Impurity of ciclesonide and preparation method thereof
A technology of ciclesonide and cycloalkyl, which is applied in the field of ciclesonide impurities and its preparation, and can solve problems such as toxicity and affecting the curative effect of ciclesonide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] The preparation of ciclesonide (compound III) is, for example, according to the method described in Chinese patent CN1626546, which is fully incorporated by reference herein.
[0081] The instruments used for mass spectrometry were Agilent 1200 high performance liquid chromatography system and Agilent G6410A tandem triple quadrupole mass spectrometer. The ion source was electrospray ion source in positive ion mode. Split the HPLC eluate, allowing approximately 1 μg / ml into the ion source of the mass spectrometer.
[0082] The NMR analysis of formula Ⅱ compound is in CDCl 3 The instrument used was a Bruker Avance500 nuclear magnetic resonance spectrometer. TMS is used as a proton resonance (δ 1 H 0.00) and solvent internal reference, CDCl 3 As the carbon resonance internal standard (δ 1 C 77.00).
[0083] The following abbreviations are used and have the following meanings:
[0084] abbreviation Meaning δ chemical shift CDCl 3 deutera...
Embodiment 1
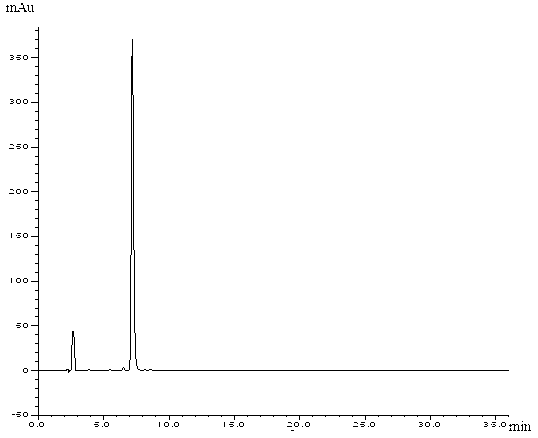
[0086] Embodiment 1: the HPLC analysis method of ciclesonide
[0087] Take an appropriate amount of this product, add methanol to dissolve, dilute with methanol to make a solution containing about 0.4mg per 1ml, as the test solution, and measure according to high performance liquid chromatography (Chinese Pharmacopoeia 2010 edition two appendix VD). Octadecylsilane bonded silica gel was used as filler, acetonitrile-water (90:10) was used as mobile phase, and the detection wavelength was 242nm. The number of theoretical plates should not be less than 5000 based on ciclesonide. Precisely measure 20 μl of the test solution, inject it into a liquid chromatograph, record the chromatogram to 6 times the retention time of the main peak, and calculate the purity of ciclesonide with the area normalization method. If necessary, the system peak area or solvent peak area can be deducted from the blank control.
Embodiment 2
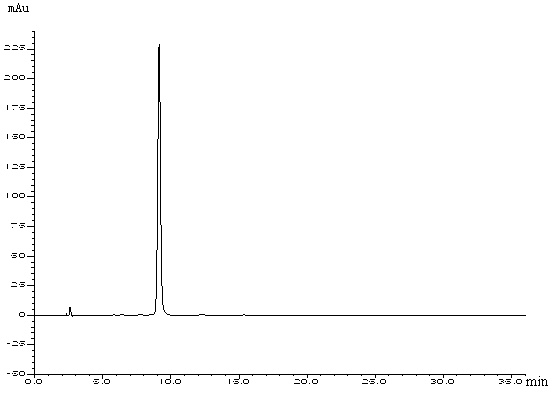
[0088] Embodiment 2: the preparation of formula II compound
[0089] Dissolve 5 g of ciclesonide (compound of formula III) in 200 ml of methanol, place it under a 254 nm ultraviolet lamp, and track the reaction progress with HPLC until the basic reaction of the raw materials is complete and the solution turns pale yellow. After the reaction solution was concentrated under reduced pressure, it was diluted with acetonitrile and separated by preparative chromatography (chromatographic column: Agilent ZORBAX SB-C 18 21.2×250mm), mobile phase: acetonitrile:water=80:20, flow rate 10ml / min, collect target components, concentrate under reduced pressure to obtain target ciclesonide photodegradation product (compound of formula II). HPLC purity was 99.0%.
[0090] M+H in mass spectrum [ESI-MS, m / z] + Peak is 541.
[0091] 13 C NMR (500MHz, CDCl 3 ) δ (ppm): 207.2, 203.5, 176.7, 165.8, 132.0, 107.4, 97.6, 81.8, 67.9, 67.2, 54.6, 54.0, 49.3, 46.2, 40.8, 40.3, 39.1, 38.6, 33.8,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com