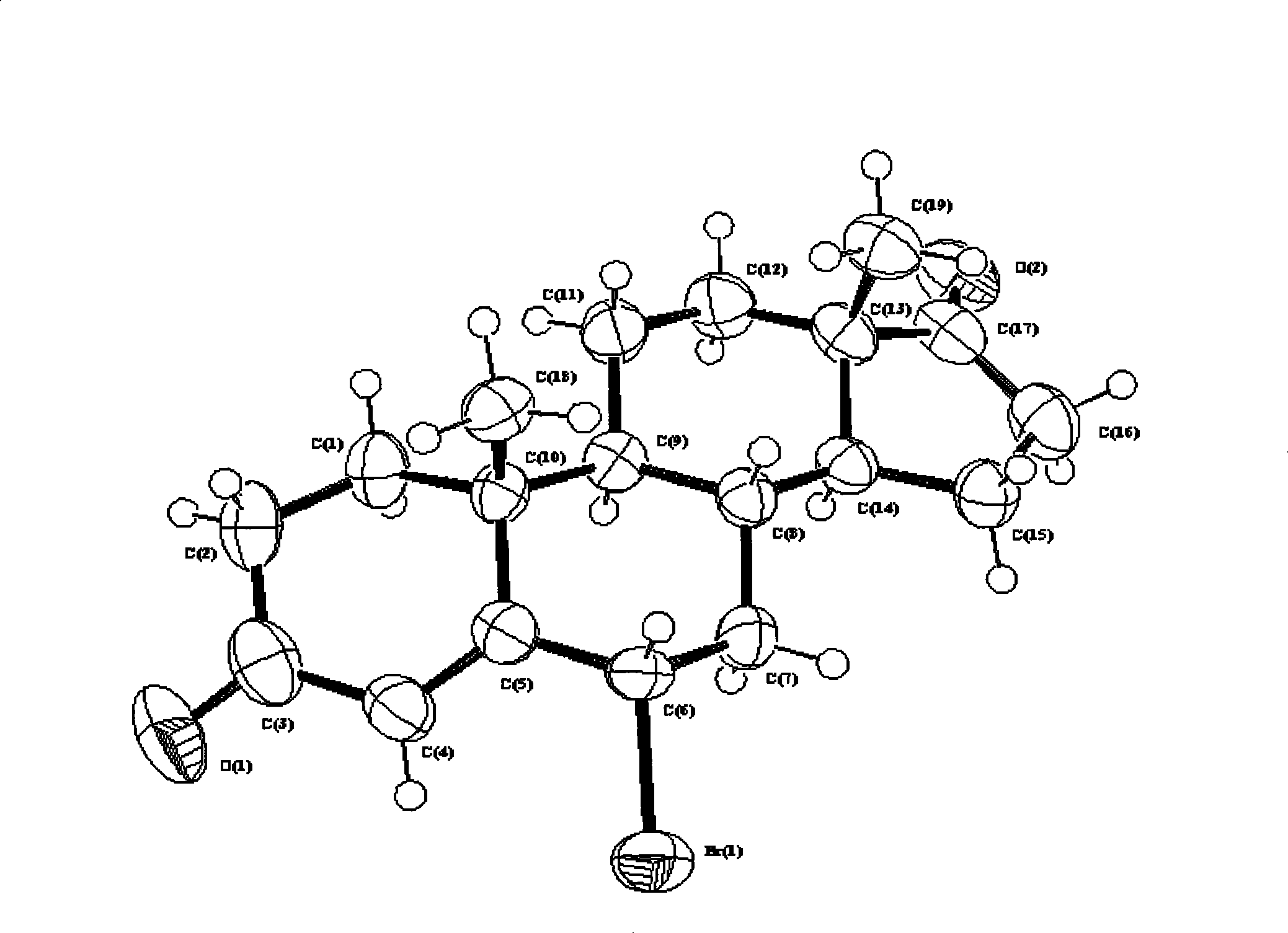
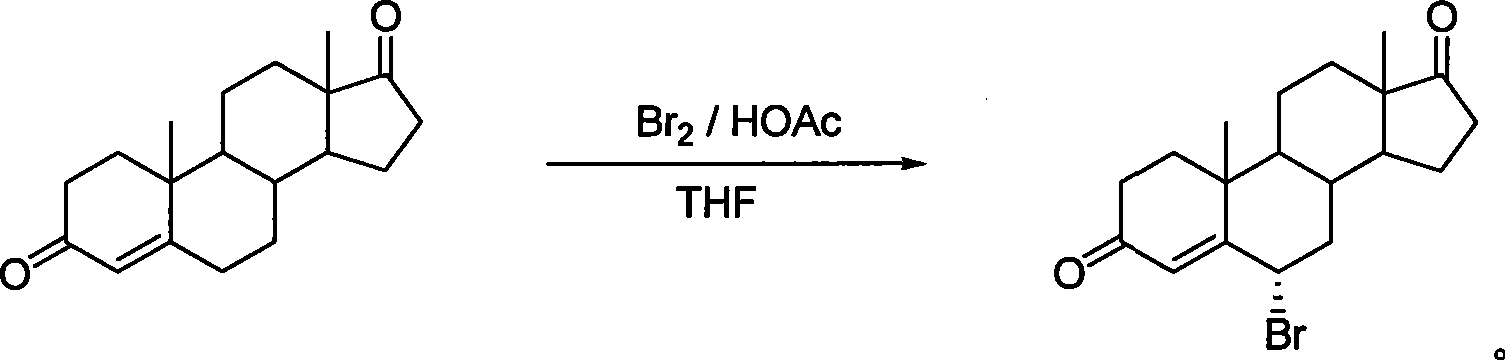
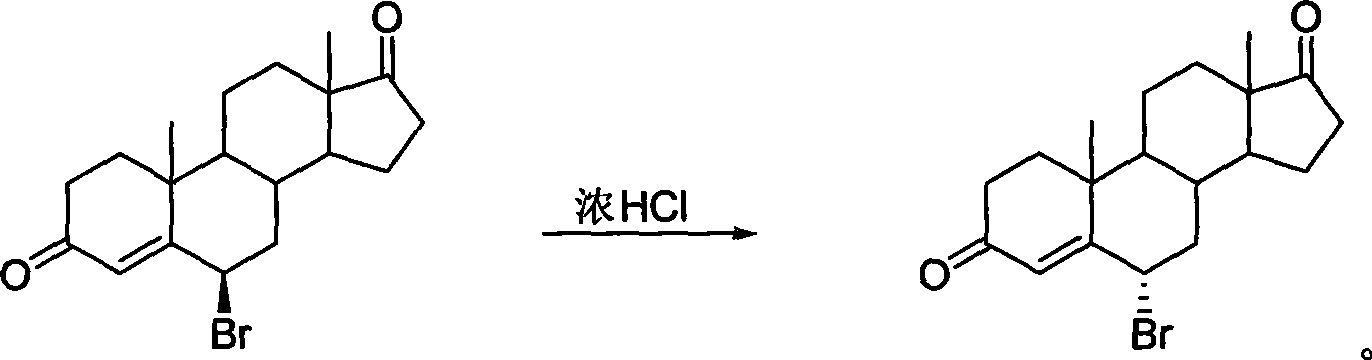
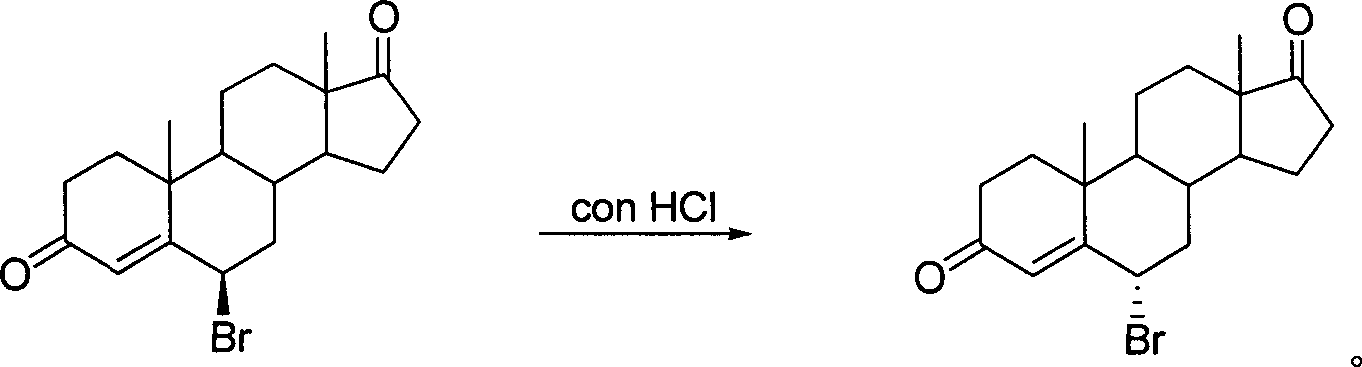
High-efficiency synthesis method of 6 alpha-bromo-carboxyandrost-4-ene-3,17-dione
InactiveCN101362788AHigh yieldMild and easy reaction conditionsSteroidsSynthesis methodsStrong acids
The invention pertains to chemical and medicine synthesis field and relates to an indirect synthesis method and a direct synthesis method of a compound 6 alpha -bromo-androstane-4-ene-3, 17-dione. The methods are: (1) the compound 6 beta-bromo-androstane-4-ene-3, 17-dione is directly transformed structurally by strong acid in aqueous medium, and a configuration pure 6 alpha-bromo-androstane-4-ene-3, 17-dione is obtained with a high yield; (2) androstane-4-ene-3, 17-dione is used as the material to synthesize 6 alpha -bromo-androstane-4-ene-3, 17-dione in a high yield with the condition of Br2 / HOAc / THF. The products prepared by both methods are identical; the spatial configuration is confirmed by X-diffraction, the synthesis methods of the invention have the advantages of moderate and convenient reaction conditions, cheap and available agents, convenient operation, good reproducibility and high yield. The methods of the invention can be used as a source of reference and be used in the preparation and synthesis of the 6 alpha-bromides of steroids with 4-ene- 3-one system.
Owner:FUDAN UNIV
Efficient preparation method of delta<9, 11>-canrenone
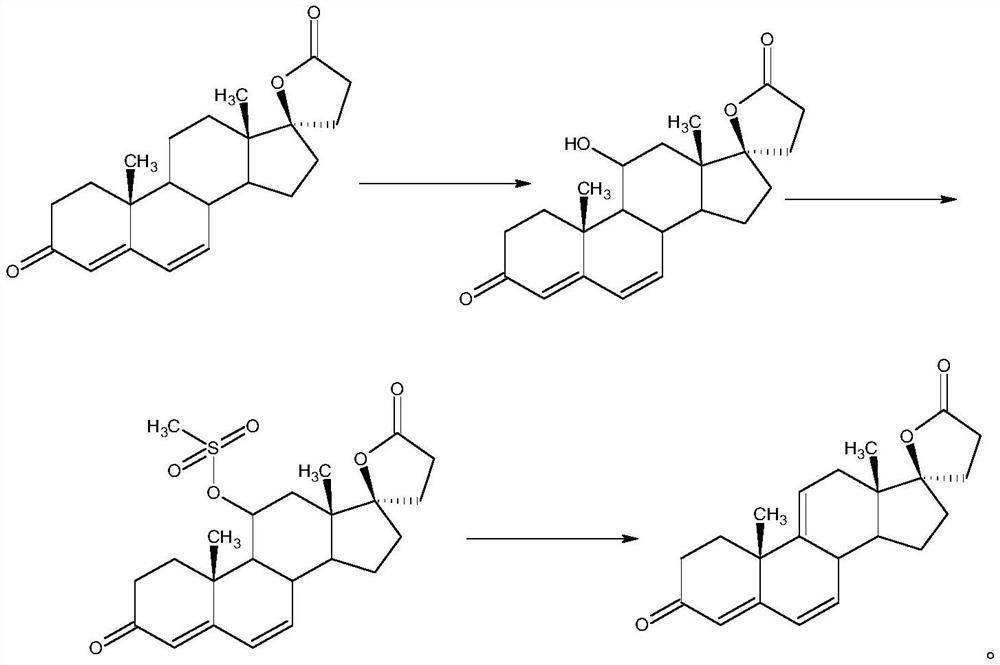
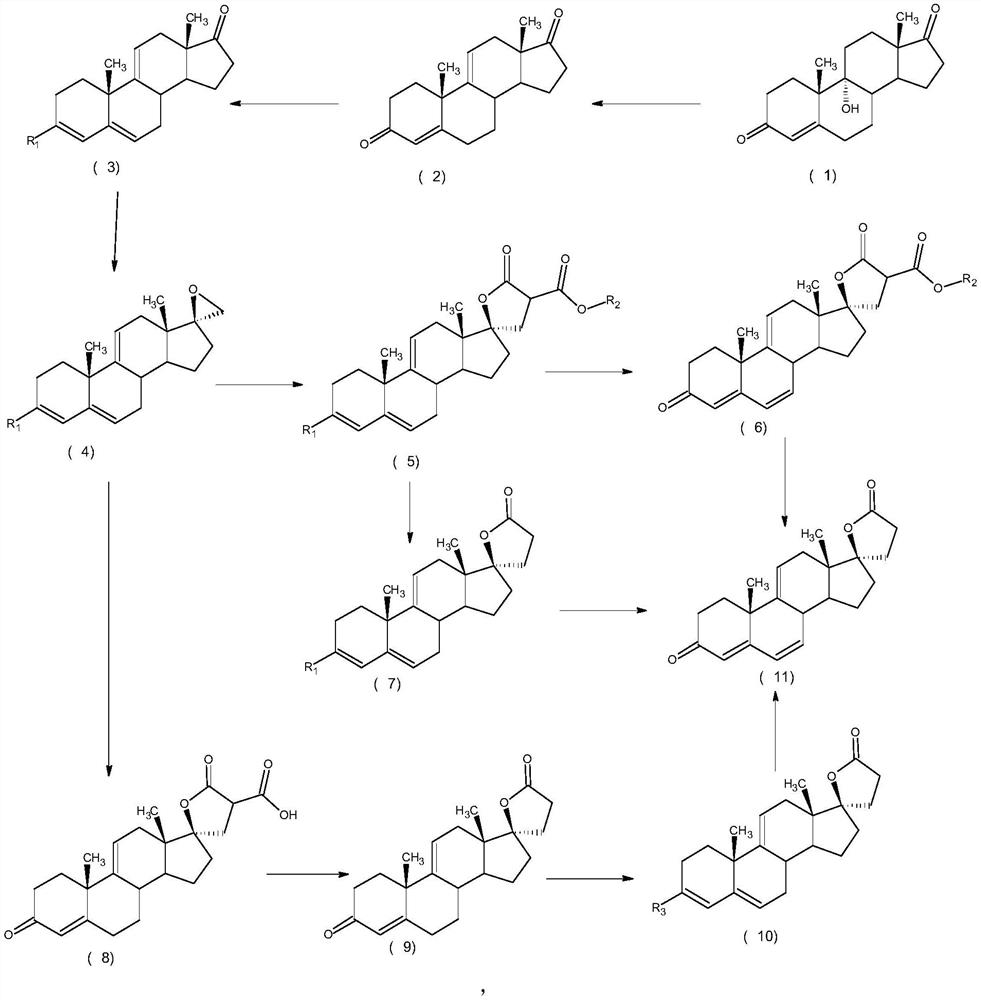
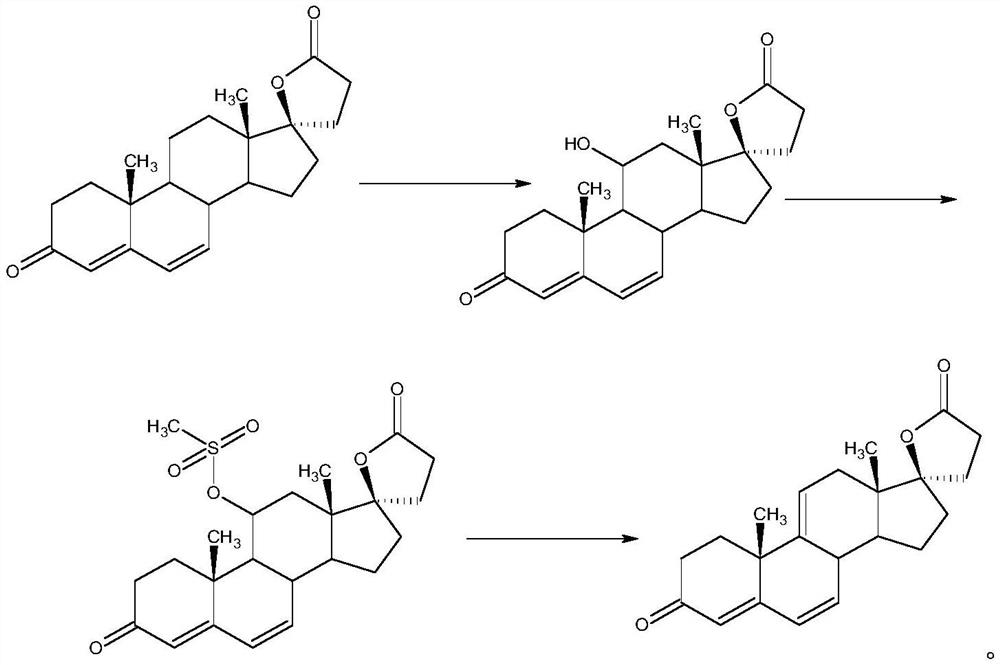
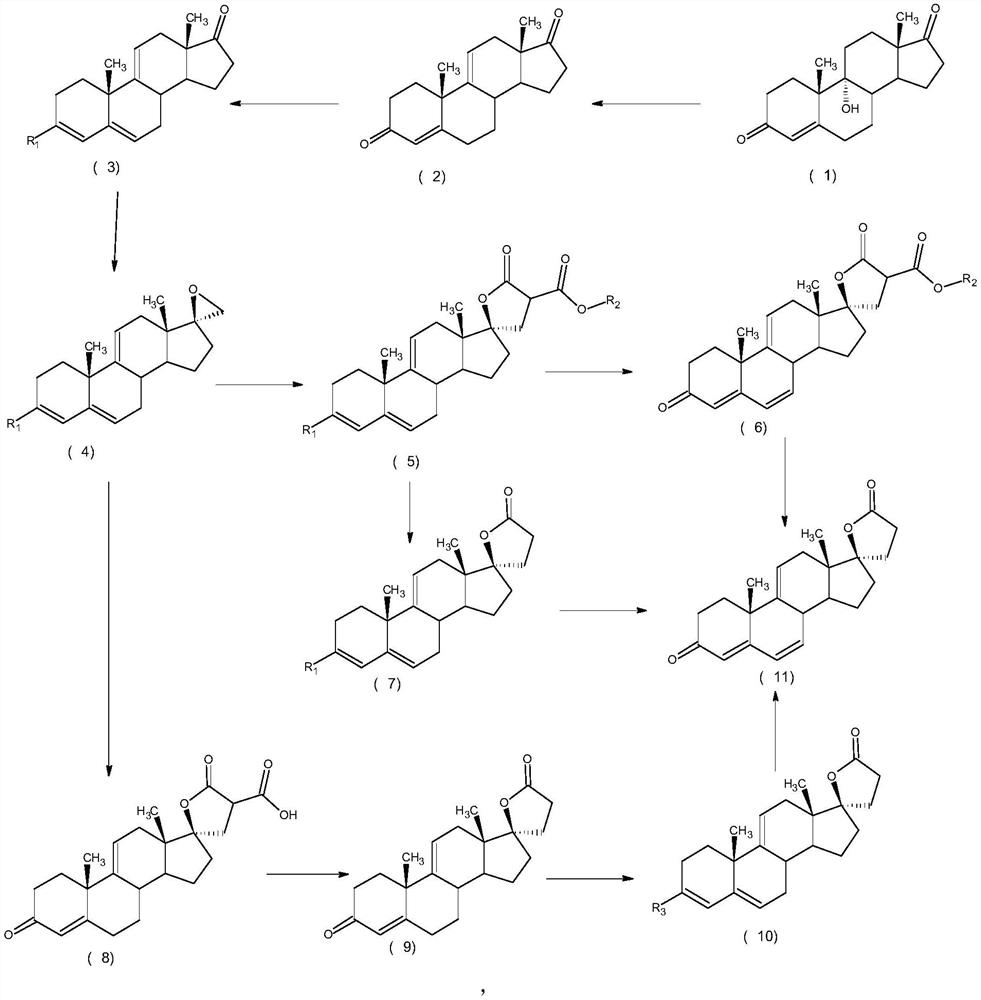
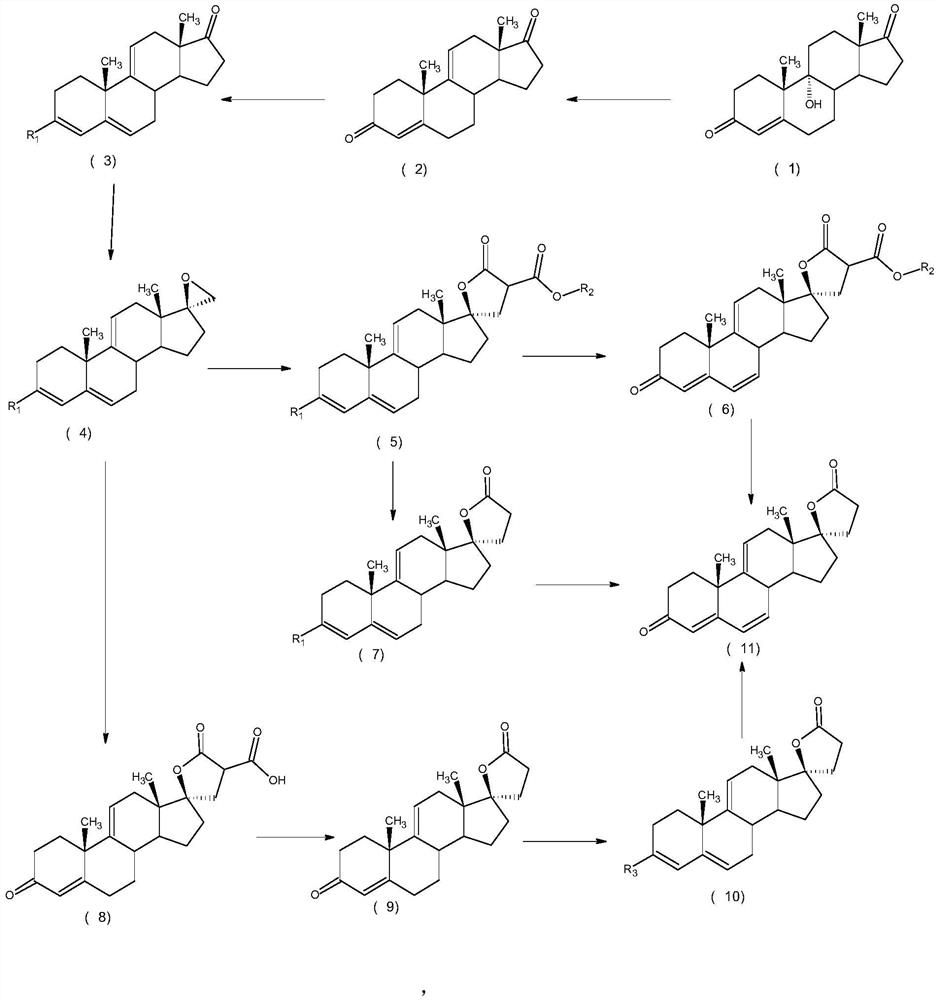
ActiveCN112062805AReduce process stepsEasy to purifyLactone steroidsDouble bondPharmaceutical Substances
The invention discloses an efficient preparation method of delta<9, 11>-canrenone, and belongs to the technical field of preparation of intermediates of medicines. The method comprises the following steps of: by taking 9 alpha-hydroxyl-4-androstenedione as a raw material, firstly removing 9-site hydroxyl through dehydration reaction to generate delta<9, 11> double bonds, then protecting 3-site carbonyl, then performing epoxidation on 17-site carbonyl, condensing with malonic acid diester to form a lactone ring, and performing oxidative decarboxylation or decarboxylation oxidation reaction to obtain delta<9, 11>-canrenone. According to the method, the raw materials are cheap and easy to obtain, the cost is low, reaction products in all steps are easy to purify, the total mass yield of the final product is higher than 80%, and the method is high in operability, extremely high in commercial competitiveness, suitable for industrial large-scale production and good in economic benefit.
Owner:ZHEJIANG SHENZHOU PHARMA
6 alpha-bromo- androstane-4-ene-3,17-dione high efficient synthesis method
The invention discloses an indirect and direct synthesizing method of 6 alpha-bromine-androster-4-olefin-3, 17-diketone, which comprises the following steps: 1. using strong acid to transmit 6 beta-bromine-androster-4-olefin-3, 17-diketone in the water to obtain pure 6 alpha-bromine-androster-4-olefin-3, 17-diketone; 2. synthesizing 6 alpha-bromine-androster-4-olefin-3, 17-diketone based on androster-4-olefin-3, 17-diketone as raw material in the Br2 / HOAc / THF; affirming stereoscopic structure of two-method product consistent through X-diffraction.
Owner:FUDAN UNIV
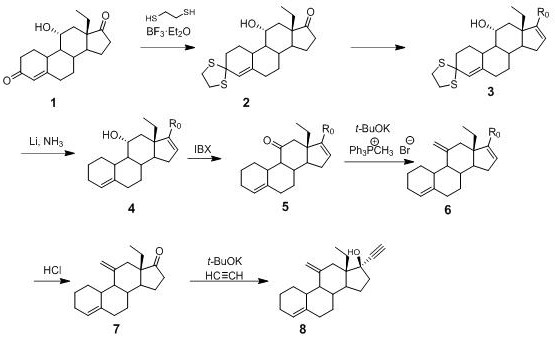
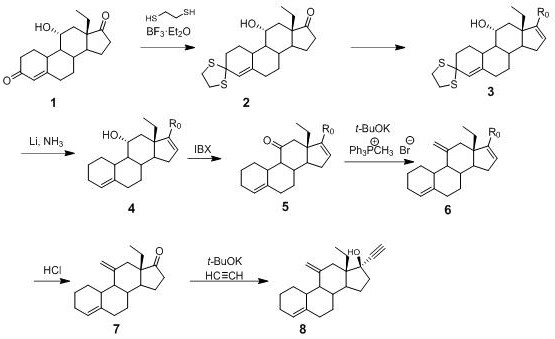
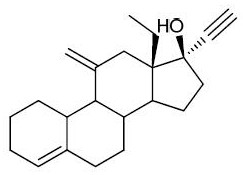
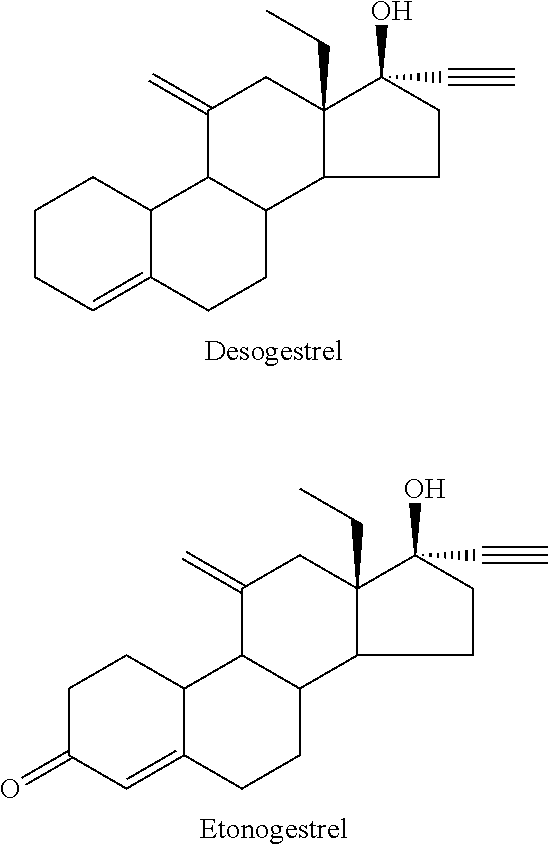
Preparation method of desogestrel
The invention relates to a preparation method of desogestrel, which comprises the following specific steps: by taking 11 alpha-hydroxy-18-methyl-estra-4-ene-3, 17-diketone) as a starting material, carrying out 3-keto protection by using ethanedithiol, carrying out 17-keto protection by using imine, removing ethanedithiol from lithium liquid ammonia, carrying out IBX oxidation, carrying out wittig alkylenation reaction, carrying out acidolysis deprotection, and carrying out ethynylation by using acetylene potassium, so as to obtain the desogestrel.
Owner:ZHEJIANG XIANJU JUNYE PHARM CO LTD +1
Synthetic method of exemestane
ActiveCN112409432AReduce usageSimplify the subsequent purification processSteroidsBulk chemical productionPtru catalystOrganic solvent
The invention discloses a synthetic method of exemestane. The method comprises the following steps: by taking 6-methyleneandrost-4-ene-3,17-dione as a raw material, directly carrying out selective [delta]1,2 dehydrogenation reaction on the raw material in an organic solvent under the protection of nitrogen, in the presence of an inorganic base and in the presence of an organic phosphorus ester compound as a ligand under the condition of heating reflux under the catalysis of a catalyst, and after the reaction is carried out for a period of time, and carrying out post-treatment to obtain the exemestane. Compared with a traditional synthesis method, the route has the advantages that 1,2 selective dehydrogenation is carried out on 6-methyleneandrost-4-ene-3,17-dione through the catalyst to prepare the exemestane, the use of benzoquinone oxidants, Jones oxidants and the like is avoided, the subsequent purification process is simplified, the yield is increased, and the pollution problem in the production process is reduced.
Owner:SHAANXI SCI TECH UNIV
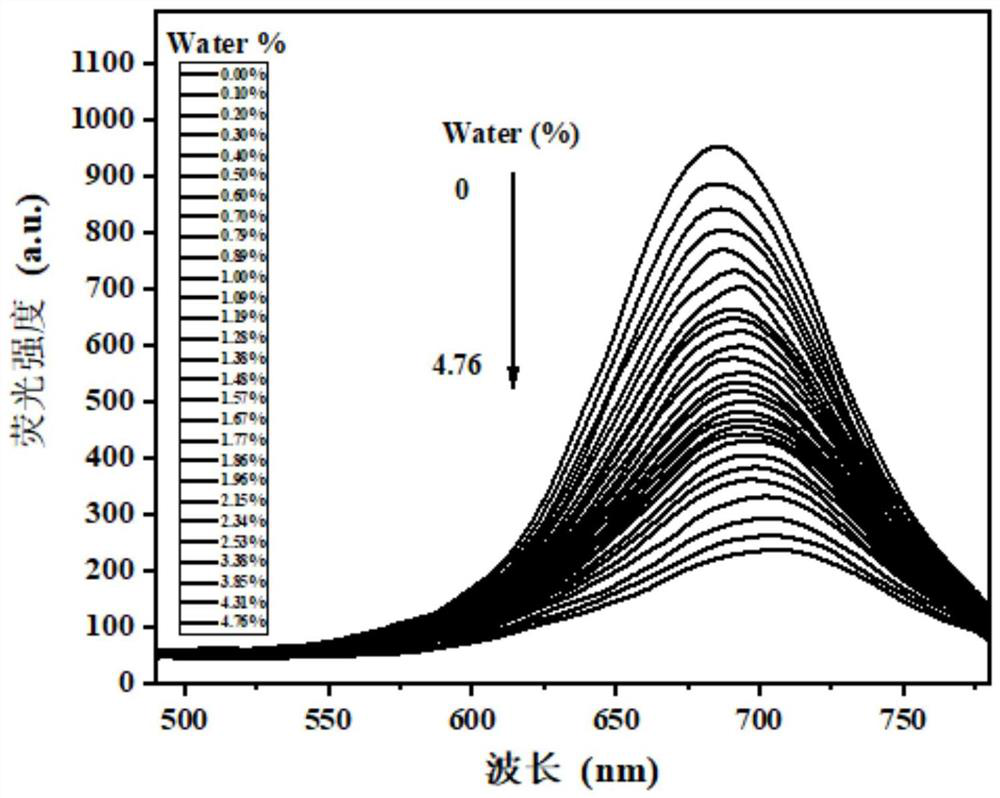
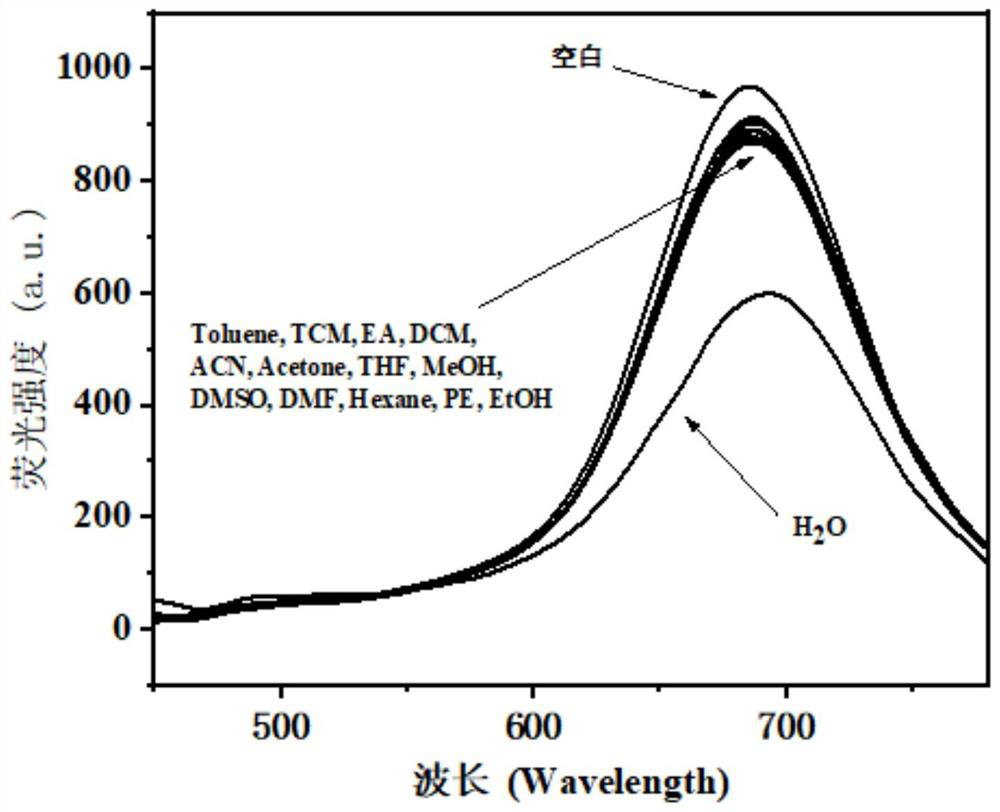
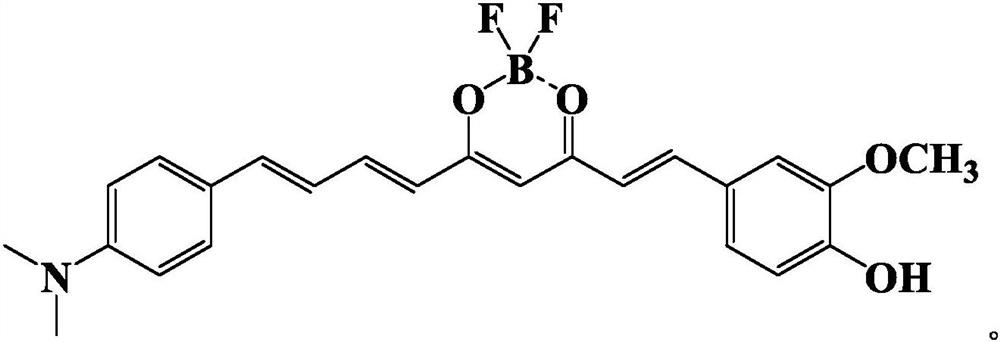
A fluorescent probe for detecting trace water in 1,4-dioxane and its preparation method
ActiveCN113185542BIncreased sensitivityHigh selectivityGroup 3/13 element organic compoundsFluorescence/phosphorescenceFluoProbesPhenyl group
The invention discloses a fluorescent probe for detecting trace water in 1,4-dioxane and a preparation method thereof. The present invention utilizes 4-dimethylaminocinnamaldehyde as a raw material, carries out aldol condensation reaction with the complex of 2,4-pentanedione and boron trifluoride ether, and then performs aldol condensation reaction with 3-methoxy-4-hydroxybenzaldehyde Condensation reaction, get fluorescent probe 1‑(3‑methoxy‑4‑hydroxyphenyl)‑9‑(4‑(dimethylaminophenyl) nonyl‑1, 6, 8‑triene‑3, 5‑ Diketone boron difluoride complex. This fluorescent probe can quickly react with traces of water in 1,4-dioxane, causing the red fluorescence of the fluorescent probe to be quenched, so it can be used to detect 1, The trace amount of water in 4-dioxane has the advantages of high sensitivity, high selectivity, quantitative and rapid detection, etc., and has a good application prospect.
Owner:NANJING FORESTRY UNIV
Preparation of 11β-hydroxy-1,4-diene-3,20-dione steroids by joint fermentation of Absidia and Arthrobacter
ActiveCN105779555BHigh yieldReduce stepsMicroorganism based processesFermentationArthrobacterDienedione
Owner:TIANJIN JINYAO GRP
A kind of method for preparing 16α-hydroxyprednisolone
ActiveCN111253457BTo avoidImprove reaction efficiencyOrganic decompositionSteroidsPrednisoloneBiochemical engineering
The invention discloses a method for preparing 16α-hydroxyprednisolone, which belongs to the technical field of medicine preparation and processing. The method uses 21-hydroxypregna-1,4,9(11), 16-tetraene-3,20-dione-21-acetate as the starting material, and undergoes oxidation, bromohydrin, debromination and alcoholysis , to prepare 16α‑hydroxyprednisolone. The method for preparing 16α-hydroxyprednisolone of the present invention can effectively control the generation of impurities in the reaction process by improving the deficiencies of the traditional process, the reaction process is mild, and the overall conversion rate is high; the inventive method has low requirements on the reaction device, The operation cost is low, the operation is simple and convenient, suitable for industrialized production, and has a good market prospect.
Owner:ZHEJIANG SHENZHOU PHARMA
Preparation method of dehydroxymethasone intermediate
The invention provides a preparation method of a dehydroxymethasone intermediate, which comprises the following steps: by taking 21-hydroxypregna-1, 4, 9 (11), 16-tetraene-3, 20-diketone-21-acetate as a starting material, sequentially carrying out bromine hydroxylation, epoxidation, methylation and hydrolysis reaction, so as to obtain the dehydroxymethasone intermediate 9beta, 11 beta-epoxy-21-hydroxy-16 alpha-methyl progesterone-1, 4-diene-3, 20-diketone. The preparation process route provided by the invention has fewer reaction steps, the quality and yield of the product have obvious competitiveness, dangerous chemical processes are not involved, the method is green and clean, and the atom economy is improved; the preparation process has a wide industrial application prospect.
Owner:JIANGSU YUANDA XIANLE PHARMA +1
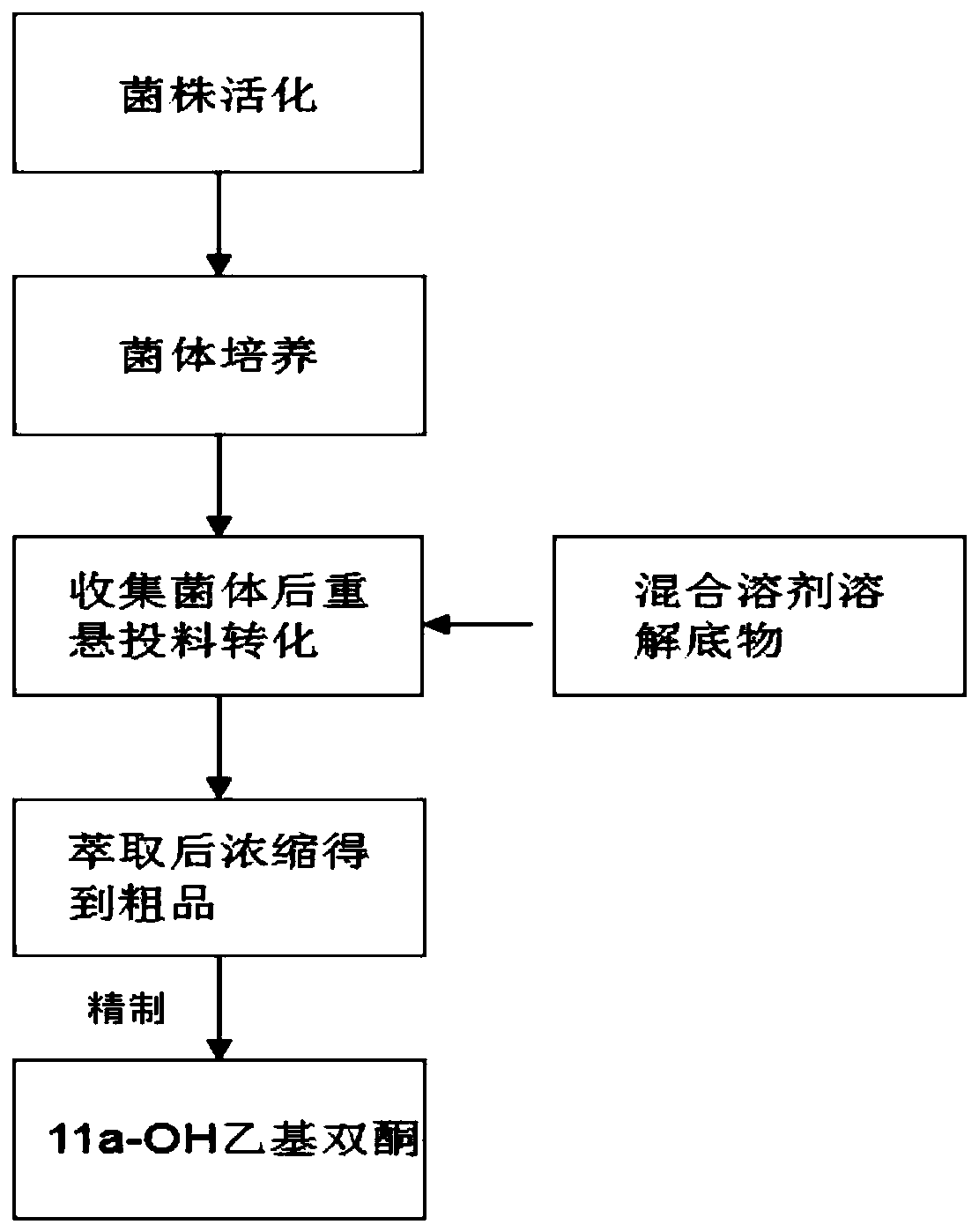
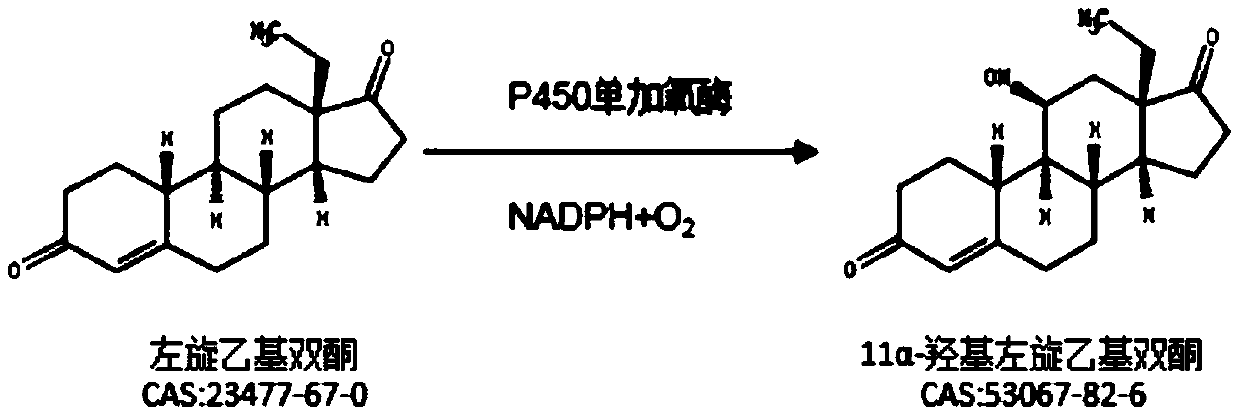
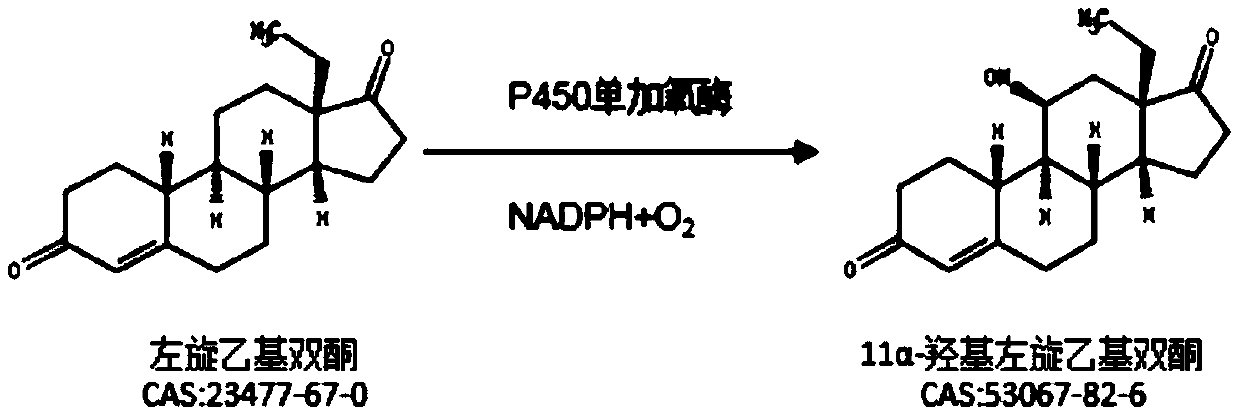
Method for reducing impurities in ethyl diketone 11a hydroxylation process by using mixed solvent
ActiveCN111593086AReduce generationImprove conversion rateFungiMicroorganism based processesBiotechnologyEthyl group
The invention relates to a method for reducing impurities in an ethyldiketone 11a hydroxylation process by using a mixed solvent. The method comprises the following steps: a Metarhizium anisopliae strain is cultured to obtain Metarhizium anisopliae thalli, and the thalli are collected; after the thalli are collected, feeding and conversion are conducted, specifically, a substrate DL-18-methyl-gon-40-en-3,17-dione is dissolved with the mixed solvent prepared from DMF and DMSO, the collected thalli are fed into the substrate, and the substrate is converted into 11a-hydroxy-18-methyl-estr-4-ene-3,17-dione. The method provided by the invention can obviously reduce the generation of impurities in the ethyl diketone biological hydroxylation product, especially the generation of 10a-OH ethyl diketone and 6 beta-ethyl diketone, increases the conversion rate, simplifies the after-treatment process and increases the average yield.
Owner:HUBEI GEDIAN HUMANWELL PHARMACEUTICAL CO LTD
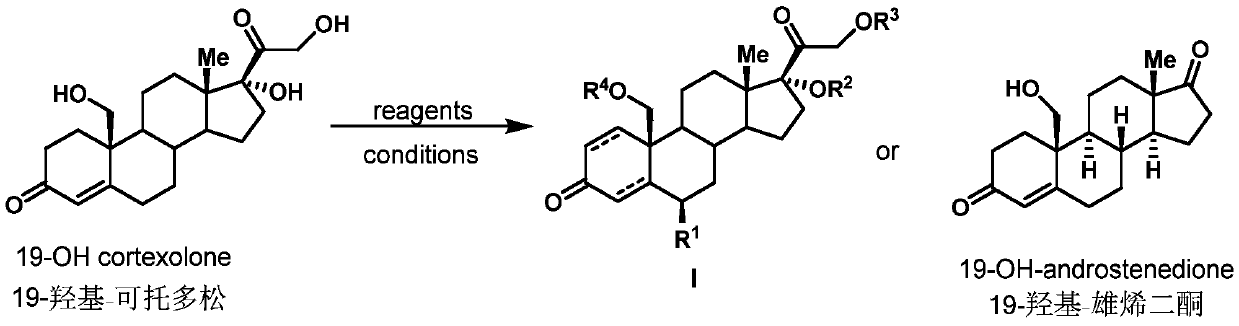
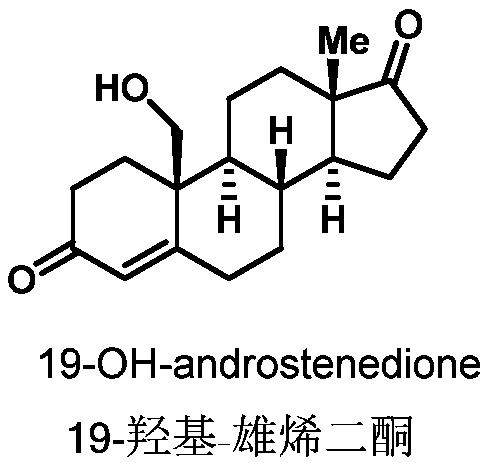
Preparation method of 19-hydroxylated cortodone derivatives and 19-hydroxyandrostenedione
Owner:WUHAN UNIV
Preparation method of 1alpha-hydroxyl dehydroepiandrosterone
ActiveCN113896756AReduce usageReduce pollutionKetal steroidsBulk chemical productionHormone drugEpiandrosterone
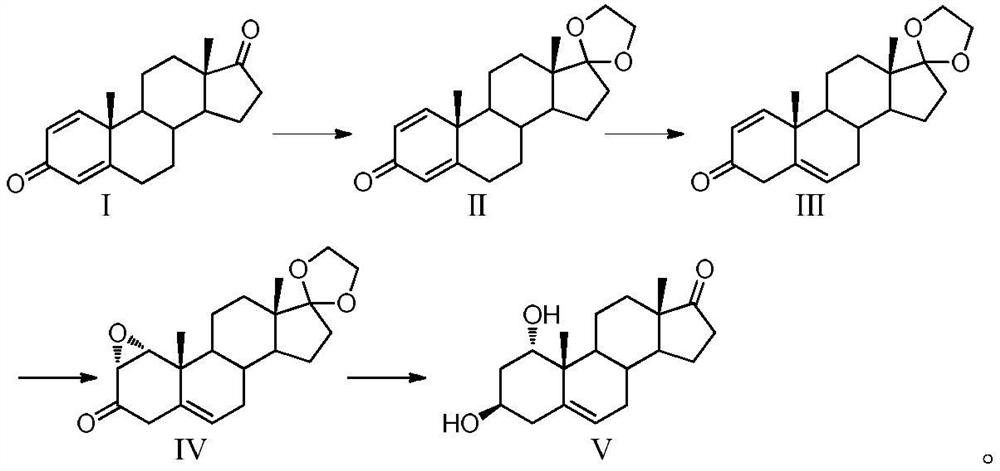
The invention belongs to the technical field of preparation of steroid hormone drugs, and particularly relates to a preparation method of 1alpha-hydroxyl dehydroepiandrosterone, which comprises the following steps of by taking a compound I (1, 4-androstenedione) as a raw material, sequentially carrying out ketalation reaction, transposition reaction, epoxy reaction and reduction hydrolysis reaction to obtain a target product V (1alpha-hydroxyl dehydroepiandrosterone). The problems of high technical requirements for removing isomers, difficulty in separation, low yield and the like in the prior art can be effectively solved.
Owner:ZHEJIANG XIANJU PHARMA +1
A high-efficiency δ 9,11 -The preparation method of canrenone
The invention discloses a high-efficiency Δ 9,11 A preparation method of canrenone belongs to the technical field of preparation of intermediates of medicines. The method is based on 9α-hydroxy-4-androstenedione as a raw material, and the 9-position hydroxyl group is first eliminated by dehydration reaction to generate Δ 9,11 Double bond, then protect the 3-position carbonyl group, then epoxidize the 17-position carbonyl group, and condense with malonic acid diester to form a lactone ring, and obtain Δ after oxidative decarboxylation or decarboxylation oxidation reaction 9,11 ‑Canrenone, the raw materials of the method of the present invention are cheap and easy to obtain, the cost is low, the reaction products involved in each step are easy to purify, and the total mass yield of the final product is higher than 80%. The method of the present invention has strong operability and extremely high commercial Competitive, suitable for industrialized large-scale production, and has good economic benefits.
Owner:ZHEJIANG SHENZHOU PHARMA
Process for Preparing 11-Methylene-18-Methyl-Estr-4-En-3, 17-Dione, Useful as Intermediate Compound for the Synthesis of Molecules Having Pharmacological Activity
Owner:IND CHEM SRL
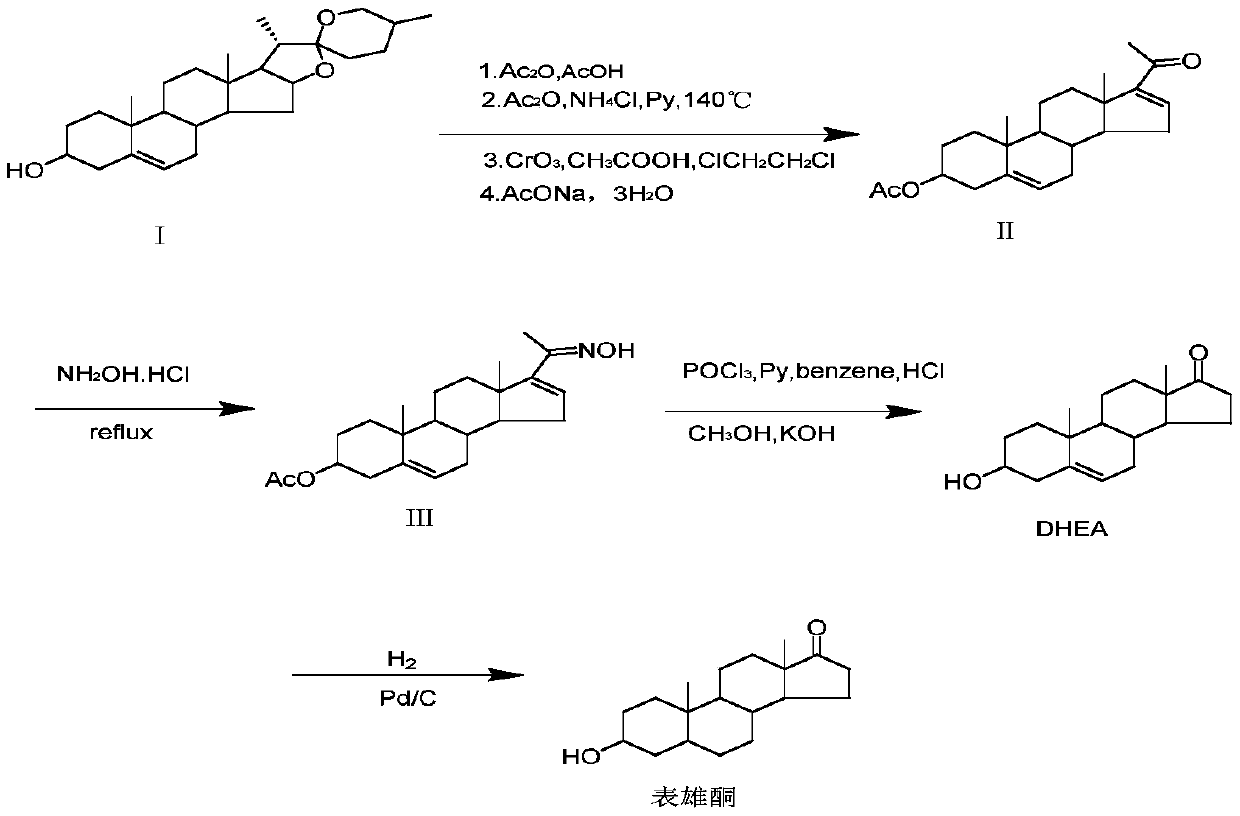
Method for preparing epiandrosterone with androstenedione as raw material
The invention relates to a method for preparing epiandrosterone by taking androstenedione as a raw material, and a target product epiandrosterone is obtained through the four-step reactions of a 3-carbonyl alkene esterification reaction, a 17-carbonyl ketal protective reaction, a hydrolysis and palladium carbon catalytic reduction reaction and an acid hydrolysis reaction of androstenedione. Compared with the existing synthesis method, the source of the raw material of the method is rich, the price is low, the synthesis condition is mild, the reduction effect of a catalyst is good, the yield ishigh, the production cost is relatively low, and the method is suitable for industrial production.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
A method for preparing progesterone using 1,4-androstenedione as raw material
The invention discloses a method for preparing progesterone by taking 1,4-androstenedione as a raw material, which comprises the following steps: 1) dissolving 1,4-androstenedione into an organic solvent, adding the acid of trimethyl orthoformate or triethyl orthoformate, and introducing nitrogen to protect the 1,4-androstenedione to synthesize the enol ether of 1,4-androstenedione, namely 3-methoxy-androstane 3,5-diene-20-ketone; and 2) dispersing (1-methoxy ethyl)-triphenylphosphine salt in a reaction medium, an organic solvent, adding alkali at low temperature, performing a Wittig reaction of the 3-methoxy-androstane 3,5-diene-20-ketone synthesized in the step 1), and purifying and crystallizing to obtain progesterone. By adopting the 1,4-androstenedione as the raw material, the method solves the problem that of lack in raw materials for synthesizing steroid drugs such as progesterone, and improves the utilization rate of 1,4-androstenedione and the yield of progesterone; the preparation process is simple.
Owner:HUNAN KEYUAN BIO PRODS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com