Process for fermentation of phytosterols to androstadienedione
A technology of androsadienedione and phytosterol, which is applied in the field of biotransformation and can solve expensive and time-consuming problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
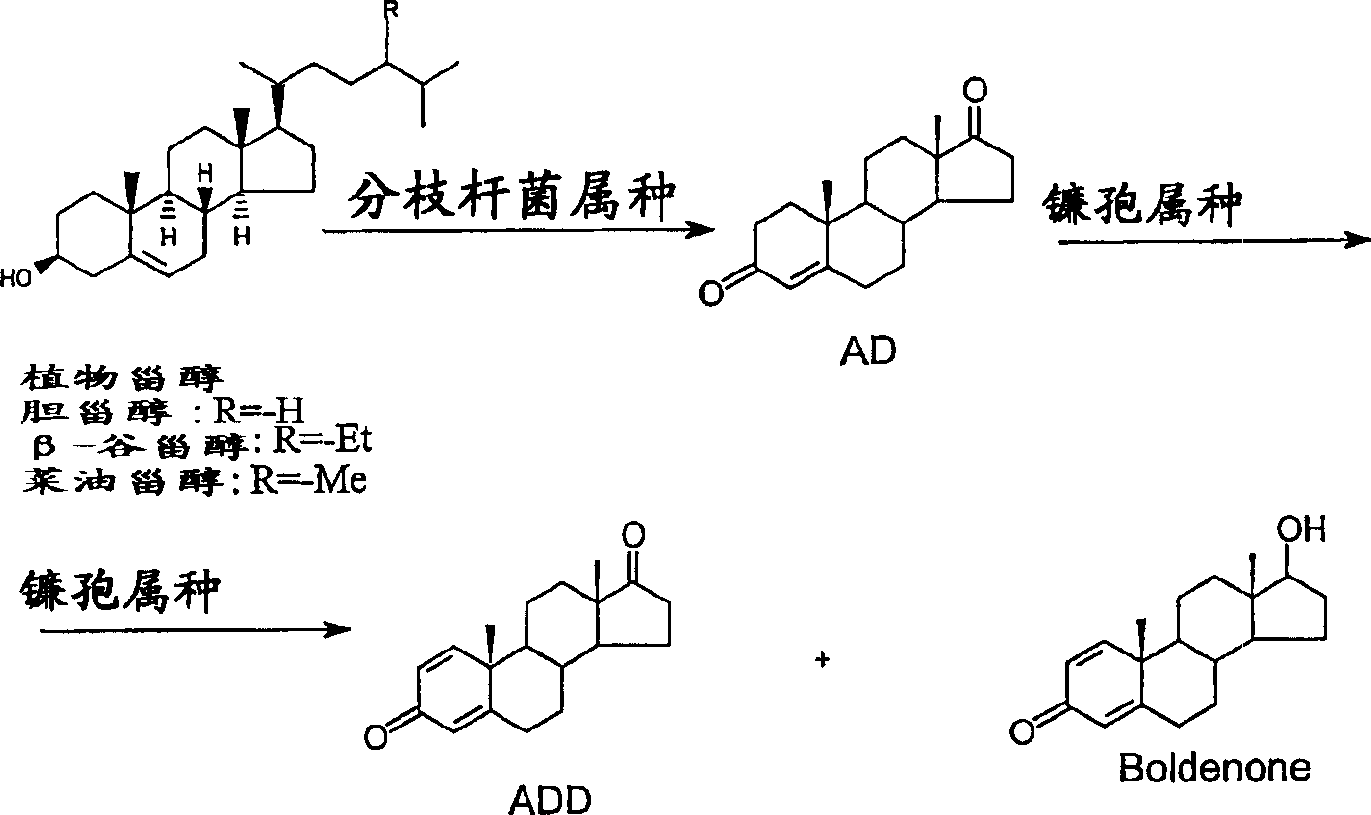
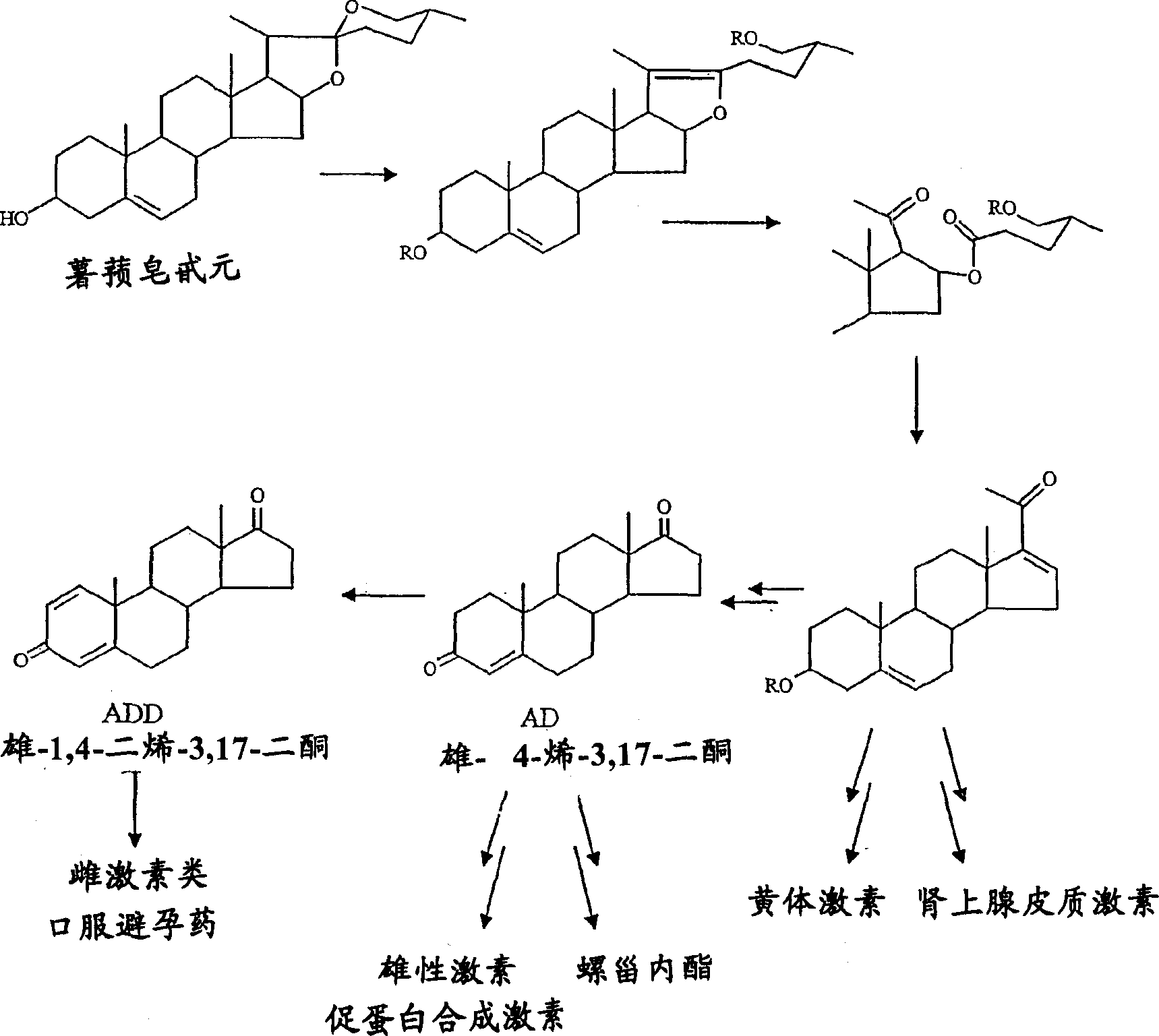
[0054] Example 1 Bioconversion of phytosterols to ADD using Mycobacterium species and Fusarium solani
[0055] Biotransformation using mycobacterial species (stage 1)
[0056] Preparation of seed cultures of mycobacterial species
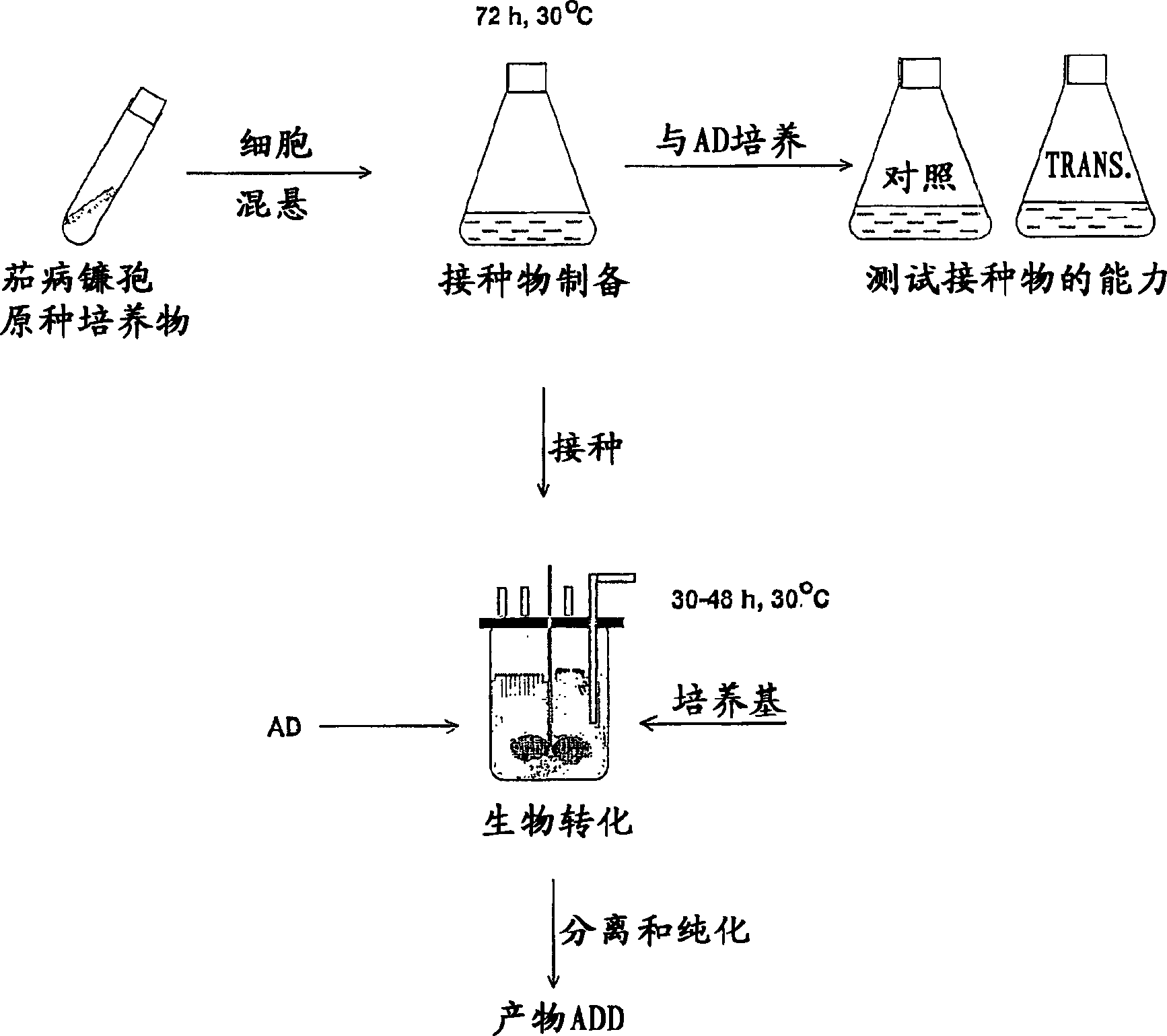
[0057] Inoculum for biotransformation was prepared in liquid media. Erlenmeyer flasks were inoculated from agar slants (cultured in glass test tubes). For working volumes up to 40 L, use 1 L Erlenmeyer flasks each containing 200 mL of seed medium, and for volumes of 50 L and larger, use 2 L Erlenmeyer flasks each containing 400 mL of medium. Inoculations were performed under sterile conditions (in a biohazard hood). Pipette sterile water (5 mL) onto the agar slants. Gently scrape off the bacterial culture with the end of a pipette. The suspension culture was then removed and placed in Erlenmeyer flasks. Bacterial multiplication was performed on a rotary shaker (200 rpm, 1" throw, 30°C room temperature). After 72 hours of incubation, the seed c...
Embodiment 2
[0121] Example 2 One-stage method for bioconversion of AD to ADD using Fusarium solani
[0122] Preparation of seed cultures of Fusarium solani
[0123] The fungal culture Fusarium solani was propagated in liquid medium (10 g / L corn steep liquor, 10 g / L glucose, pH 6.5). Erlenmeyer flasks (1 L volume, 0.2 L medium) were inoculated from cultures grown on oat flour agar at 30°C for 120 hours. Broth cultures were propagated in a rotary shaker (26°C, 120 rpm, 1"throw) for 72 hours.
[0124] Fusarium can also be subcultured from liquid to liquid medium until passage 4-5 or until the next culture turns pink.
[0125] Shake flask inoculums were stored at 4°C for no more than 1 month without significant changes in activity.
[0126] Preparation of Biotransformation Medium for Fusarium solani
[0127] The method is performed using isolated AD.
[0128] The composition of the biotransformation medium was as follows:
[0129] Medium: Biotransformation Medium
[0130] ...
Embodiment 3
[0142] The extraction of embodiment 3 fermented broth
[0143] Harvest the fermentation broth into a separatory funnel. Ethyl acetate (6.0 L) was added to the broth and shaken for 2-3 minutes. After 15 minutes the mixture separated into two layers (ethyl acetate upper layer and aqueous lower layer). The ethyl acetate layer was removed and transferred to another container. The aqueous bottom layer was extracted with a second portion of ethyl acetate (4.0 L), the upper layer (ethyl acetate) was removed and combined with the first ethyl acetate extract. The combined ethyl acetate extracts were concentrated on a rotary evaporator to form a red viscous oil (-450ml) which was used for further purification.
[0144]Each ethyl acetate extract and aqueous residue was subjected to steroid analysis by HPLC. The results of the two-stage method are listed in Table 5 and the results of the one-stage method are shown in Table 6.
[0145] Distribute ADD from oil
[0146] Hexanes (1.25 L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com