Preparation method of dehydroxymethasone intermediate
A technology for deoxymethasone and intermediates, which is applied in the field of preparation of deoxymethasone intermediates, can solve problems such as long reaction routes and unsatisfactory product yields, and achieve high economic value, low price, and reduced production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
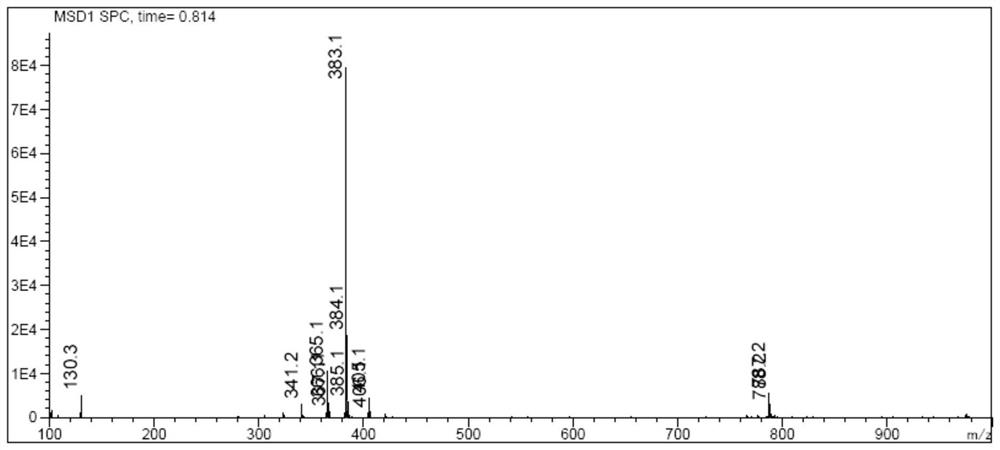
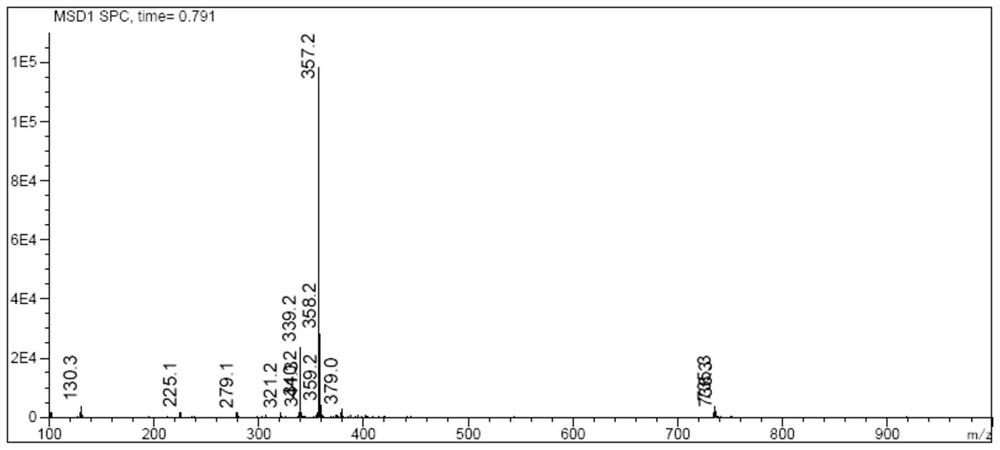
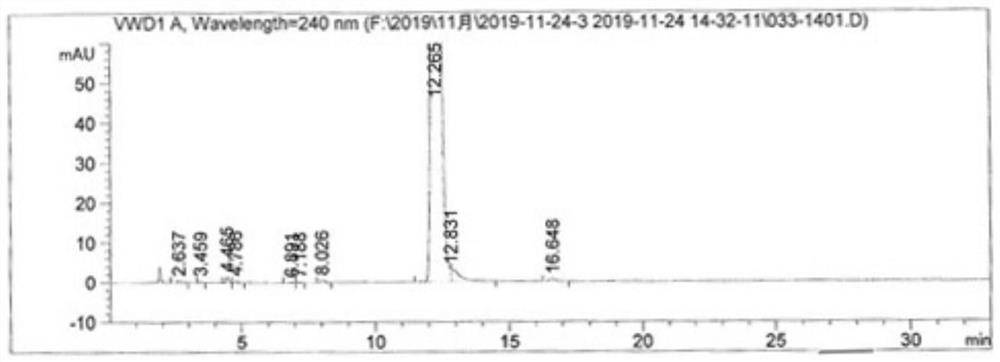
Image
Examples
Embodiment 1
[0028] The preparation of embodiment 1 compound II
[0029] Dissolve 50g of compound I (21-hydroxypregna-1,4,9(11),16-tetraene-3,20-dione-21-acetate) in 500ml of acetone, cool to 10°C and add 10mL of Chloric acid and 100 mL of water. Add 30g NBS in two batches, TLC (ethyl acetate:methanol=4:1) after the reaction is detected, add 40% NaOH solution (80gNaOH+200mL water) dropwise, raise the temperature to 35°C, and pour in 1000mL water after the insulation reaction is complete. Filtration and drying yielded 49.7 g of compound II.
Embodiment 2
[0030] The preparation of embodiment 2 compound III solution
[0031] Under the protection of nitrogen, put 400ml of tetrahydrofuran into the dry reaction tank, drop in 40g of compound II, cool down to 0-2°C, put in 4g of cuprous chloride, continue to cool down to -15--10°C, and dropwise add 150ml of Grignard reagent ( 1mol / L methylmagnesium bromide (tetrahydrofuran), heat the reaction until TLC (ethyl acetate:methanol=4:1) detects that the reaction is complete, continue to add dropwise for 10-15 minutes, stir, at -15~-10°C Incubate for more than 15 minutes to obtain a compound III solution.
Embodiment 3
[0032] The preparation of embodiment 3 compound IV
[0033] Prepare sodium hydroxide / methanol solution: under the protection of nitrogen, take 20g of sodium hydroxide and put it into 140ml of methanol, stir for later use.
[0034] Under the protection of nitrogen, add 8ml of glacial acetic acid to the compound III solution prepared in step S2 for neutralization, and the measured pH is 6-7; add sodium hydroxide / methanol solution dropwise, and set the dropwise temperature condition to -13~-10 ℃, the dropping time is controlled at 20-40 minutes, after the dropping is completed, keep warm for 1-2 hours, and perform TLC (benzene:acetone=4:1) detection. After the reaction is complete, neutralize with 30ml of glacial acetic acid, press into 1000ml of ice-purified water for water analysis, add 400ml of chloroform for extraction, filter the organic layer and emulsified layer, add 30g of neutral alumina to the filter; use 400ml for the water layer Chloroform was used for three extracti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com