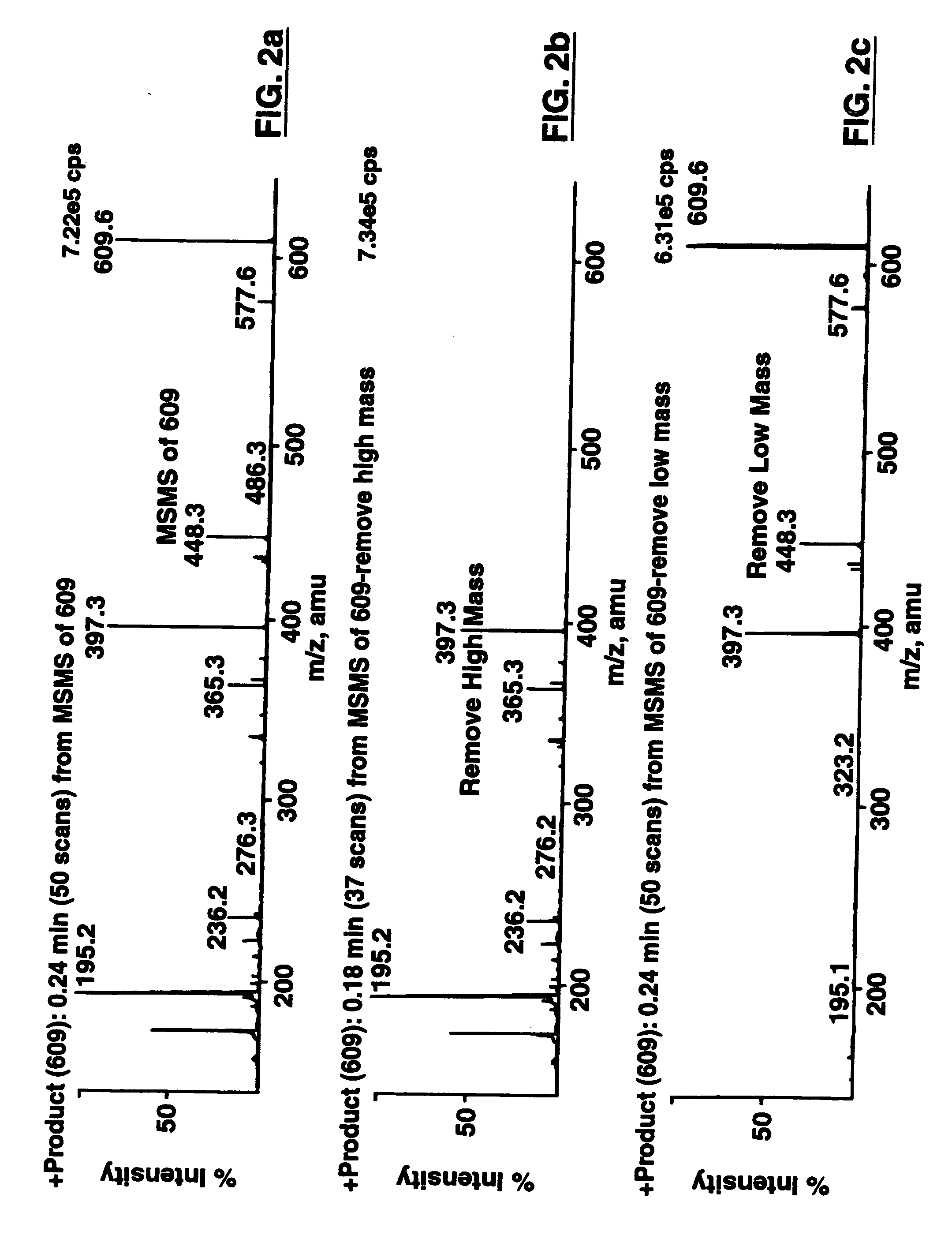
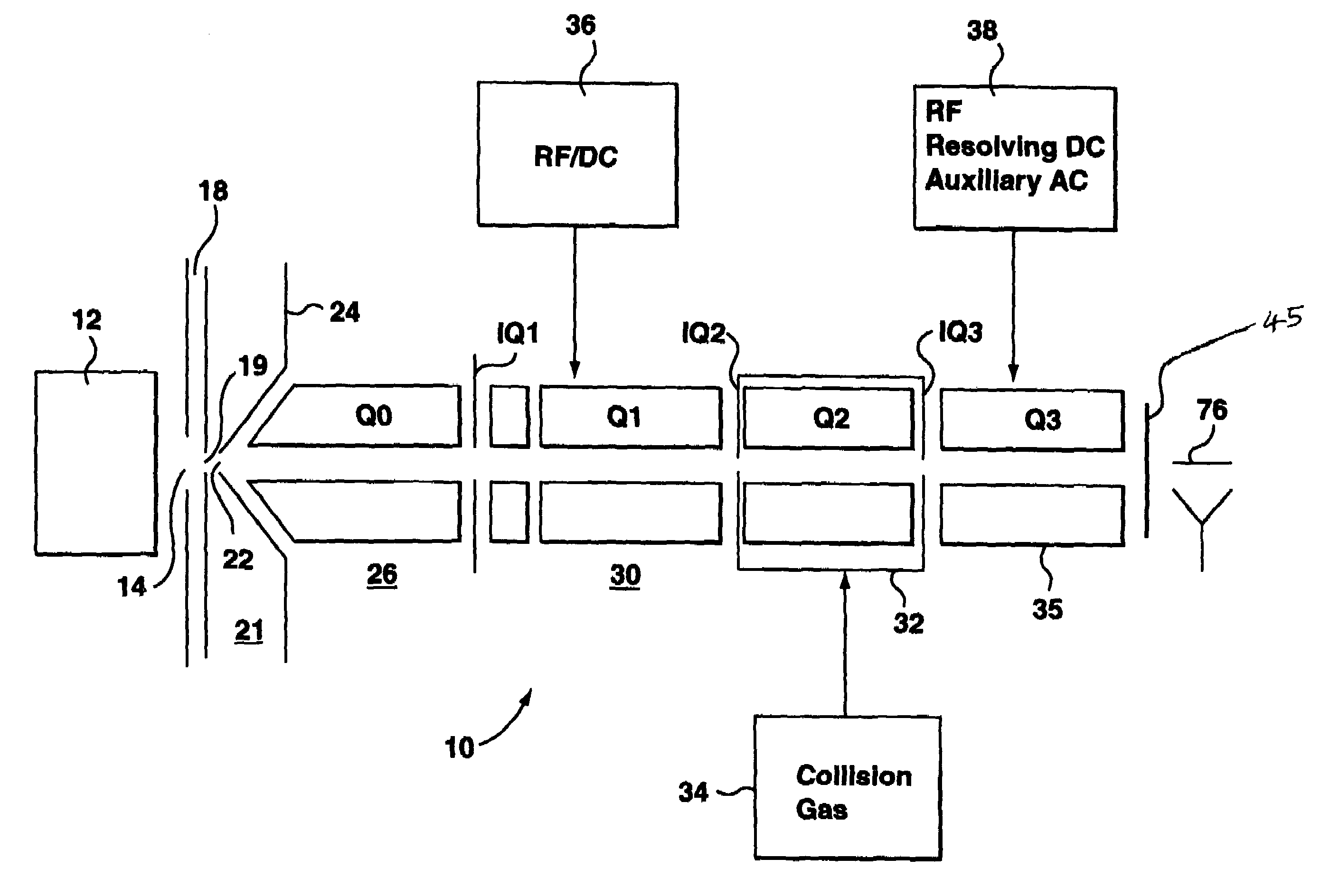
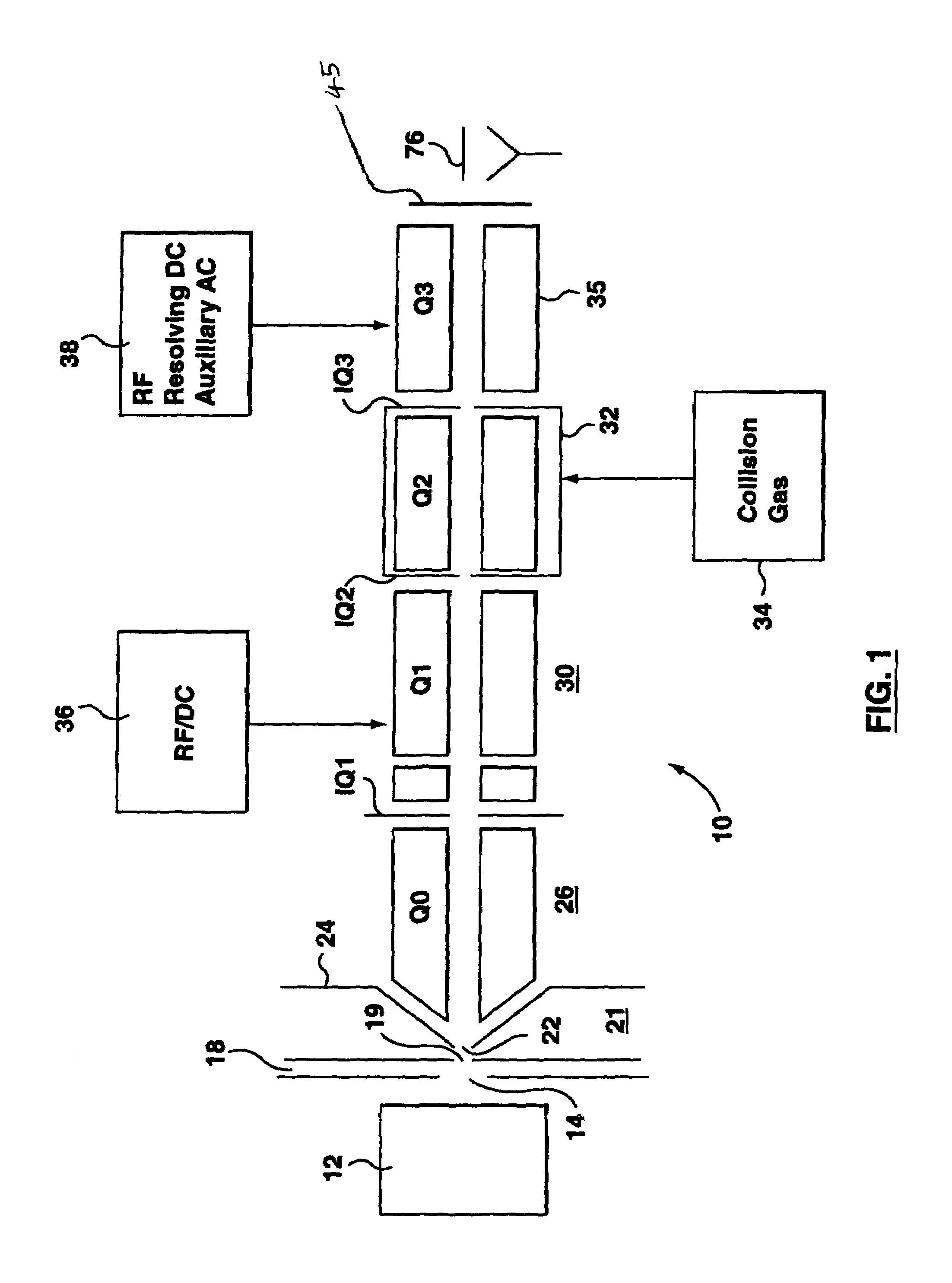
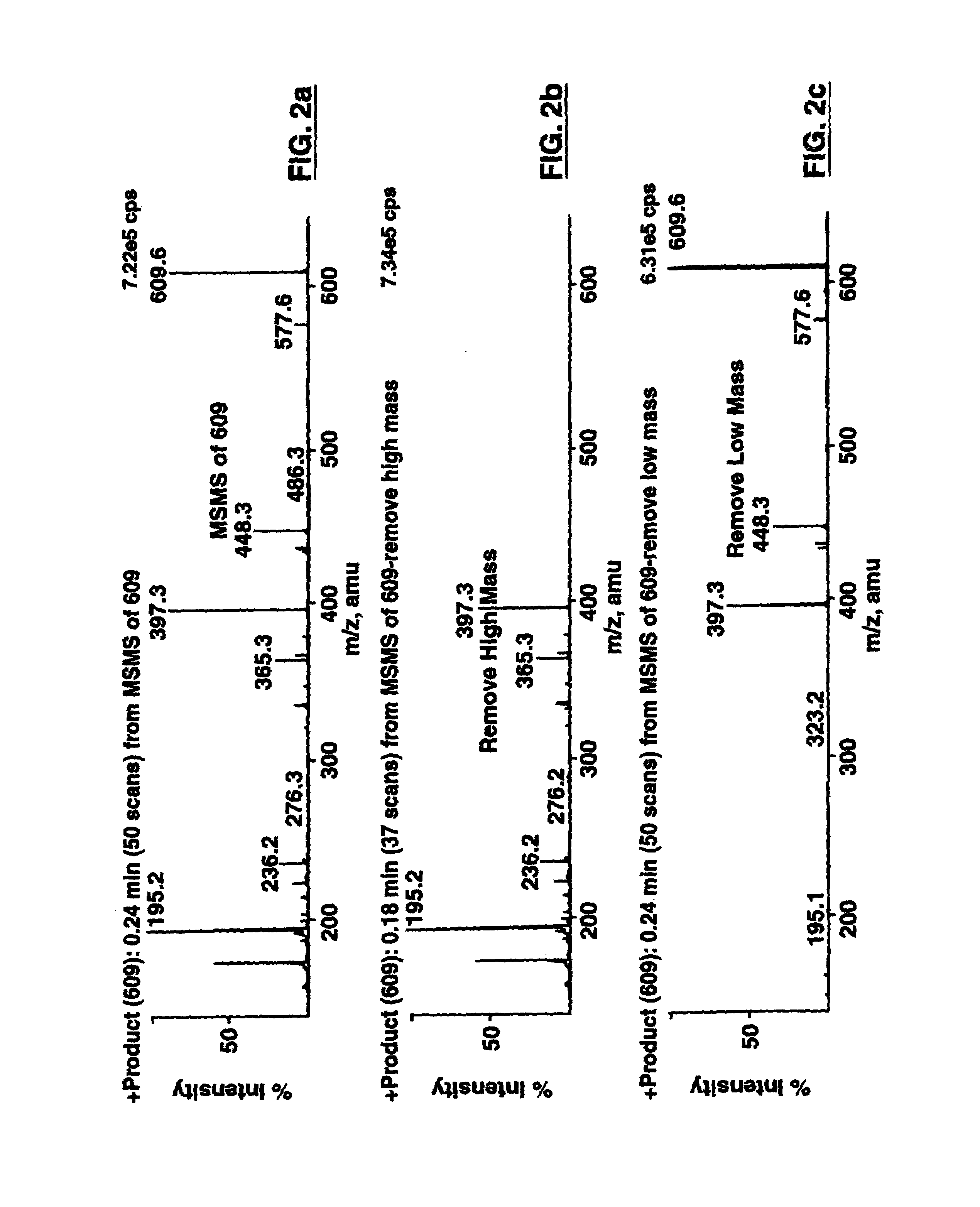
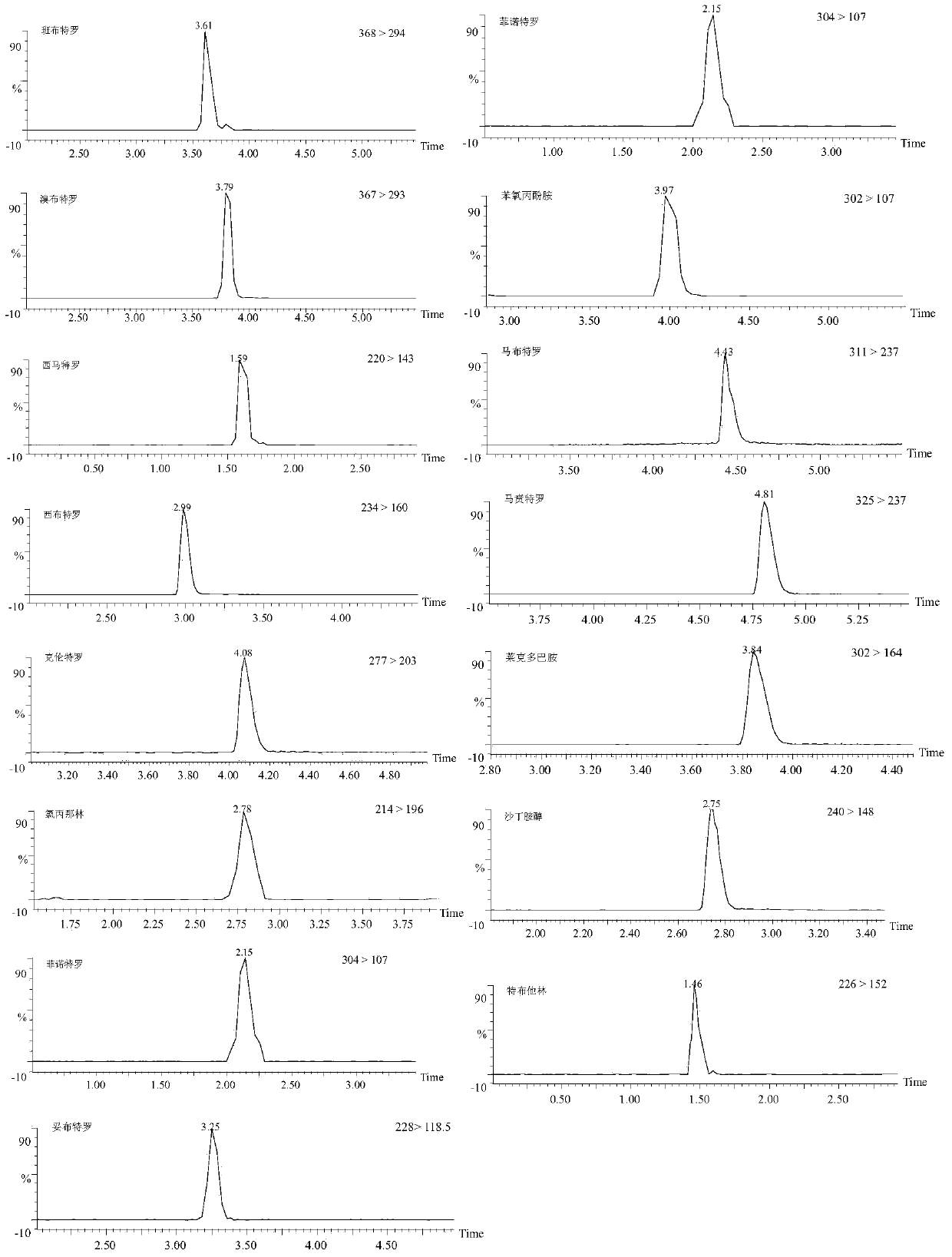
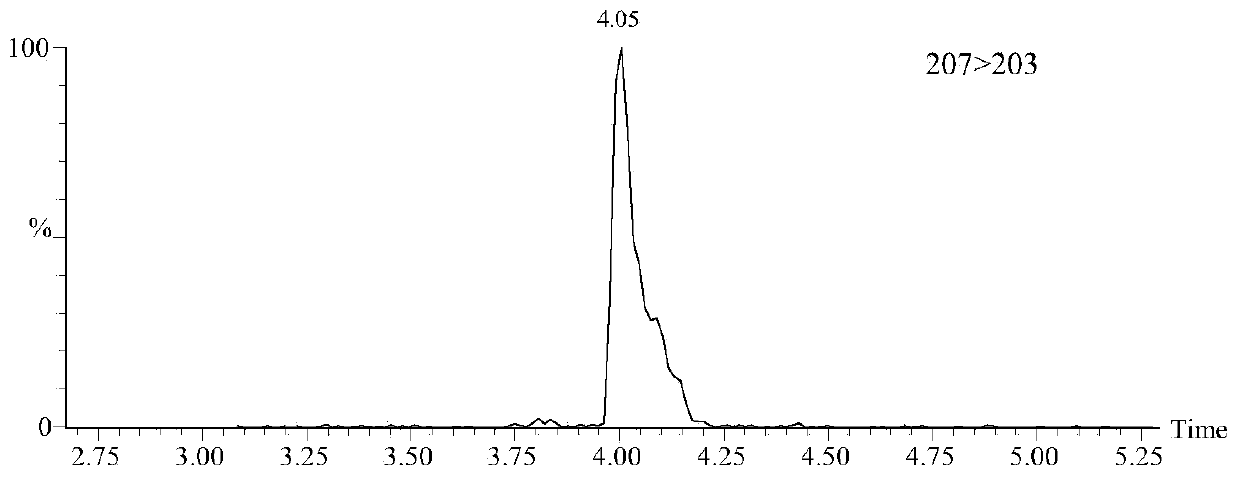
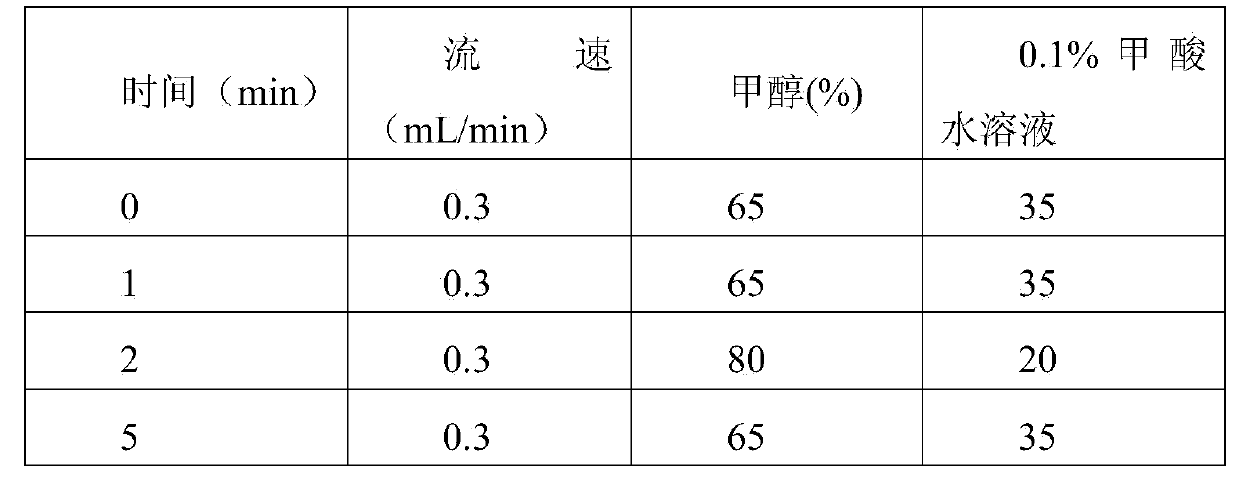
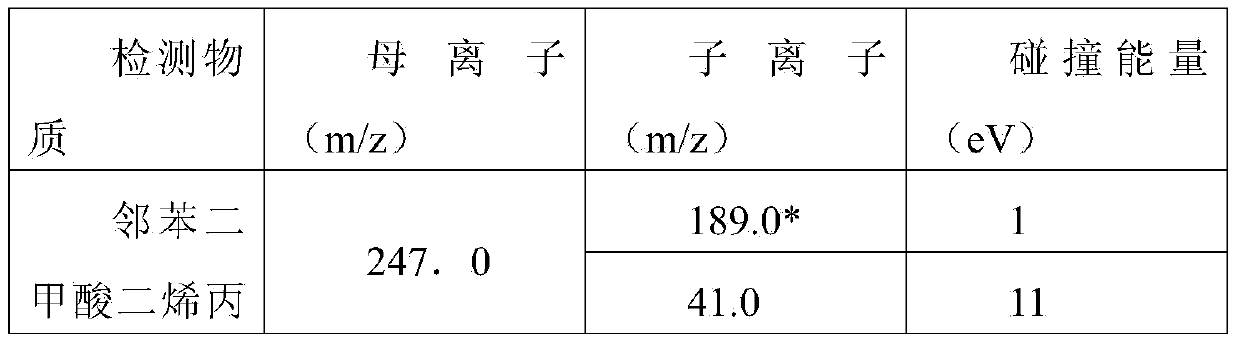
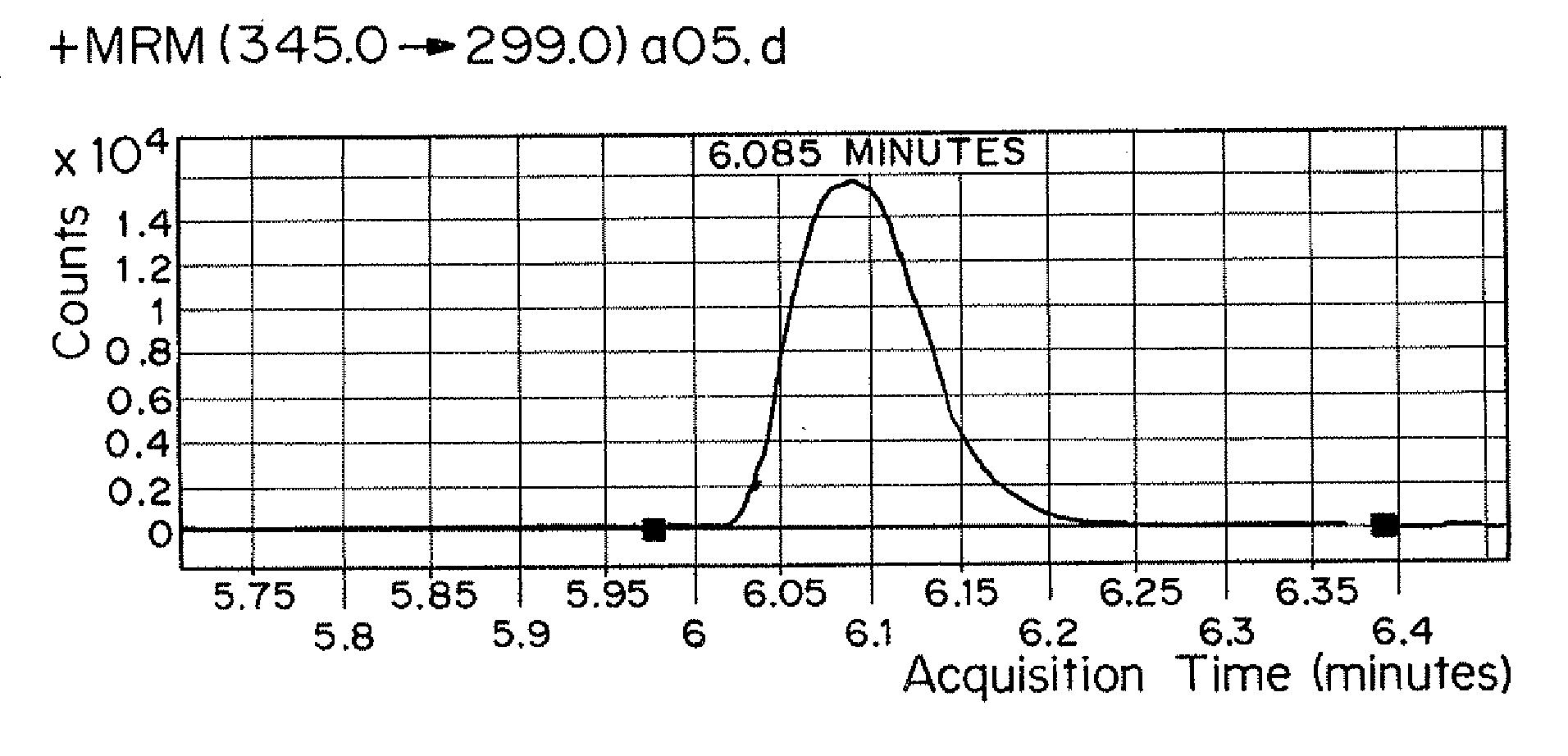
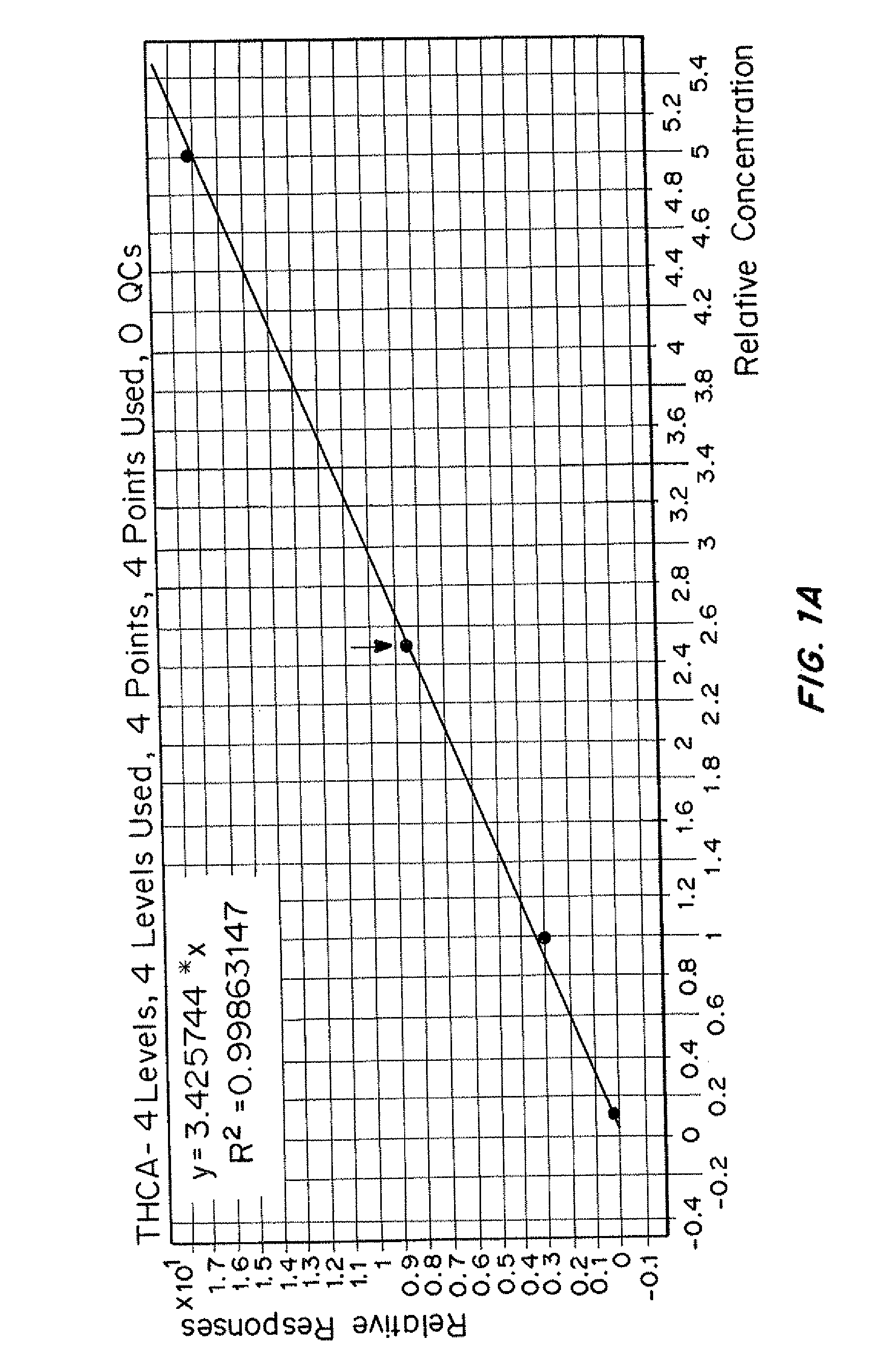
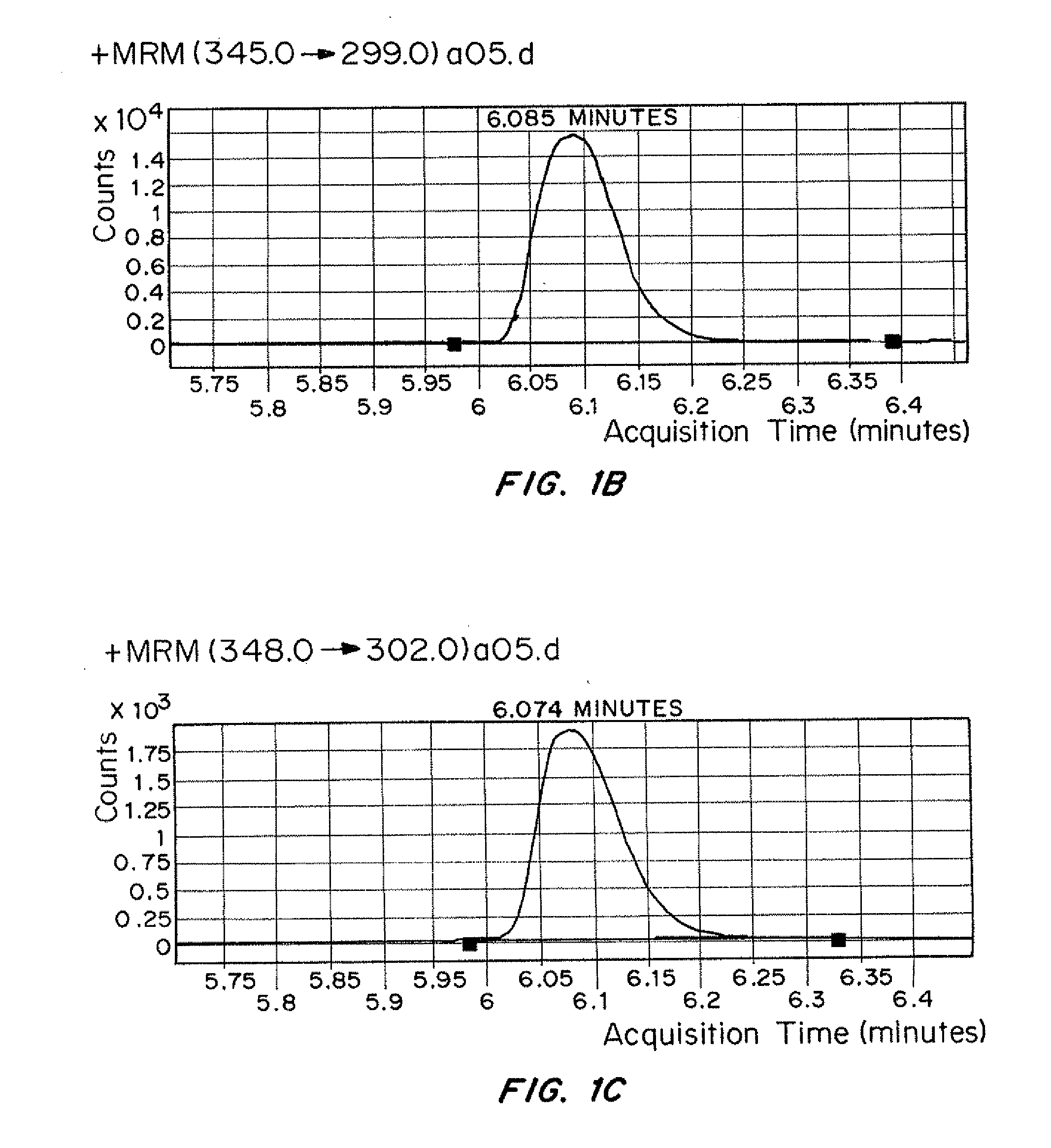
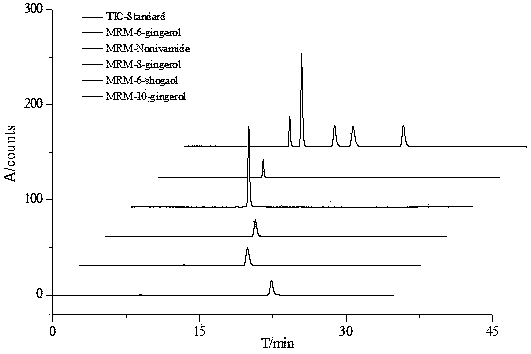
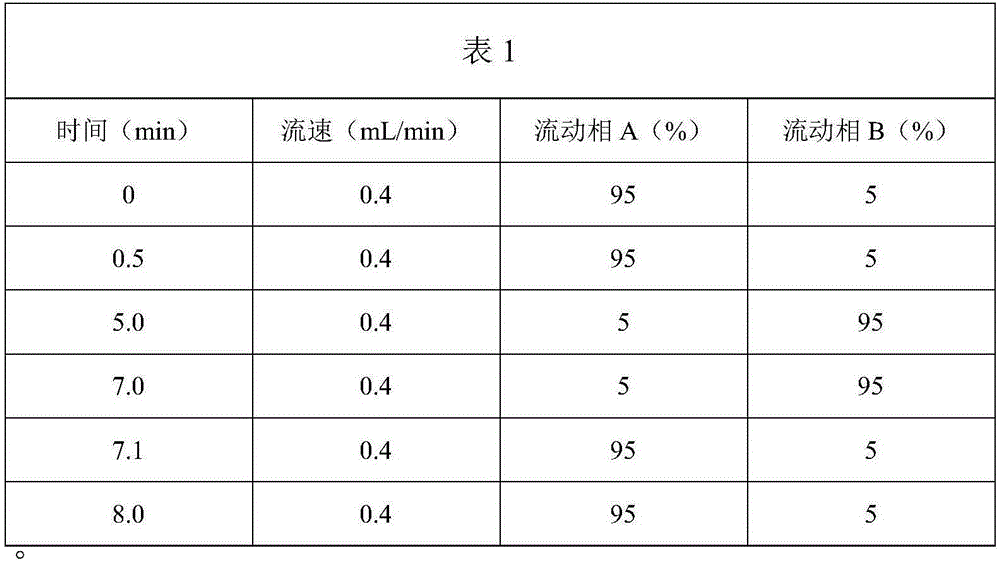
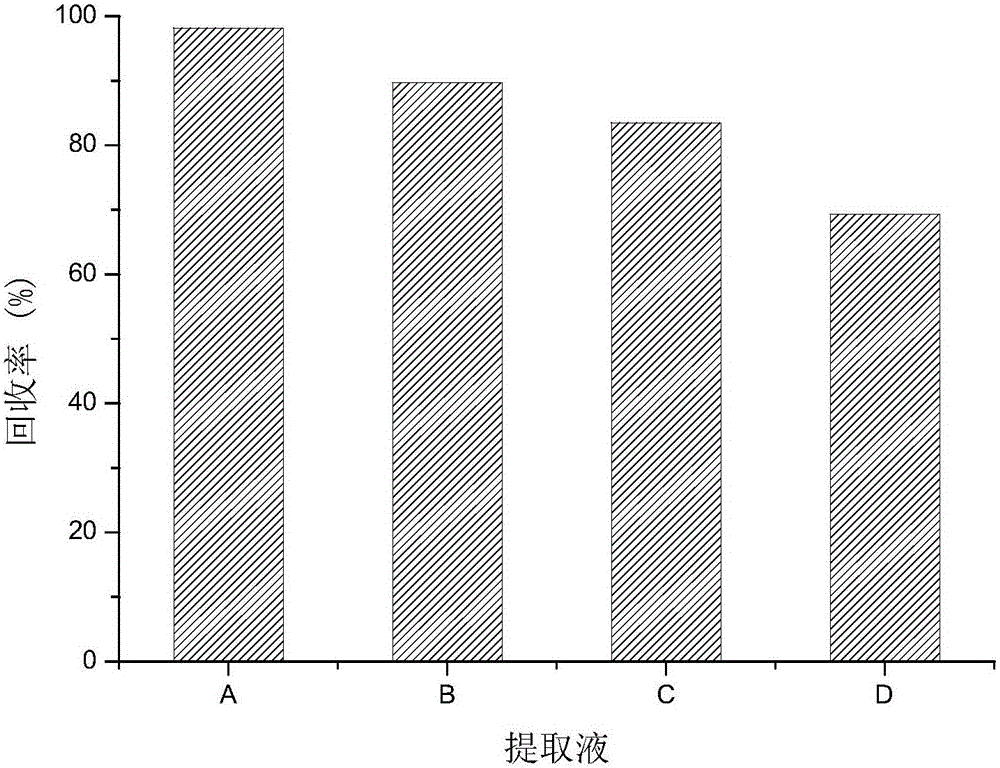
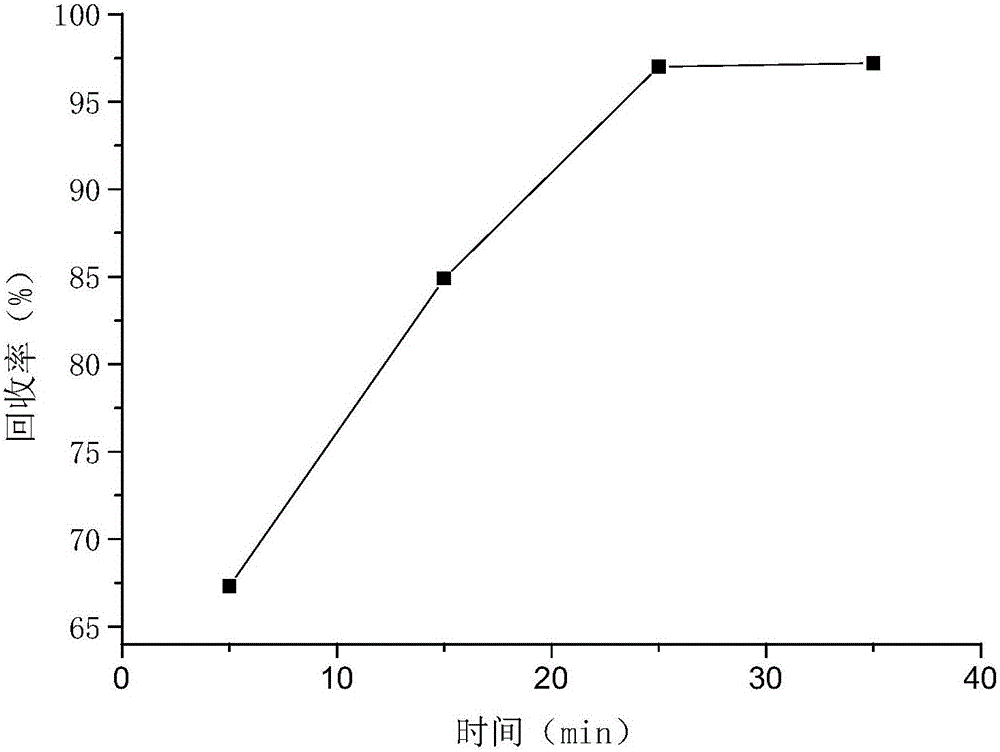
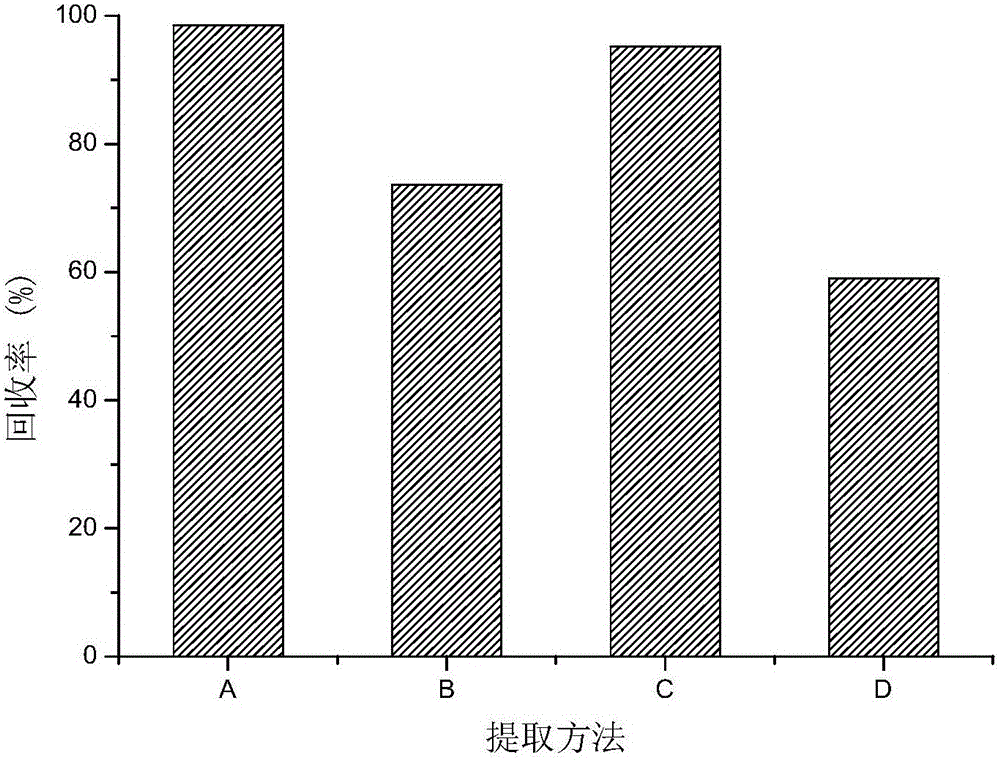
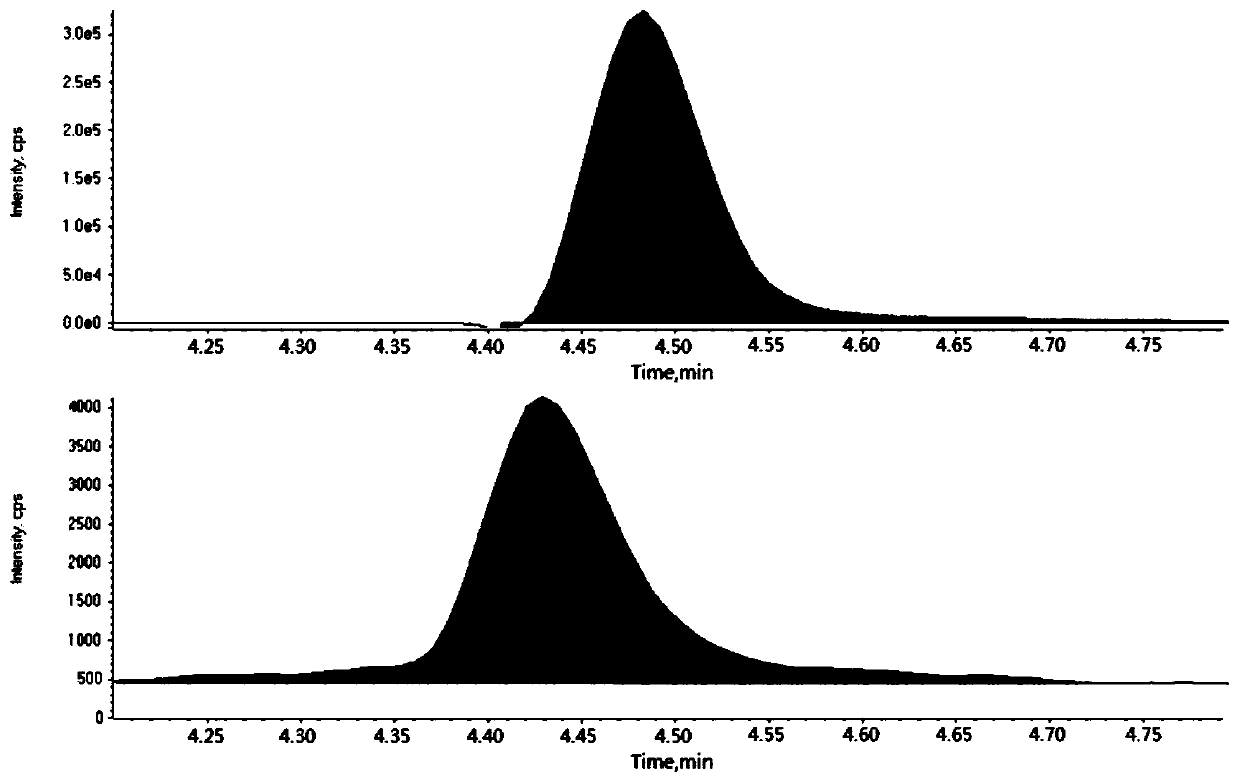
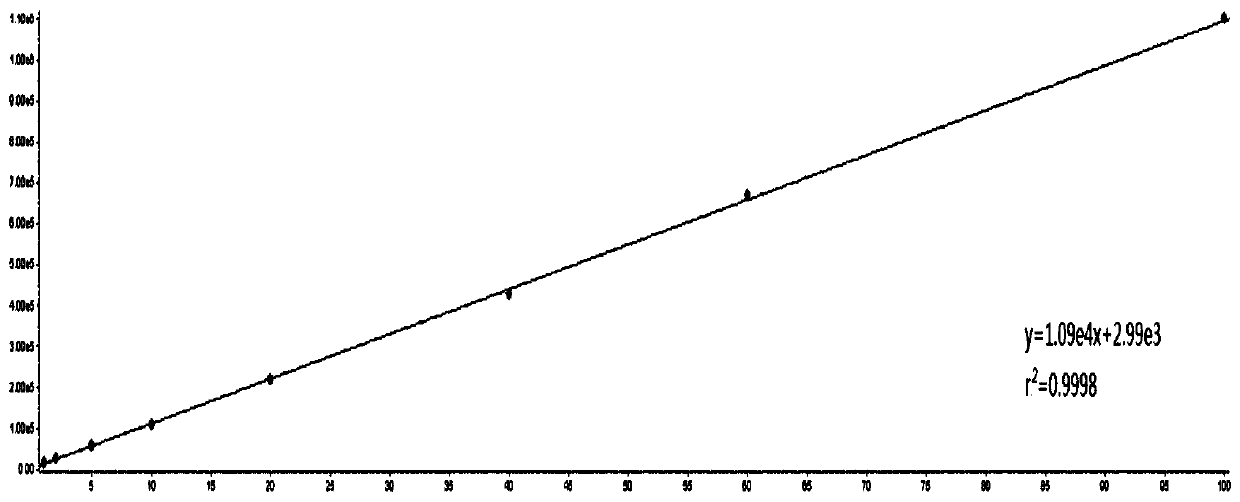
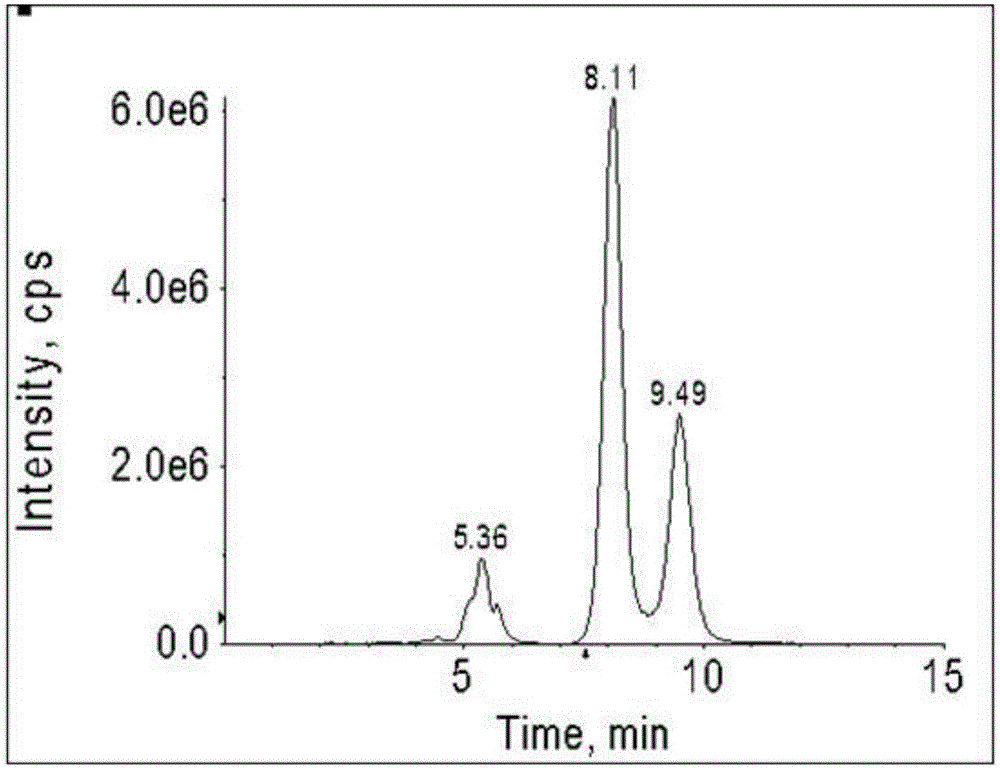
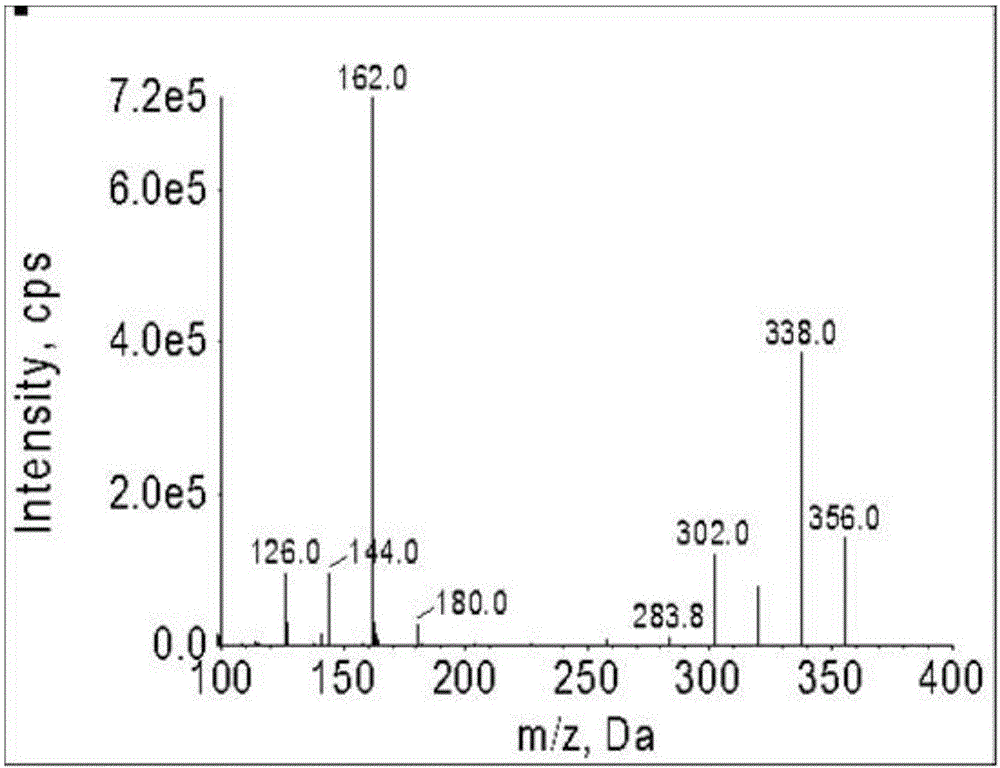
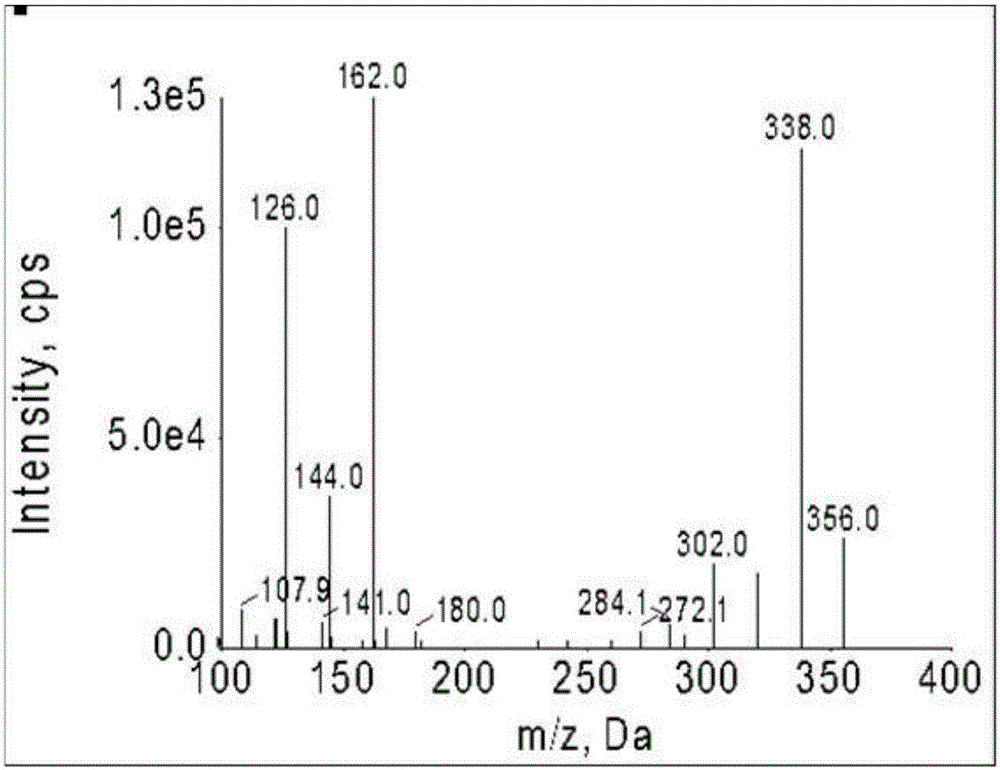
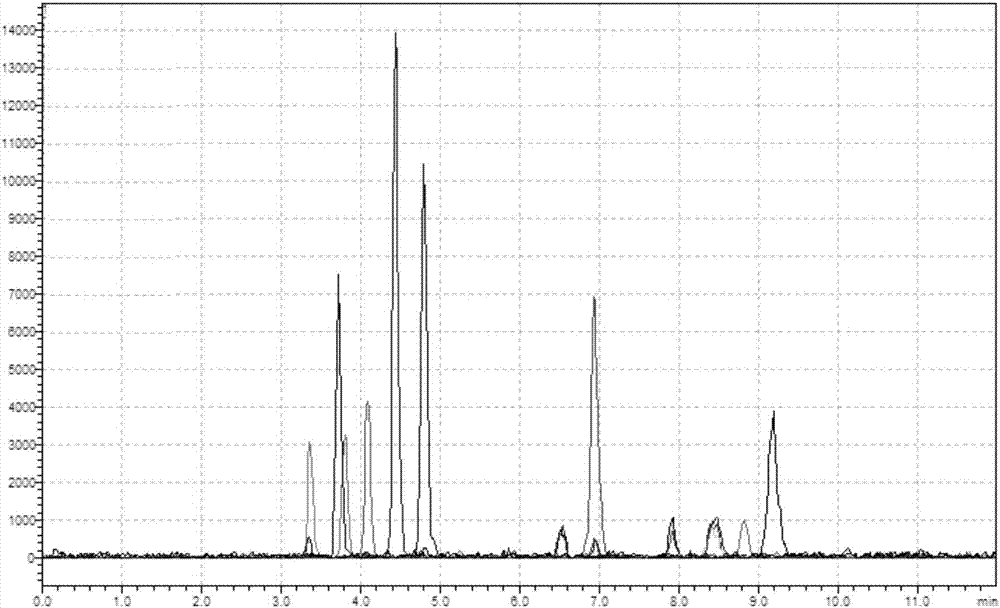
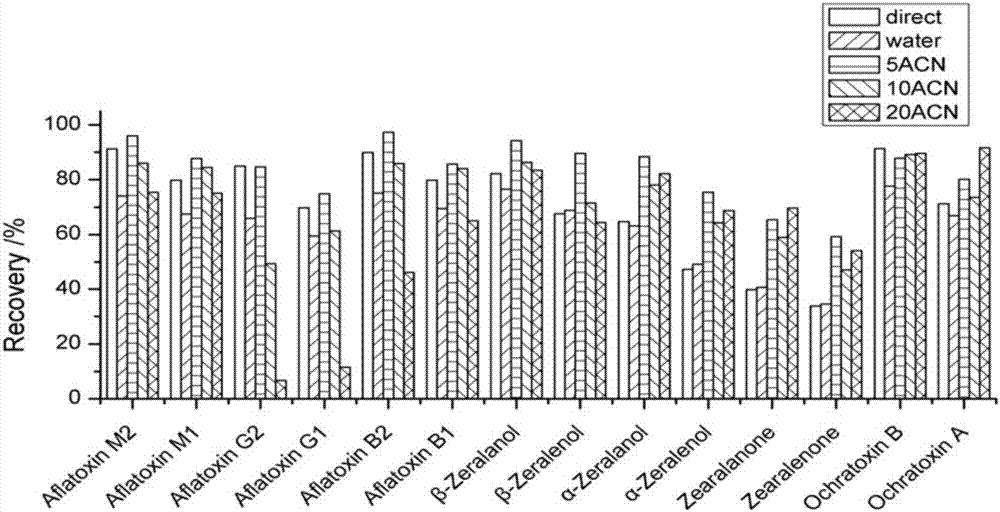
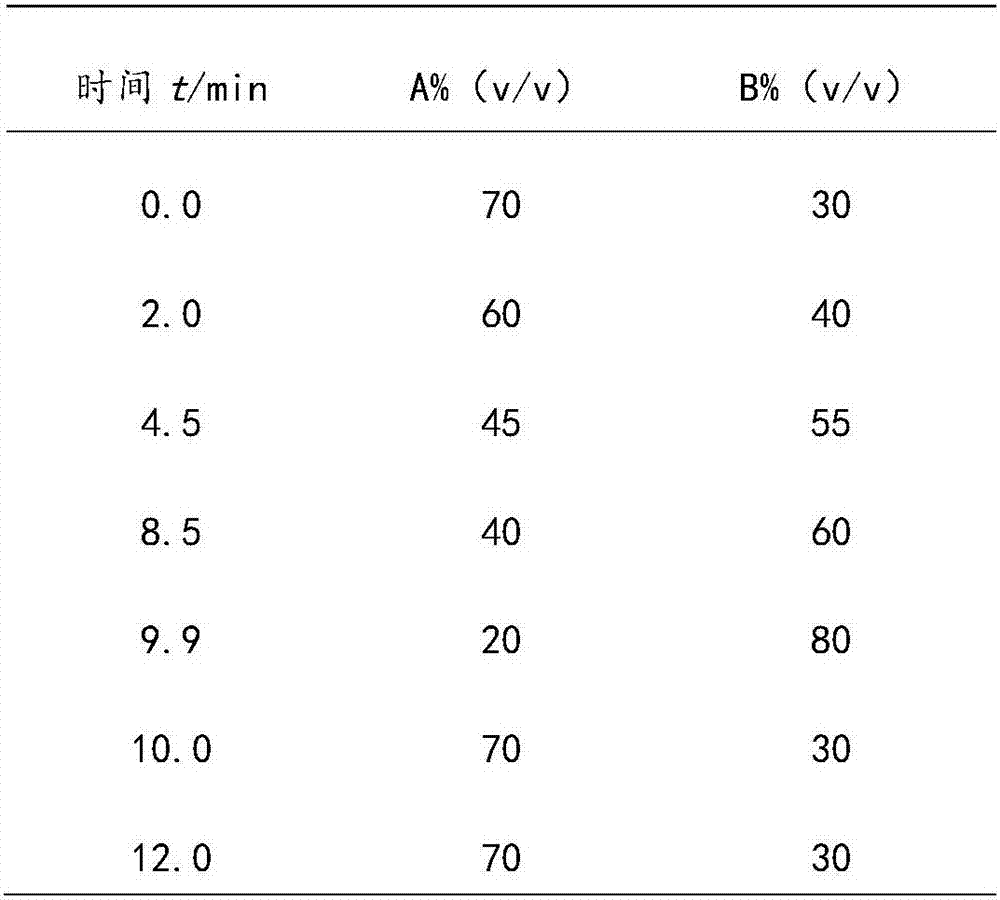
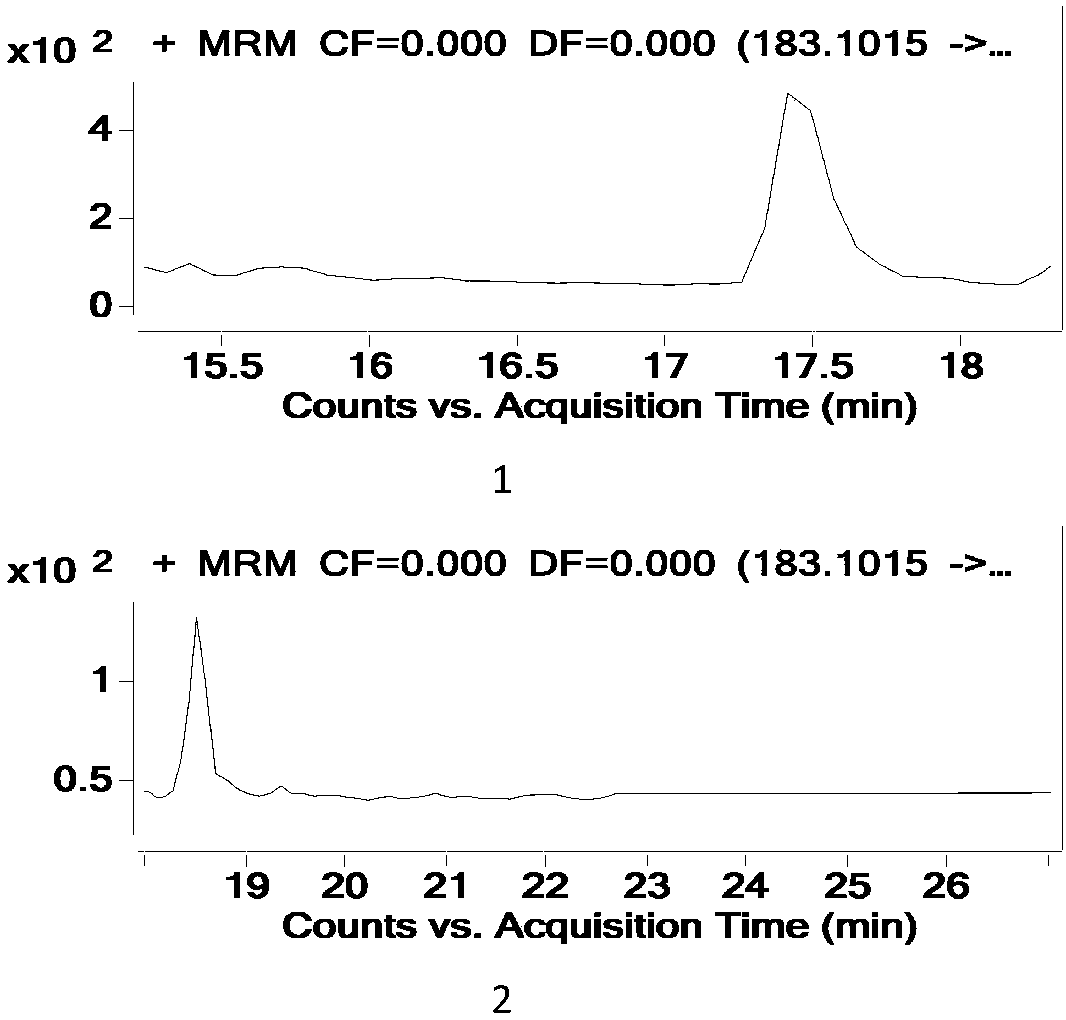
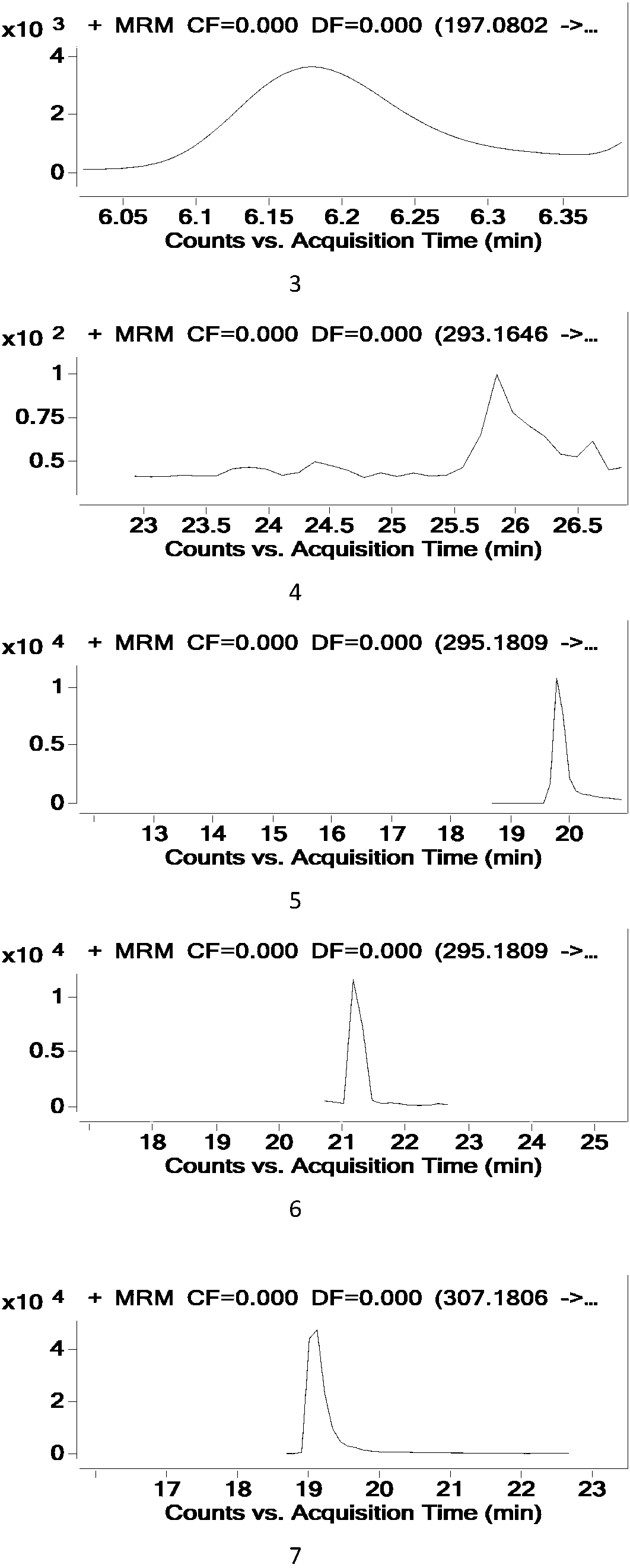
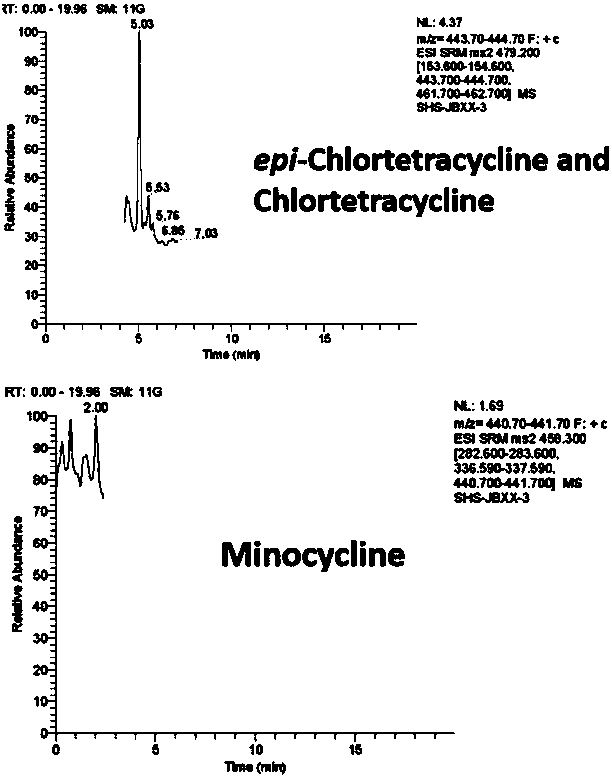
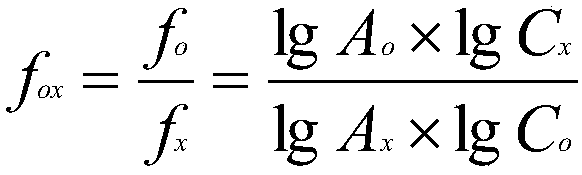Patents
Literature
109 results about "Triple quadrupole mass spectrometer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
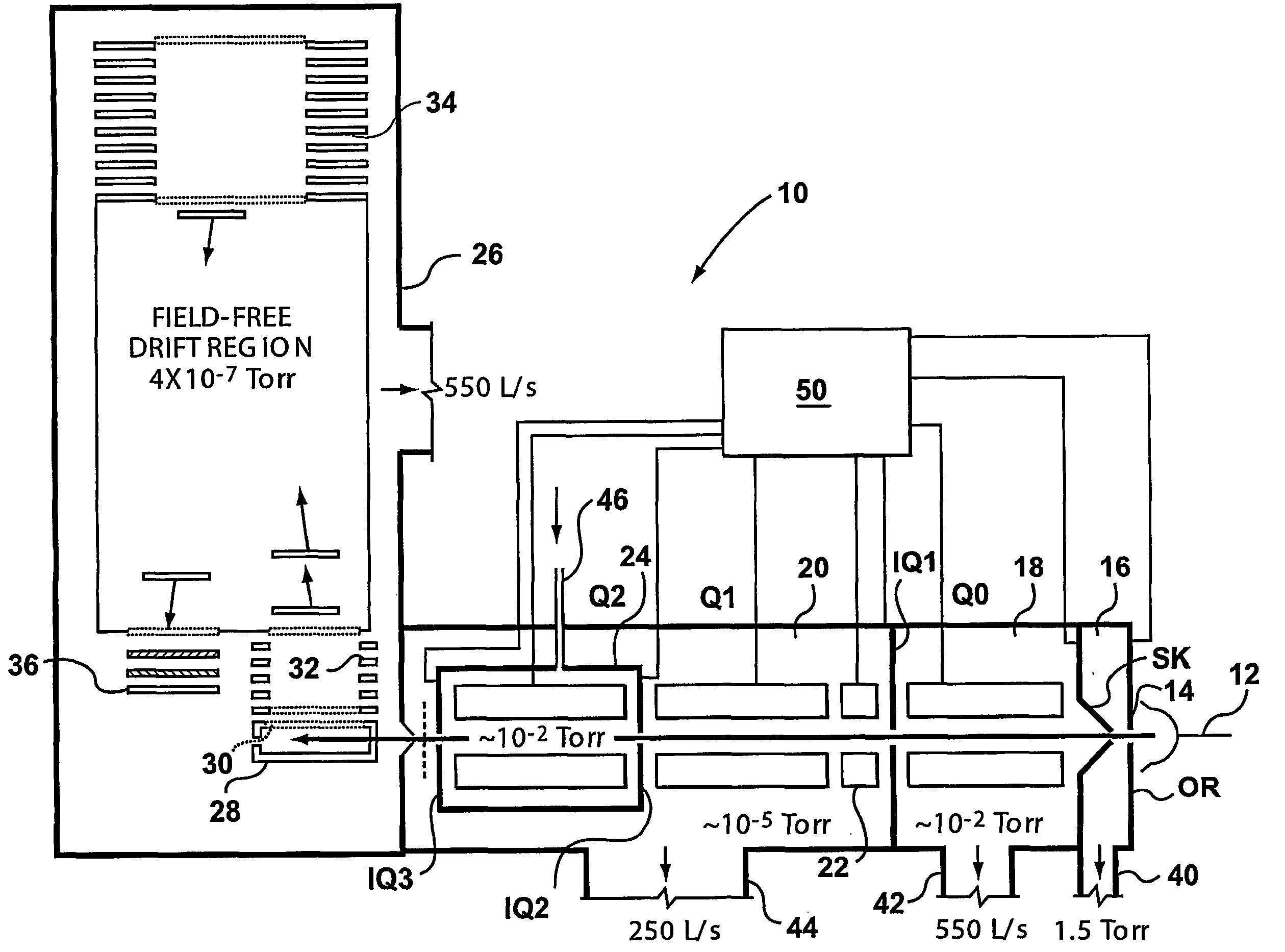
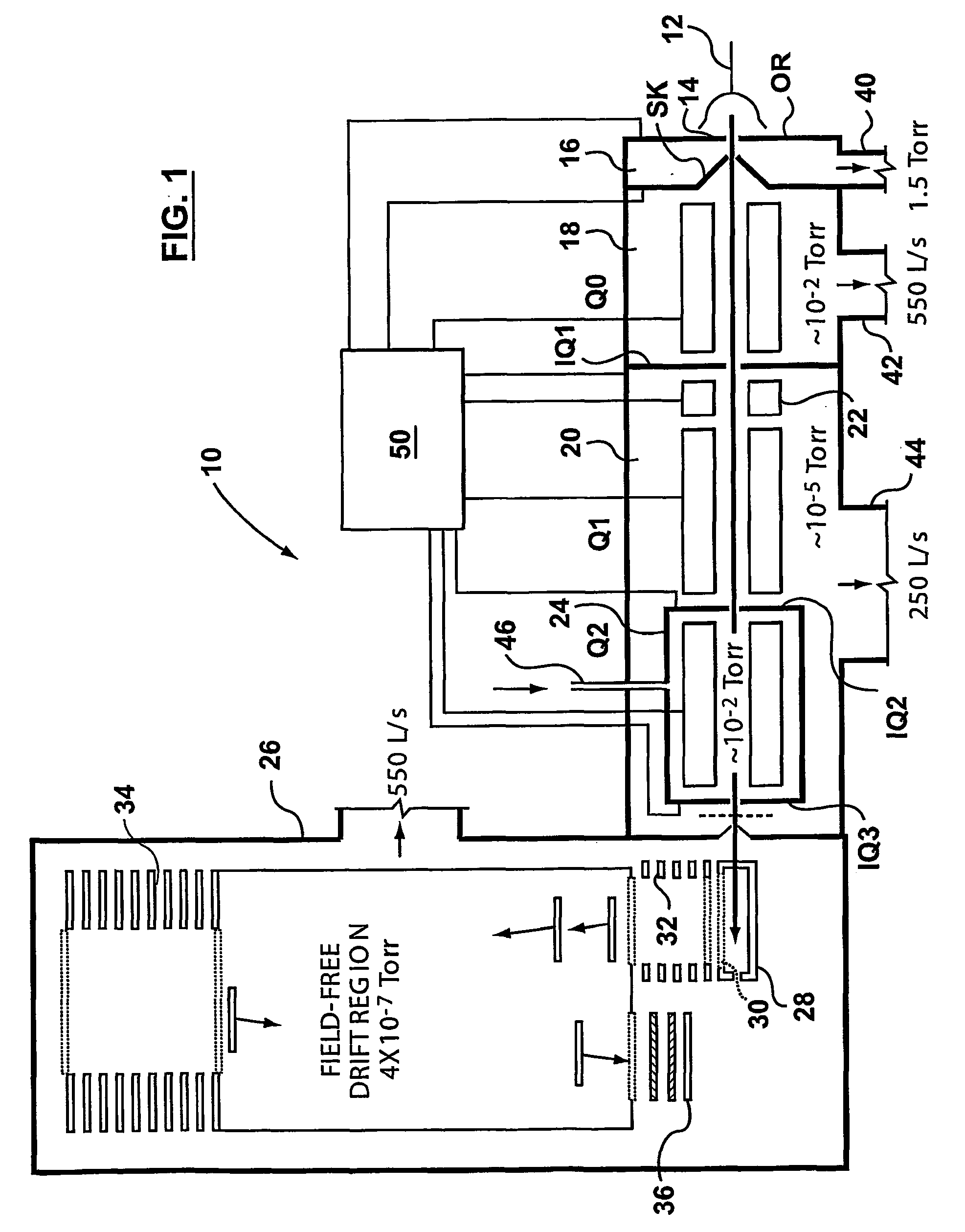
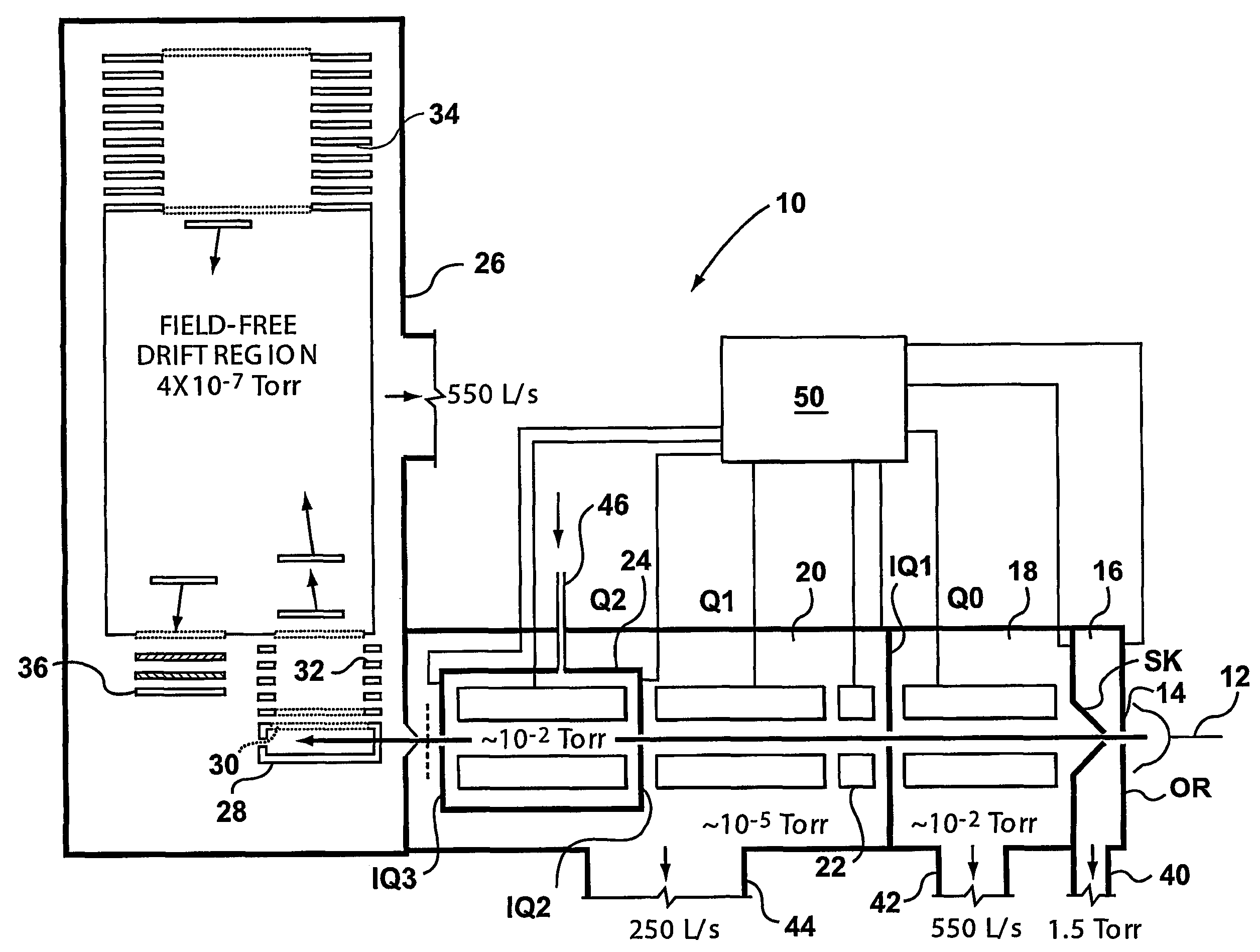
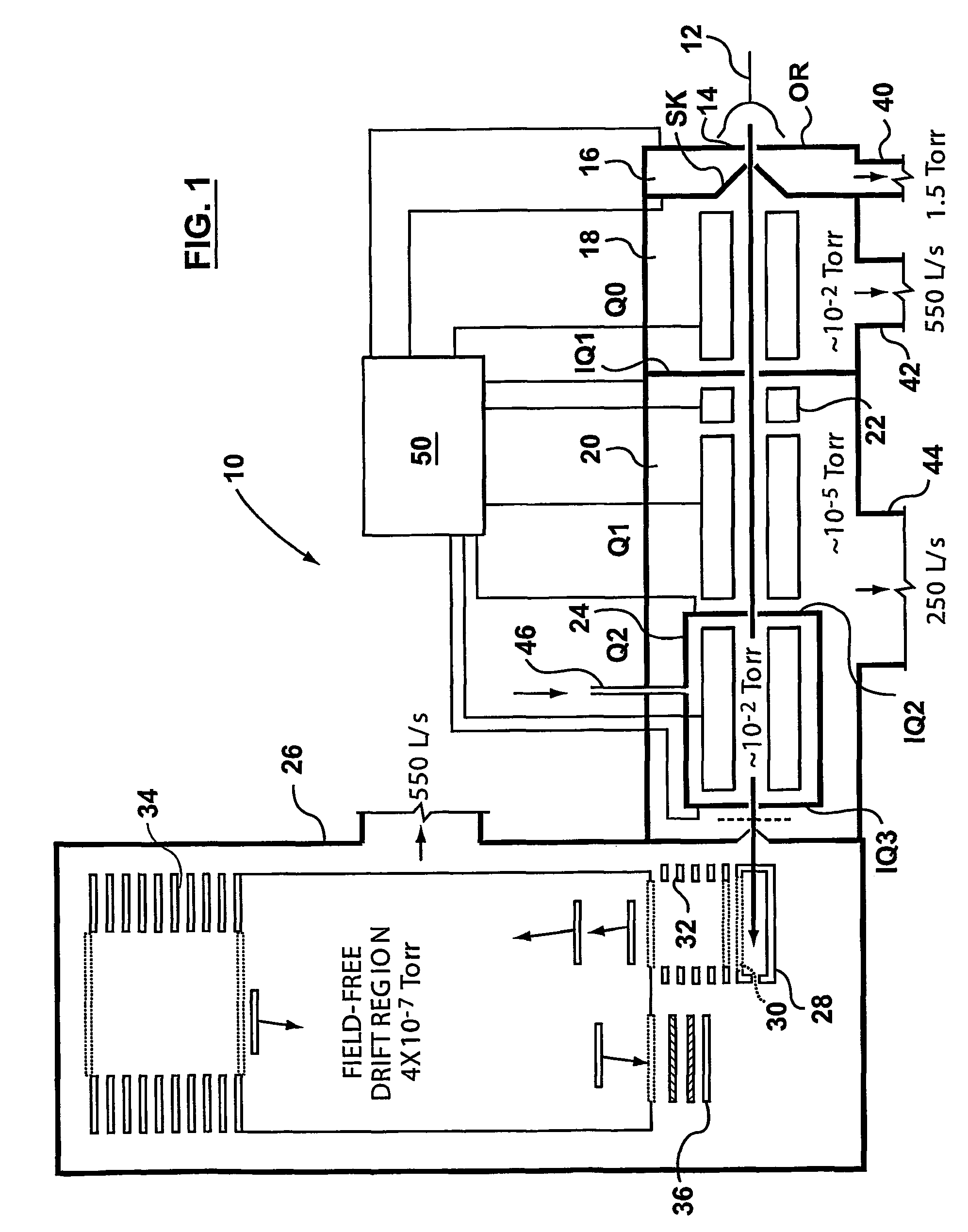
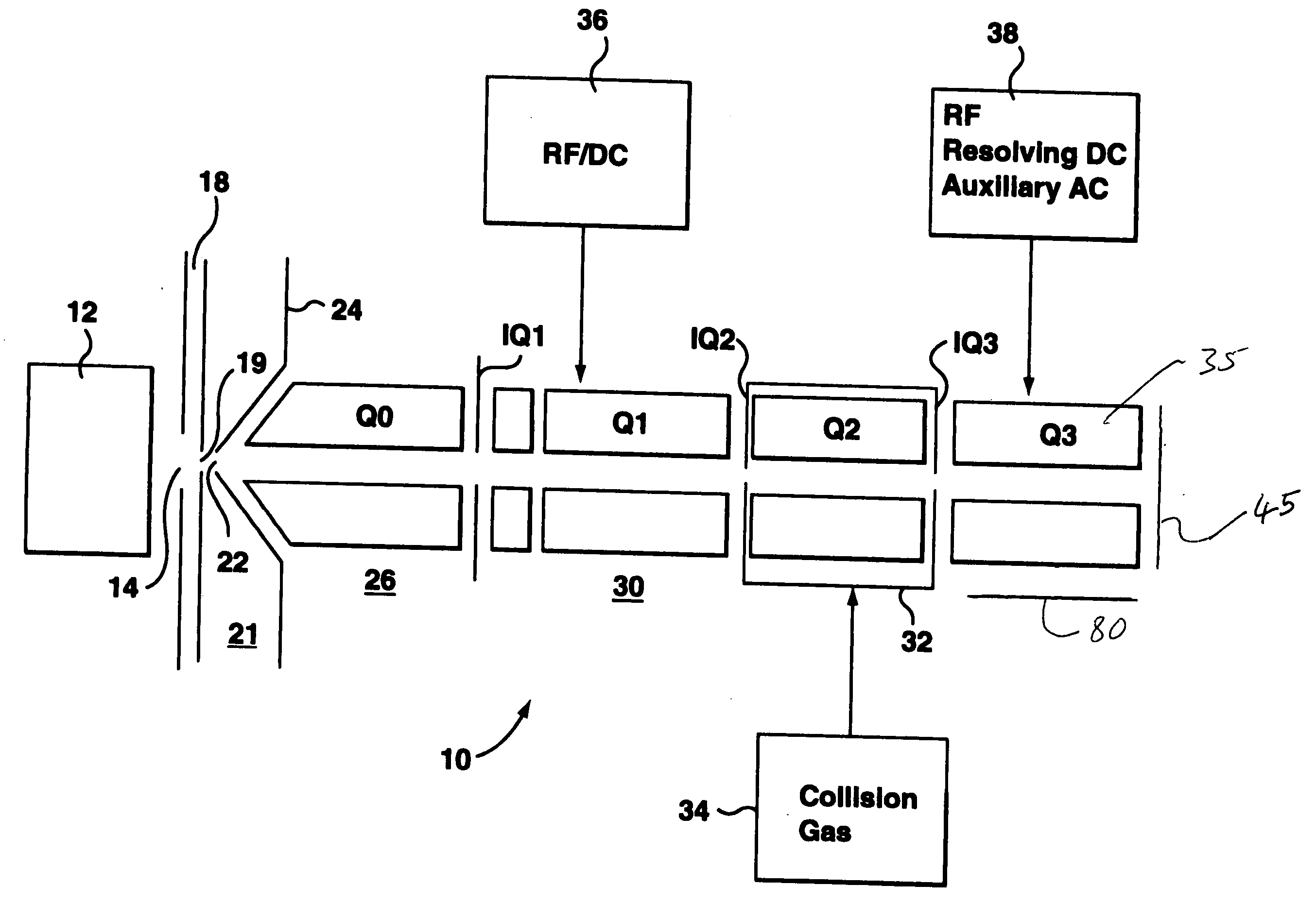
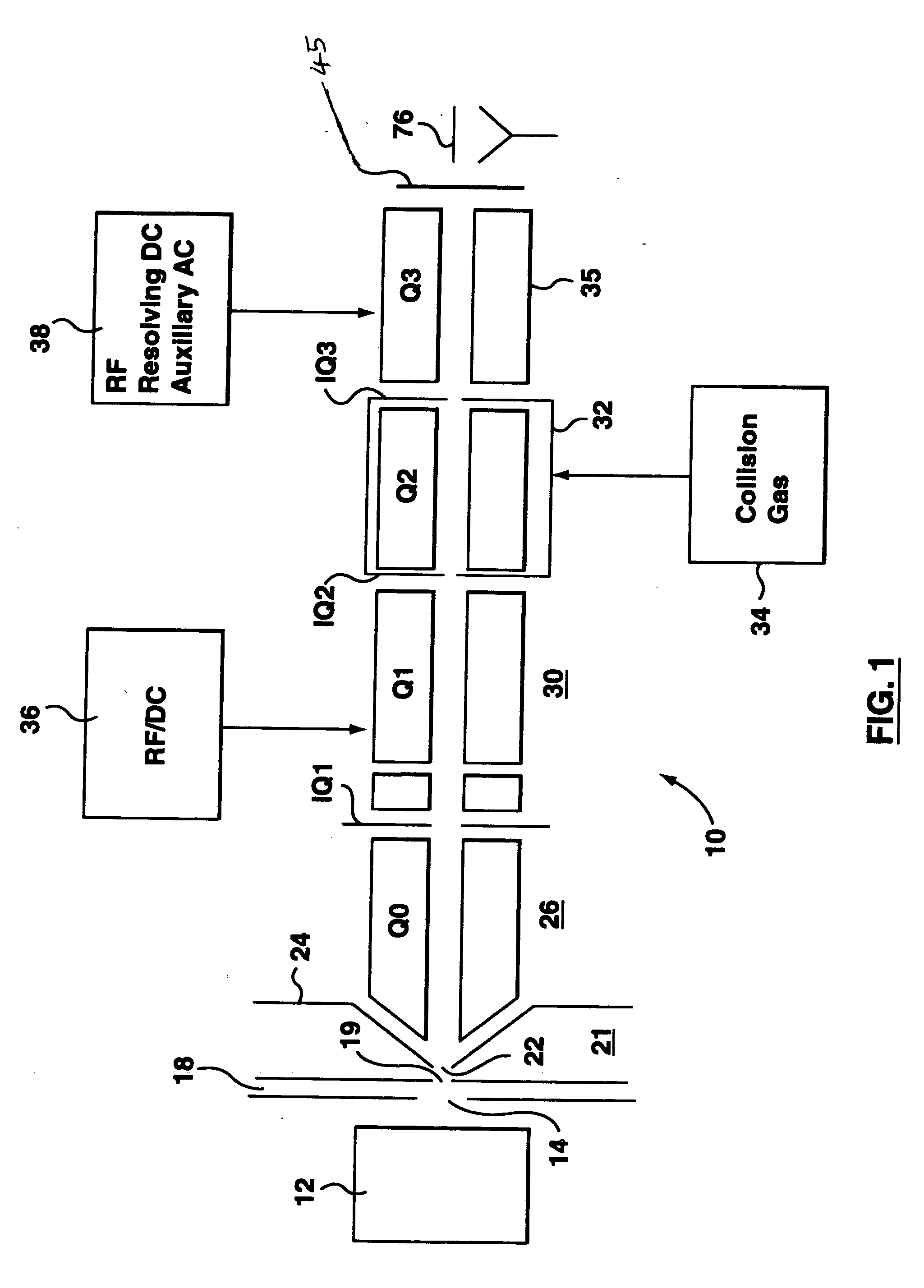
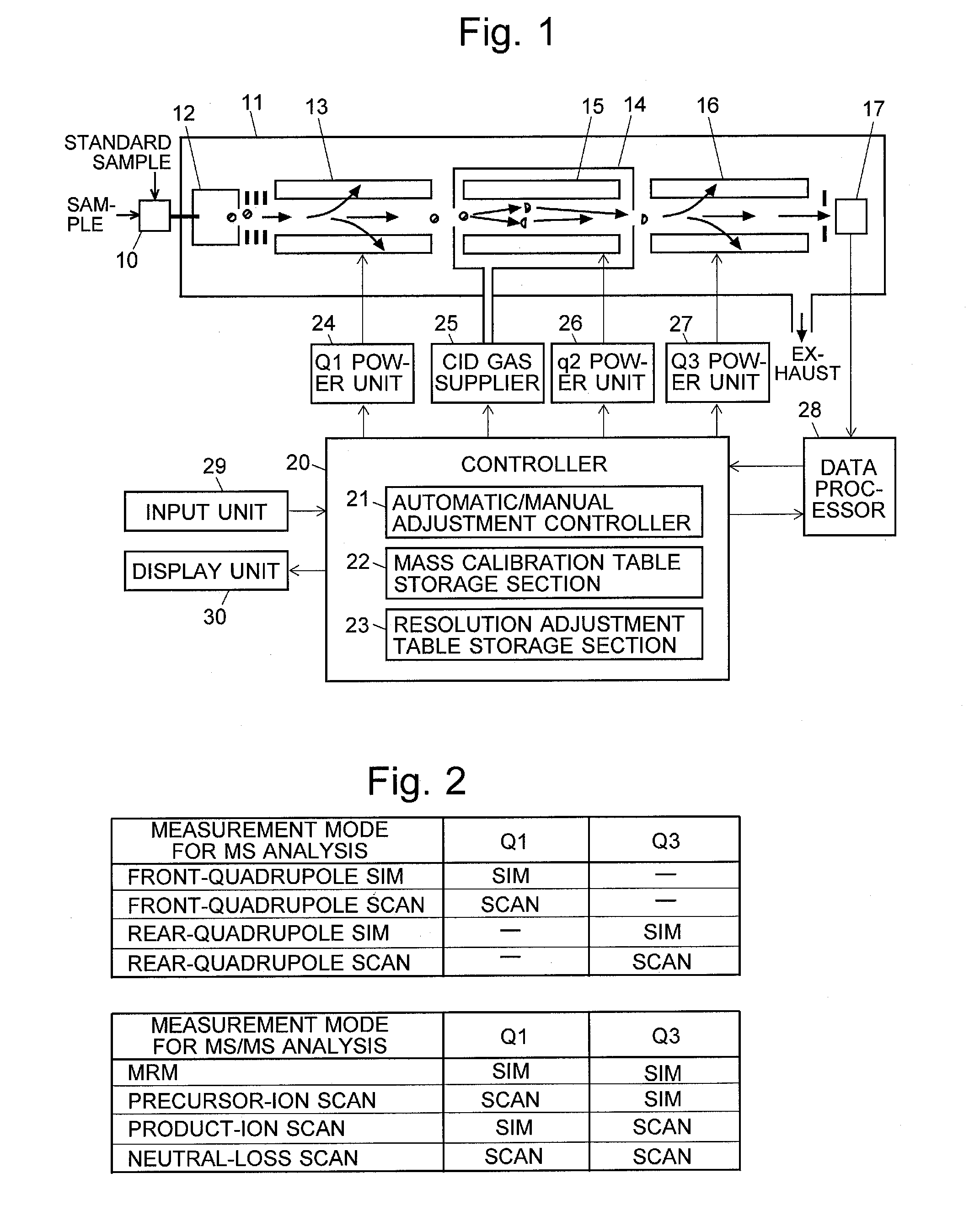
A triple quadrupole mass spectrometer (TQMS), is a tandem mass spectrometer consisting of two quadrupole mass analyzers in series, with a (non-mass-resolving) radio frequency (RF)–only quadrupole between them to act as a cell for collision-induced dissociation. This configuration is often abbreviated QqQ, here Q₁q₂Q₃.
Apparatus and method for msnth in a tandem mass spectrometer system
InactiveUS20050098719A1Simple capabilityImprove data accuracyStability-of-path spectrometersTime-of-flight spectrometersIon trap mass spectrometryTriple quadrupole mass spectrometry
A method and apparatus are provided for effecting multiple mass selection or analysis steps. Fundamentally, the technique is based on moving ions in different directions through separate components of a mass spectrometer apparatus. To effect different steps, a precursor ion is selected in a first mass selector, and then passed into a collision cell, to effect fragmentation or reaction with a gas, to generate fragment or product ions. The generated product ions are then passed back into the first mass selector, and preferably back into an upstream ion trap. The product ions then pass through the first mass selector again, to select a desired product ion, for further fragmentation and analysis. These steps can be repeated a number of times. A final mass analysis step can be effected in either a time-of-flight section or other mass analyzer. The invention enables conventional triple quadrupole mass spectrometers and QqTOF mass spectrometers to effect multiple MS steps.
Owner:MDS CO LTD +2
Apparatus and method for MSnth in a tandem mass spectrometer system
InactiveUS7145133B2Simple capabilityImprove data accuracyStability-of-path spectrometersTime-of-flight spectrometersIon trap mass spectrometryPhysical chemistry
A method and apparatus are provided for effecting multiple mass selection or analysis steps. Fundamentally, the technique is based on moving ions in different directions through separate components of a mass spectrometer apparatus. To effect different steps, a precursor ion is selected in a first mass selector, and then passed into a collision cell, to effect fragmentation or reaction with a gas, to generate fragment or product ions. The generated product ions are then passed back into the first mass selector, and preferably back into an upstream ion trap. The product ions then pass through the first mass selector again, to select a desired product ion, for further fragmentation and analysis. These steps can be repeated a number of times. A final mass analysis step can be effected in either a time-of-flight section or other mass analyzer. The invention enables conventional triple quadrupole mass spectrometers and QqTOF mass spectrometers to effect multiple MS steps.
Owner:MDS CO LTD +2
Triple quadrupole mass spectrometer with capability to perform multiple mass analysis steps
A method of analyzing a substance comprises ionizing the substance to form a string of ions. The ions are then subject to a first mass analysis step. In one embodiment, the ions are accelerated into a collision cell in known manner to form primary fragment ions. These primary fragment ions are then accelerated into a downstream mass analyzer, to promote secondary fragmentation. In another embodiment of the invention, ions are passed through the collision cell, without fragmentation, and then accelerated from the collision cell into a low pressure section, which may be a mass analyzer or a rod set for collecting and collimating ions. This is done under conditions that promote fragmentation. The operating conditions of the low pressure section can be such as to promote collection or retention of ions depending upon their mass, and more specifically to reject low mass ions. This enables primary fragment ions to be cooled, and secondary fragment ions to be formed subsequently from these ions after they have disipated some of their energy. This enables control of secondary fragmentation processes, and offers numerous opportunities for analyzing complex ions.
Owner:DH TECH DEVMENT PTE +2
Triple quadrupole mass spectrometer with capability to perform multiple mass analysis steps
InactiveUS7060972B2Stability-of-path spectrometersIsotope separationTriple quadrupole mass spectrometryMass analyzer
A method of analyzing a substance comprises ionizing the substance to form a string of ions. The ions are then subject to a first mass analysis step. In one embodiment, the ions are accelerated into a collision cell in known manner to form primary fragment ions. These primary fragment ions are then accelerated into a downstream mass analyzer, to promote secondary fragmentation. In another embodiment of the invention, ions are passed through the collision cell, without fragmentation, and then accelerated from the collision cell into a low pressure section, which may be a mass analyzer or a rod set for collecting and collimating ions. This is done under conditions that promote fragmentation. The operating conditions of the low pressure section can be such as to promote collection or retention of ions depending upon their mass, and more specifically to reject low mass ions. This enables primary fragment ions to be cooled, and secondary fragment ions to be formed subsequently from these ions after they have disipated some of their energy. This enables control of secondary fragmentation processes, and offers numerous opportunities for analyzing complex ions.
Owner:DH TECH DEVMENT PTE +2
Milk and milk product tetracycline antibiotic residual quantity checking method
ActiveCN101290306AShort detection timeHigh sensitivityComponent separationTesting medicinal preparationsTriple quadrupole mass spectrometryGradient elution
The invention relates to a method for detecting the residue amount of terracycline antibiotics in milk and dairy products. The method utilizes an ultra performance liquid chromatography - electrospray tandem triple quadrupole mass spectrometer to determine the residue amount of the terracycline antibiotics. The method is as follows: a sample is extracted from Na2EDTA-McIlvaine buffer solution (pH4.0); proteins are removed by trichloroacetic acids; a columella is extracted through an HLB solid phase and then purified and enriched; the sample is separated by a chromatographic column, with the column temperature of 30 DEG C; gradient elution is performed by utilization of water solution (v / v) which contains 0.1 percent of methanoic acids and acetonitrile as a moving phase; and quantitative detection is performed by adoption of the multi-reaction monitoring means. The detection limit of instruments is between 1.0 and 2.0 mu g / kg; a related coefficient r reaches over 0.999 within the linear range of between 1 and 100 mu g / kg; and the recovery rate is between 81.7 and 100.7 percent (the addition levels are 10 mu g / kg, 50 mu g / kg and 100 mu g / kg). The method has the advantages of quickness, accuracy, high sensitivity and wide application scope.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Liquid chromatography-mass spectrometry method for rapidly detecting content of beta-agonists and sample pretreatment method
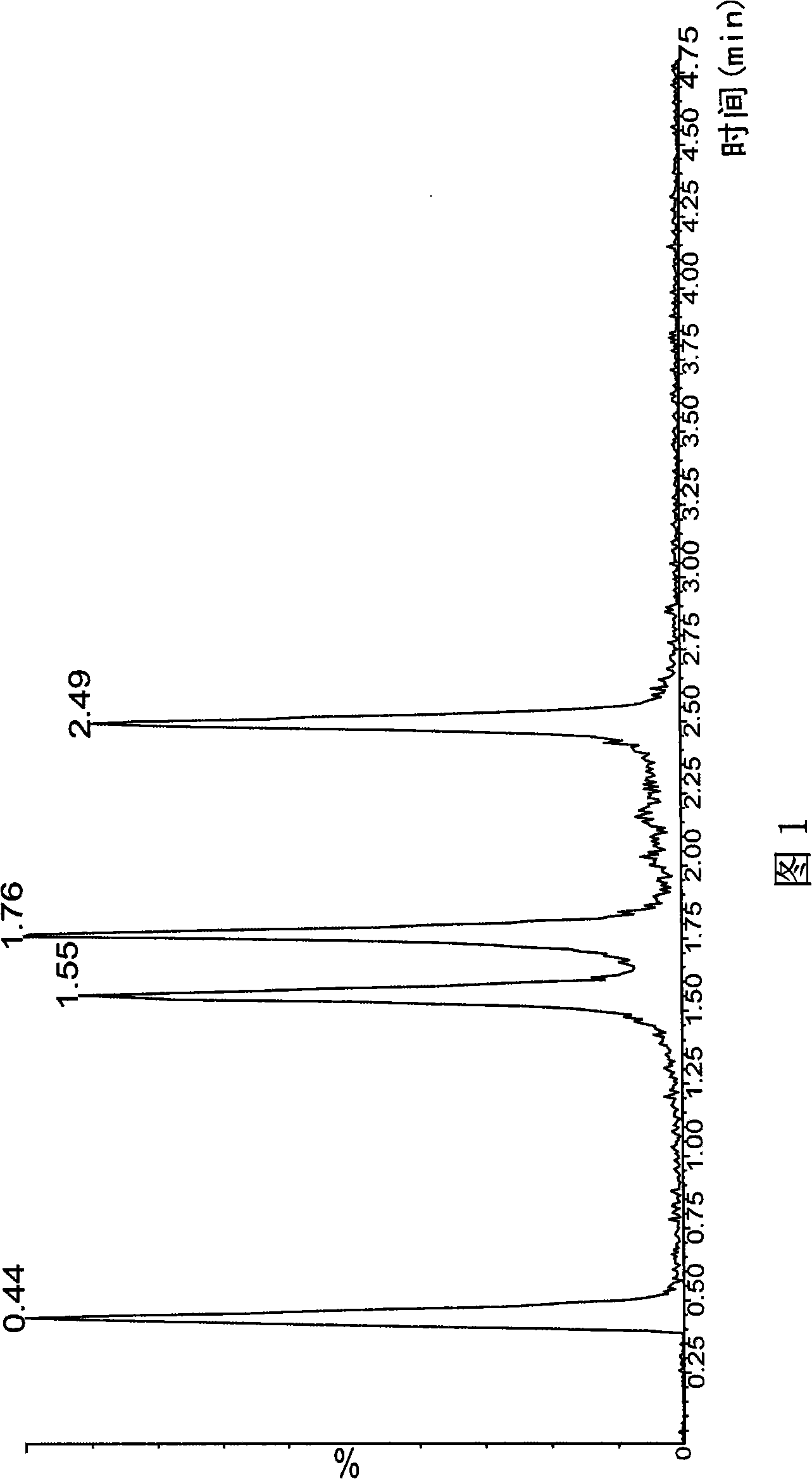
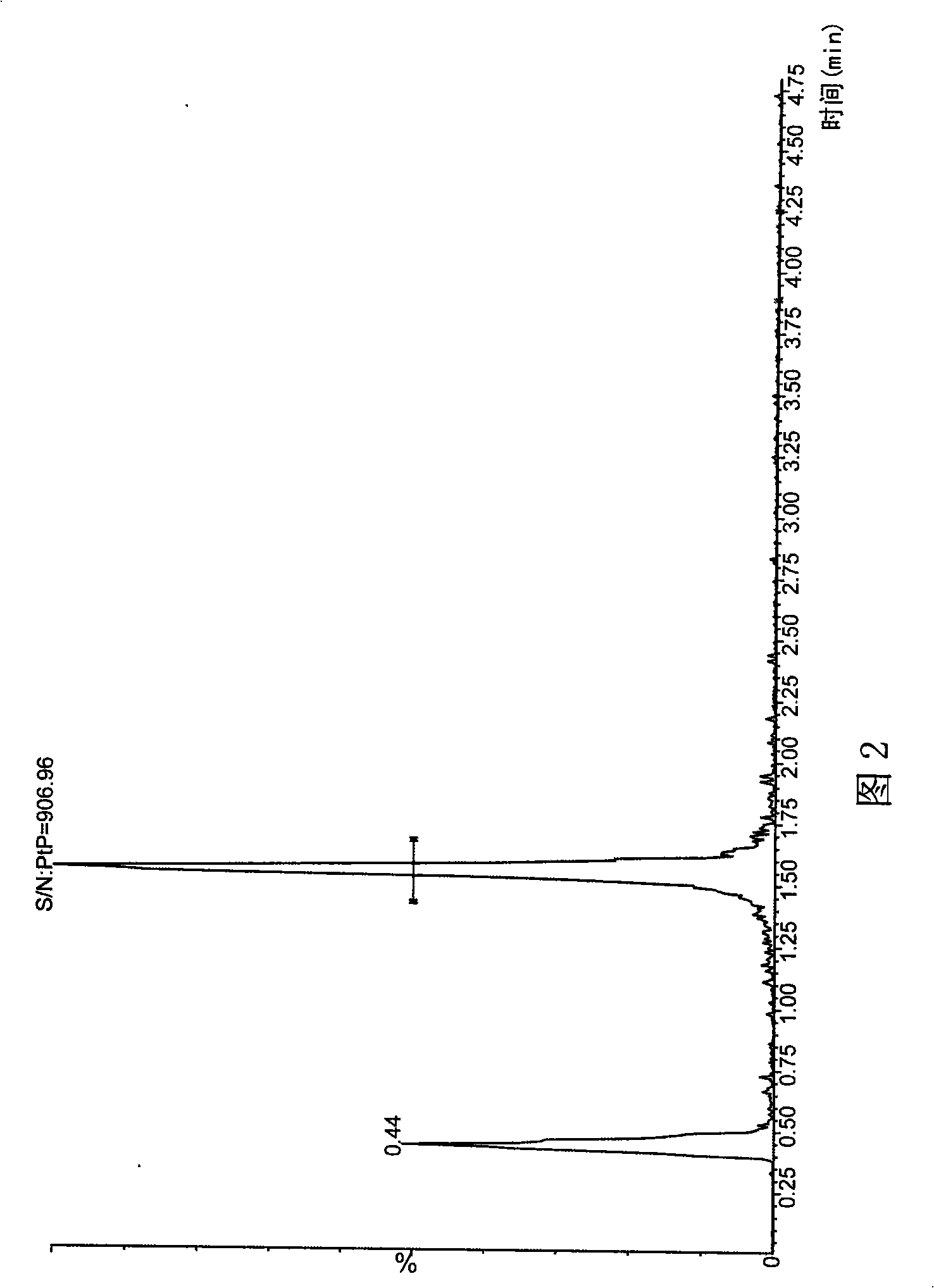
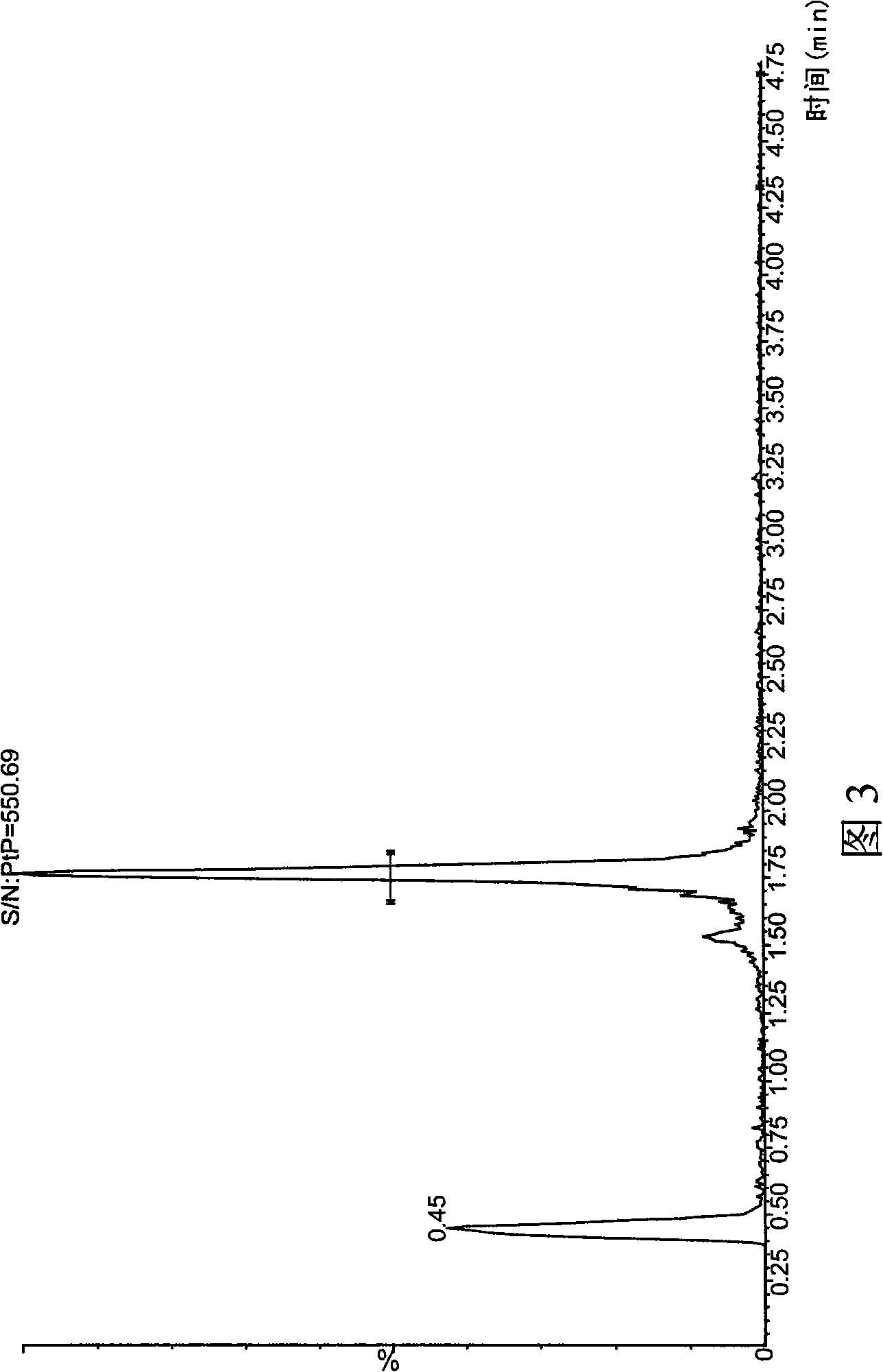
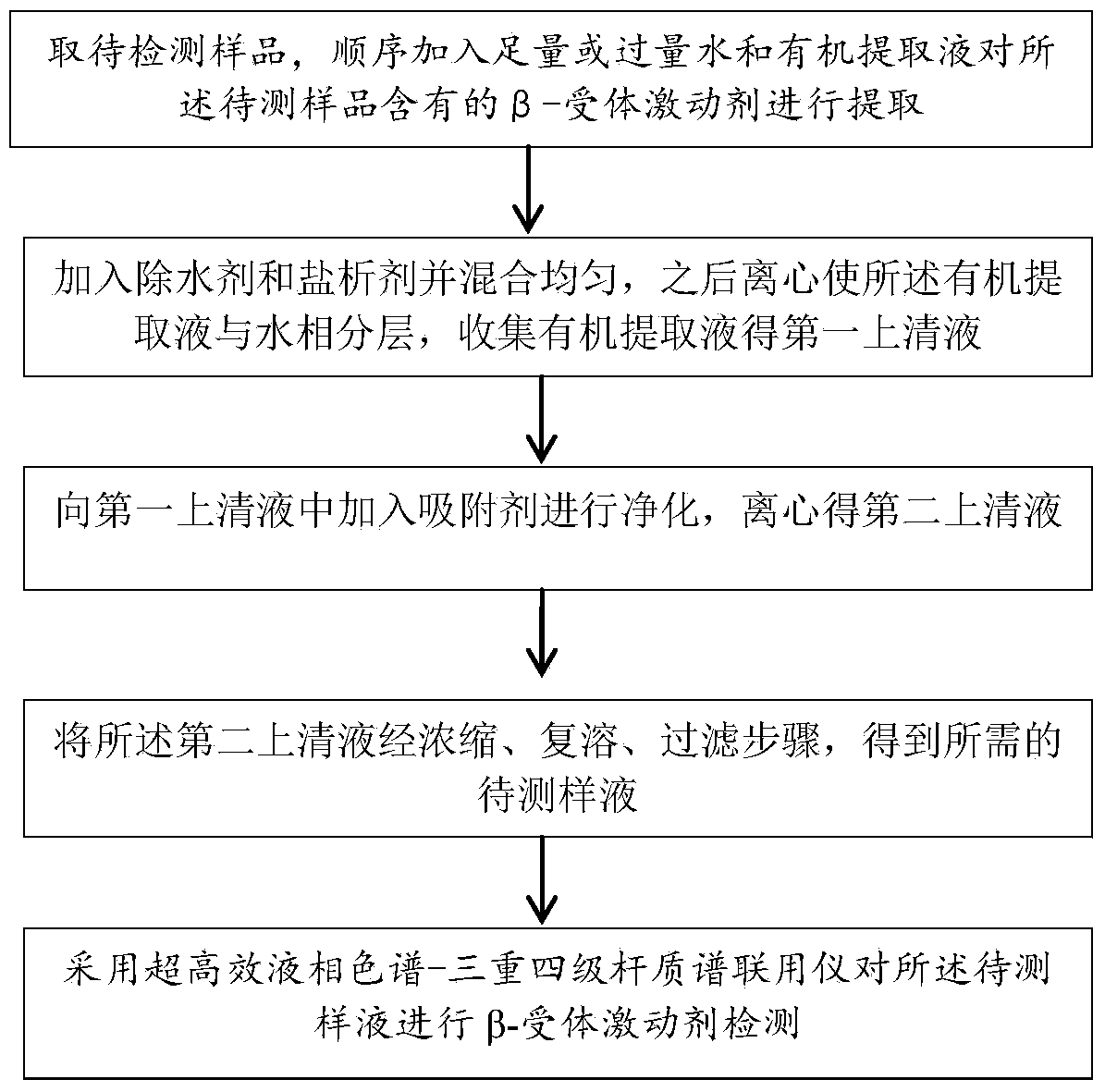
The invention provides a liquid chromatography-mass spectrometry method for rapidly detecting the content of beta-agonists. The liquid chromatography-mass spectrometry method comprises the following steps: pretreating a sample, namely, sequentially adding water and an organic extracting solution in the sample for extracting a beta-receptor stimulant; then adding a salting-out agent and a water removing agent to ensure that the organic extracting solution and a water phase demix, centrifuging to obtain a first supernate, adding an adsorbing agent in the first supernate for purification, centrifuging to obtain a second supernate, concentrating, re-dissolving and filtering the second supernate to prepare a sample to be detected; and then detecting the beta-receptor stimulant contained in an animal-derived food by adopting an ultra-high performance liquid chromatography-triple quadrupole mass spectrometer. According to the sample pretreatment method suitable for liquid chromatography-mass spectrometry detection, provided by the invention, interfering molecules are removed through extraction and purification so that high accuracy is achieved in sample detection.
Owner:BEIJING ENTRY EXIT INSPECTION & QUARANTINE BUREAU INSPECTION & QUARANTINE TECH CENT
Method for measuring diallyl phthalate migration in food contact materials
ActiveCN103424489AAccurately Measure MigrationQuantitatively accurate and reliableComponent separationFood contact materialsPhysical chemistry
The invention discloses a method for measuring diallyl phthalate migration in food contact materials. The method includes the steps: firstly, preparing a standard working solution, a food stimulant test solution and a blank test solution; secondly, adopting a high performance liquid chromatograph-tandem triple quadrupole mass spectrometer to perform liquid chromatograph-tandem mass spectrum measurement on the three solutions in the first step, drawing a regression curve of the standard working solution by taking concentration x of phthalate in the standard working solution as an abscissa and corresponding measured quantitative ion peak area y as an ordinate, and calculating slope a and intercept b of the regression curve according to a linear equation, of y=ax+b, obtained by the curve; thirdly, according a formula that c=[(y-y)-b] / a, calculating concentration of diallyl phthalate in the food stimulant test solution. By the aid of the method, migration of the diallyl phthalate in the food contact materials can be measured accurately, quantification is accurate and reliable, and reproducibility is good.
Owner:常州进出口工业及消费品安全检测中心
Method for detecting residual quantity of norfloxacin antibiotic in milk
InactiveCN101609073AShort detection timeHigh sensitivityComponent separationPreparing sample for investigationTriple quadrupole mass spectrometryColumn temperature
The invention relates to a method for detecting residual quantity of norfloxacin antibiotic in milk. The method utilizes an ultra-performance liquid chromatography (UPLC)-electrospray tandem triple quadrupole mass spectrometer to determine the residual quantity of norfloxacin antibiotic. A sample is extracted by Na2 EDTA-Mellvaine buffer solution (pH 4.0), subjected to protein removal by trichloroacetic acid, purified and concentrated by a PEP solid phase extraction columella, separated by adopting a chromatographic column with column temperature of 30 DEG C, subjected to gradient elution by adopting aqueous solution (v / v) containing 0.1% formic acid and acetonitrile as a flowing phase, and then quantitated by adopting a multiple reaction monitoring manner. The instrument detection limit is 1.0-2.0 mug / Kg; and within the linear range of 1-100 mug / Kg, the correlation coefficient r can reach more than 0.999, and the coefficient of recovery is 80%-105% (adding level of 10, 50 and 100 mug / Kg). The method has the advantages of rapidness, accuracy, high sensitivity and wide application scope.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Methods for detecting substances in biological samples
InactiveUS20120153138A1Low costAccurate detectionIsotope separationMass spectrometersMedicineTriple quadrupole mass spectrometry
Methods for simultaneously detecting multiple drug classes in a biological sample are described herein. In one embodiment, the biological sample is taken from an animal, such as a human. Suitable classes of drugs include drugs prone to abuse and prescription medications. In one embodiment, the biological sample is enzymatically hydrolyzed to liberate free drug in the sample. Once the biological sample has been prepared, the sample is extracted using solid-phase extraction (SPE). Following solid phase extraction (SPE), the resulting eluate containing the compounds to be detected is diluted and injected into a liquid chromatograph coupled to a triple quadrupole mass spectrometer. Multiple classes of drugs can be analyzed simultaneously in less than about 13 minutes, preferably less than about 11 minutes, more preferably less than about 10 minutes, more preferably less than about 9 minutes, most preferably less than about 8 minutes.
Owner:ELAB CONSULTING SERVICES
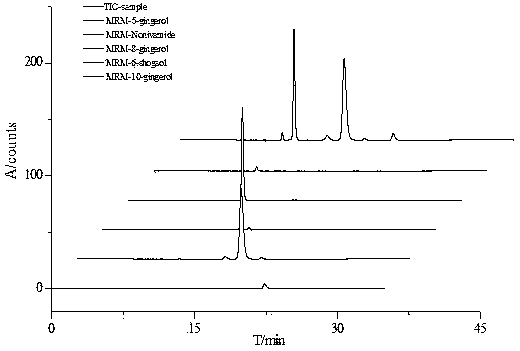
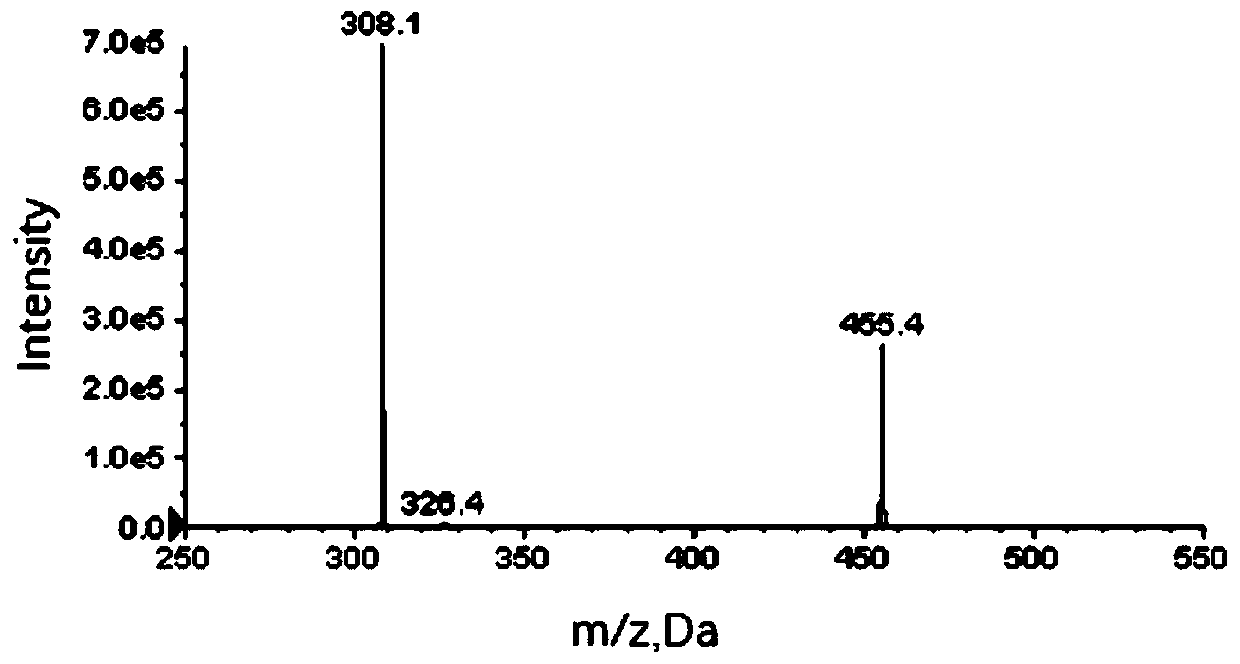
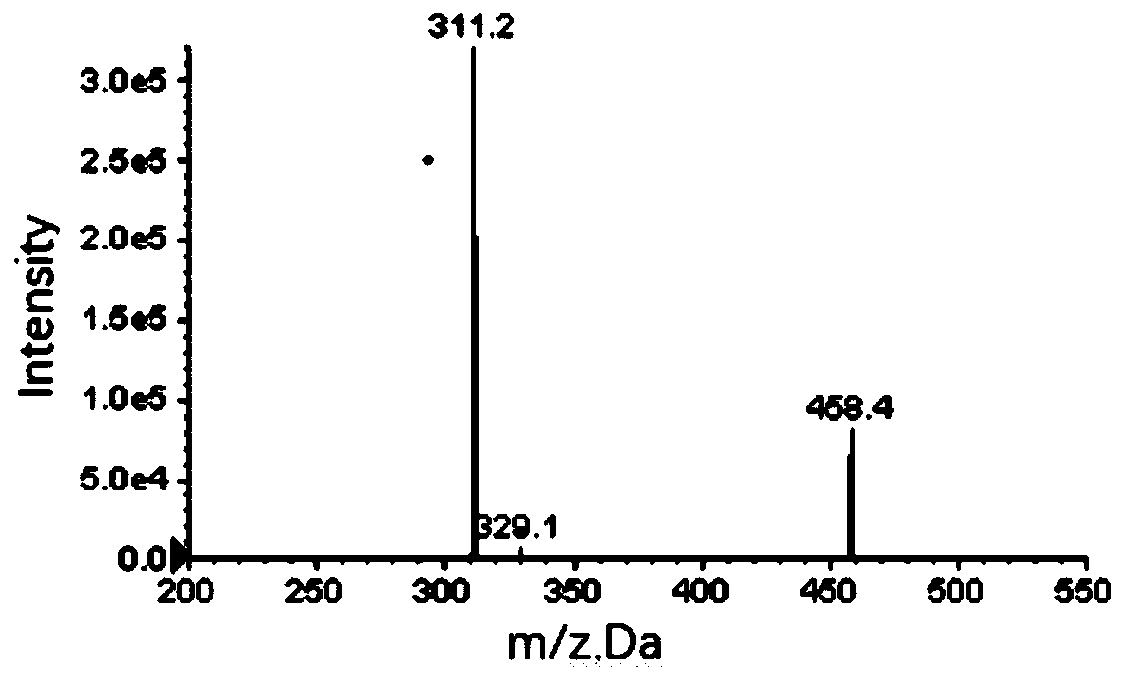
Method for measuring content of gingerol in ginger medicinal materials and preparations thereof
ActiveCN108414665AAccurate evaluationHigh sensitivityComponent separationMass spectrometry detectorHplc mass spectrometry
The invention discloses a method for measuring the content of gingerol in ginger medicinal materials and preparations thereof. By virtue of the separation and analysis technology of ultra-high performance liquid chromatography in series with a triple quadrupole mass spectrometer detector and an ultraviolet detector as well as a one-measurement multi-evaluation method, a pure substance N-vanillyl nonane amide reference substance which does not exist in a sample, has stable properties and is easy to obtain is adopted as an internal reference substance so as to establish a relative correction factor between the component and four gingerol components in the sample, thereby realizing measurement of the contents of the four gingerol components in the ginger medicinal materials and the preparations thereof by virtue of calculation of the correction factor. The method disclosed by the invention is simple in operation, high in sensitivity, accurate and efficient and low in cost, can be used forobjectively and accurately evaluating the quality of the ginger medicinal materials and the preparations thereof, can be used for quality control, can solve the problem that the quality of the medicinal materials and the preparations thereof can not be objectively and reasonably controlled due to the lack of reference substances, and has important significance for controlling quality and ensuringcurative effects.
Owner:河南省纳普生物技术有限公司
Method for rapid determination of blood concentration of methotrexate
InactiveCN110320302AThe pre-processing process is simpleImprove accuracyComponent separationData acquisitionBlood plasma
The invention relates to a method for rapid determination of blood concentration of methotrexate. The method comprises the following steps: firstly, adding a to-be-determined plasma sample into ammonium acetate -formic acid water solution, then, adding methanol solution containing MTX-d3 and eddying for 25s-35s, then, performing centrifugal separation, and then, collecting liquid supernatant, injecting the liquid supernatant into a rapid liquid chromatography system to perform separation analysis, then, injecting into a triple quadrupole mass spectrometer detector for detection, further performing data acquisition and processing through a chromatography software, and obtaining the blood concentration of the methotrexate. The method for rapid determination of blood concentration of methotrexate of the invention is rapid and accurate, highly sensitive, simple to operate and low-cost, and is suitable for clinical routine blood concentration monitoring. Preprocessing of the sample is simple, the amount of needed sample is low, so the method is suitable for clinical routine detection and pharmacokinetic study. An interior label is added in a sample preprocessing process, thus, accuracyof MTX quantification is improved, and specificity is high and sensitivity is high.
Owner:北京陆道培生物技术有限公司
Method for detecting mycotoxin in fermented tea
ActiveCN106950328AFix extraction issuesSolve measurement problemsComponent separationMycotoxinFiltration
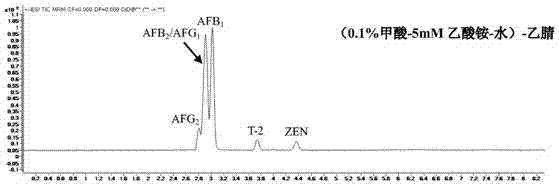
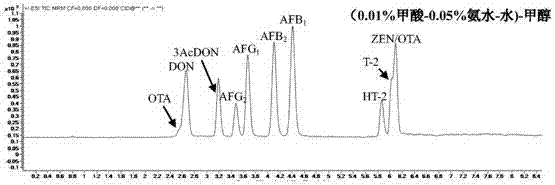
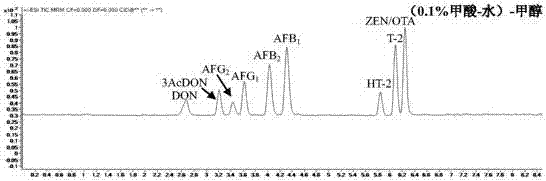
The invention discloses a method for detecting mycotoxin in fermented tea. The method comprises dissolving a fermented tea sample in a polar solution, carrying out salting-out, carrying out centrifugation, carrying out filtration, putting the filtrate in 1290 / 6460 ultra-high performance liquid chromatography-triple quadrupole mass spectrometer, carrying out analysis through a mobile phase (0.1% formic acid-5 mmol / L ammonium acetate-water)-methanol, wherein the mobile phase A is 0.1% formic acid-5 mmol / L ammonium acetate-water and the mobile phase B is methanol, and carrying out separation through a gradient elution method comprising improving a concentration of the mobile phase B step by step so that the mycotoxin in the fermented tea sample reaches to the base line in short time and then is separated. The method realizes effective extraction and determination of various mycotoxins in fermented tea, can detect a variety of mycotoxins at the same time and has the characteristics of high detection efficiency, low environmental pollution, simple operation and promotion and use easiness.
Owner:中山市食品药品检验所
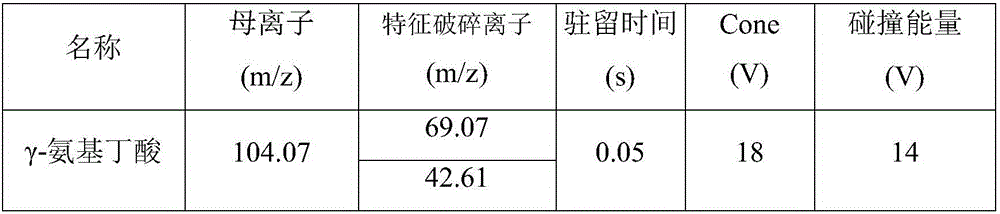
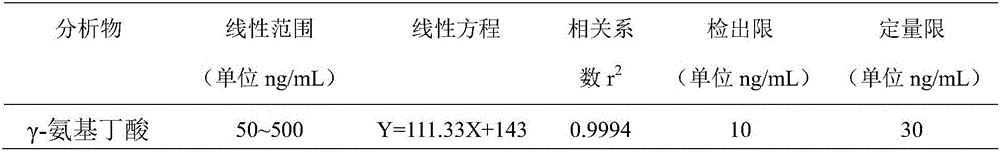
Method for fast detecting gamma-aminobutyric acid in baijiu
InactiveCN106442766AQuick judgmentQualitatively accurateComponent separationAcetic acidIsocratic elution
The invention discloses a method for fast detecting gamma-aminobutyric acid in baijiu. The method is characterized in that an ultra-high performance liquid chromatography serially connected triple quadrupole mass spectrometer is used for detecting the gamma-aminobutyric acid in baijiu; after the rotary evaporation for alcohol removal on a baijiu sample to be tested, an ACQUITY UPLC BEH C18 1.7 mum 2.1*50mm Column liquid chromatography column is used; an acetonitrile (A)+0.1 percent acetic acid water solution (B) is used as a flowing phase to perform isocratic elution; after a sample is subjected to ultra performance liquid chromatography separation, detection is performed through the triple quadrupole mass spectrometer. The method provided by the invention has the advantages that the method is simple; the qualitative and quantitative detection on gamma-aminobutyric acid in the baijiu can be accurately performed; the scientific basis is provided for the accurate judgment and fast detection of the gamma-aminobutyric acid in the baijiu.
Owner:ANHUI RUISIWEIER TECH
Method for determining tris (2,3-dibromopropyl) phosphate content of water
The invention provides a method for determining tris (2, 3-dibromopropyl) phosphate content of water, and belongs to the field of organic phosphate flame retardant. The method mainly comprises the following steps: (1) conducting solid-phase extraction on a sample; (2) eluting and collecting the extraction column; (3) concentrating the eluent obtained in the step (2) to a constant volume; and (4) determining the concentrated liquid with a high-performance liquid chromatograph-triple quadrupole mass spectrometer. The method provided by the invention improves determination method of tris (2,3-dibromopropyl) phosphate in water environment; and through ultra performance liquid chromatograph-triple quadrupole mass spectrometry combination, the method for detecting tris (2,3-dibromopropyl) phosphate method has more advantages than other methods. The present invention using ultra performance liquid chromatograph has larger separation speed than gas chromatograph and general high performance liquid chromatograp; triple quadrupole mass spectrometry for SRM scanning realizes higher selectivity, more accurate qualitative diagnosis, and improved sensitivity. Therefore, the method provided by the invention has obvious advantages compared with other gas-phase method and liquid mass chromatography method.
Owner:NANJING UNIV
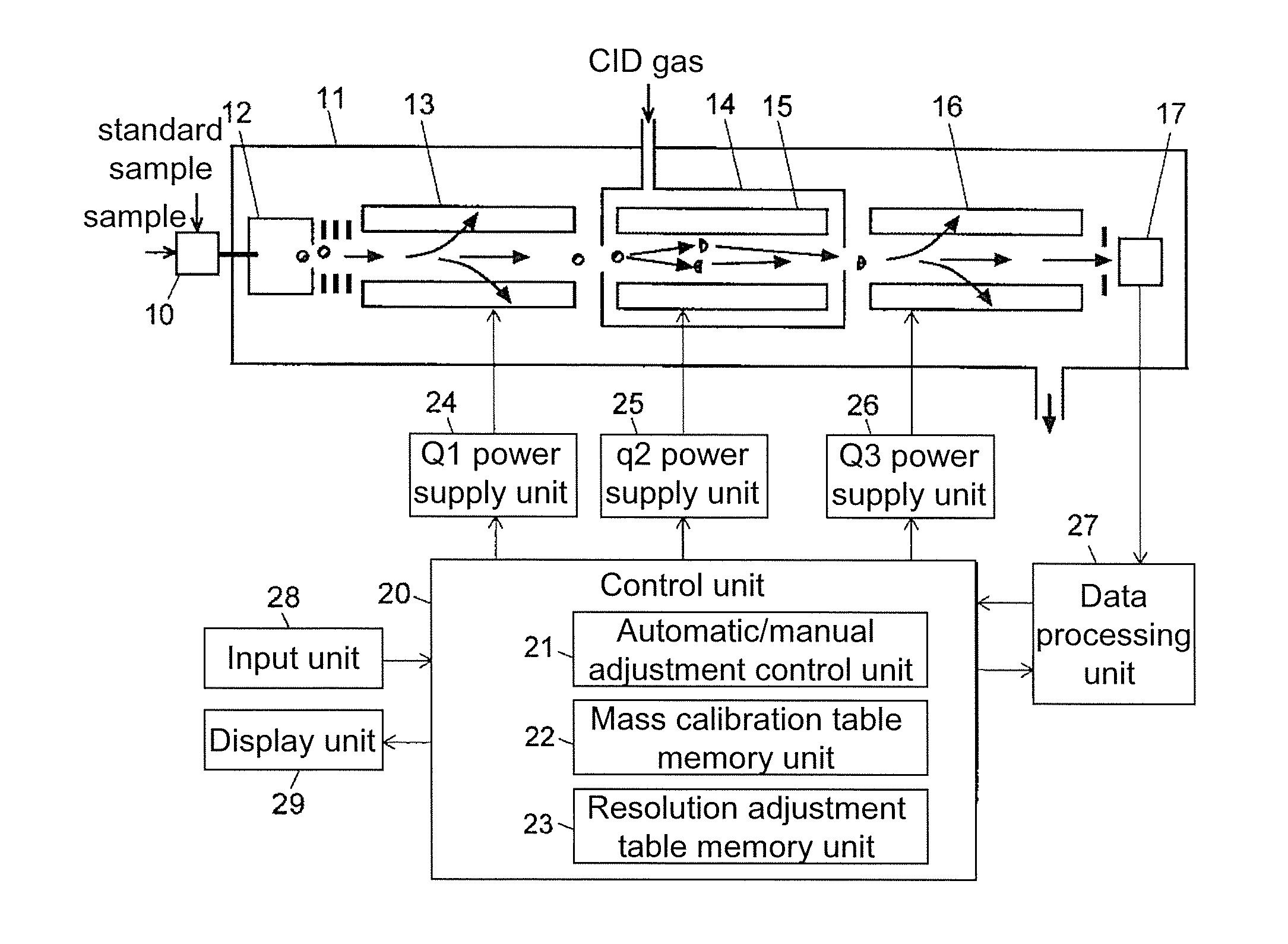
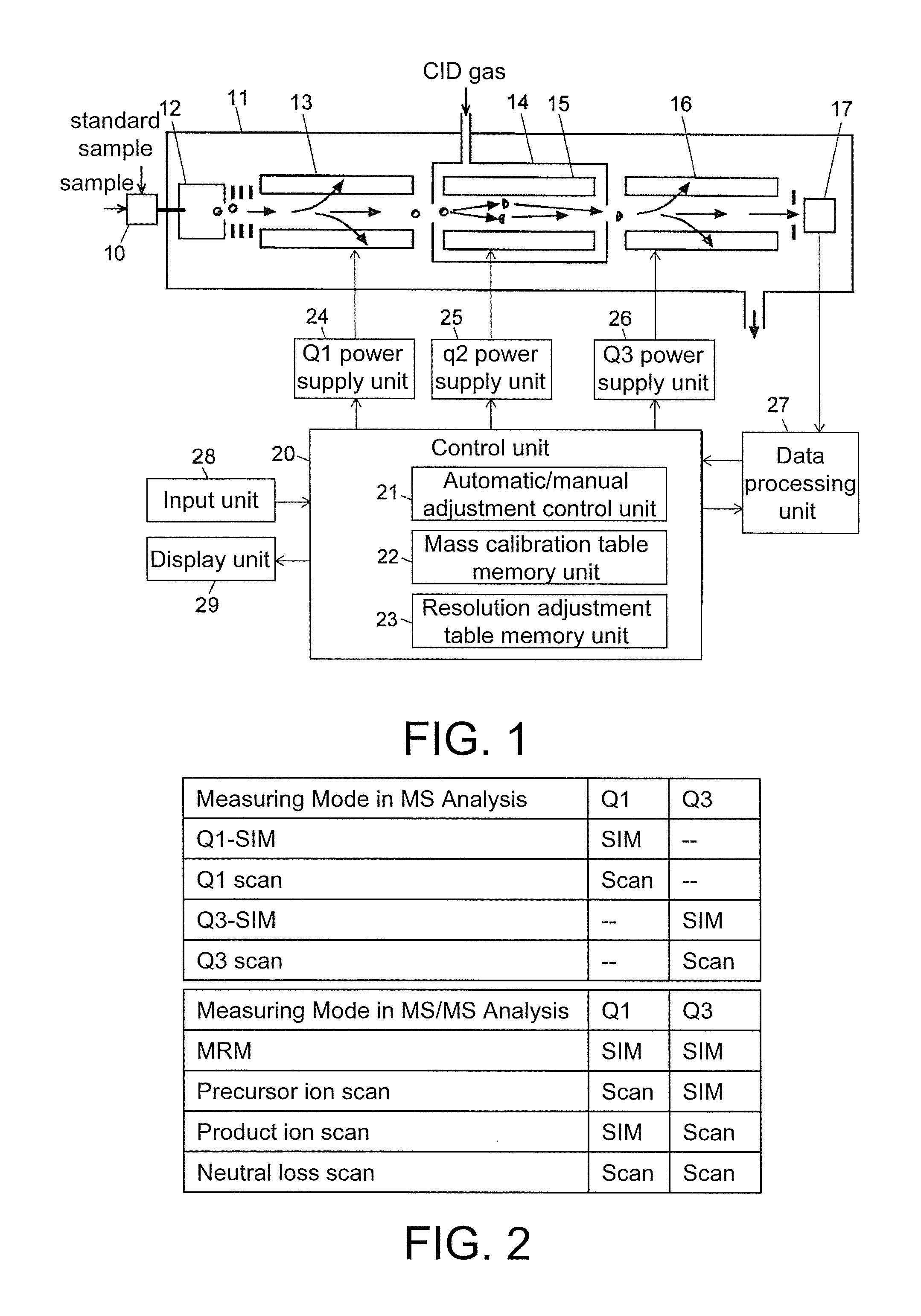
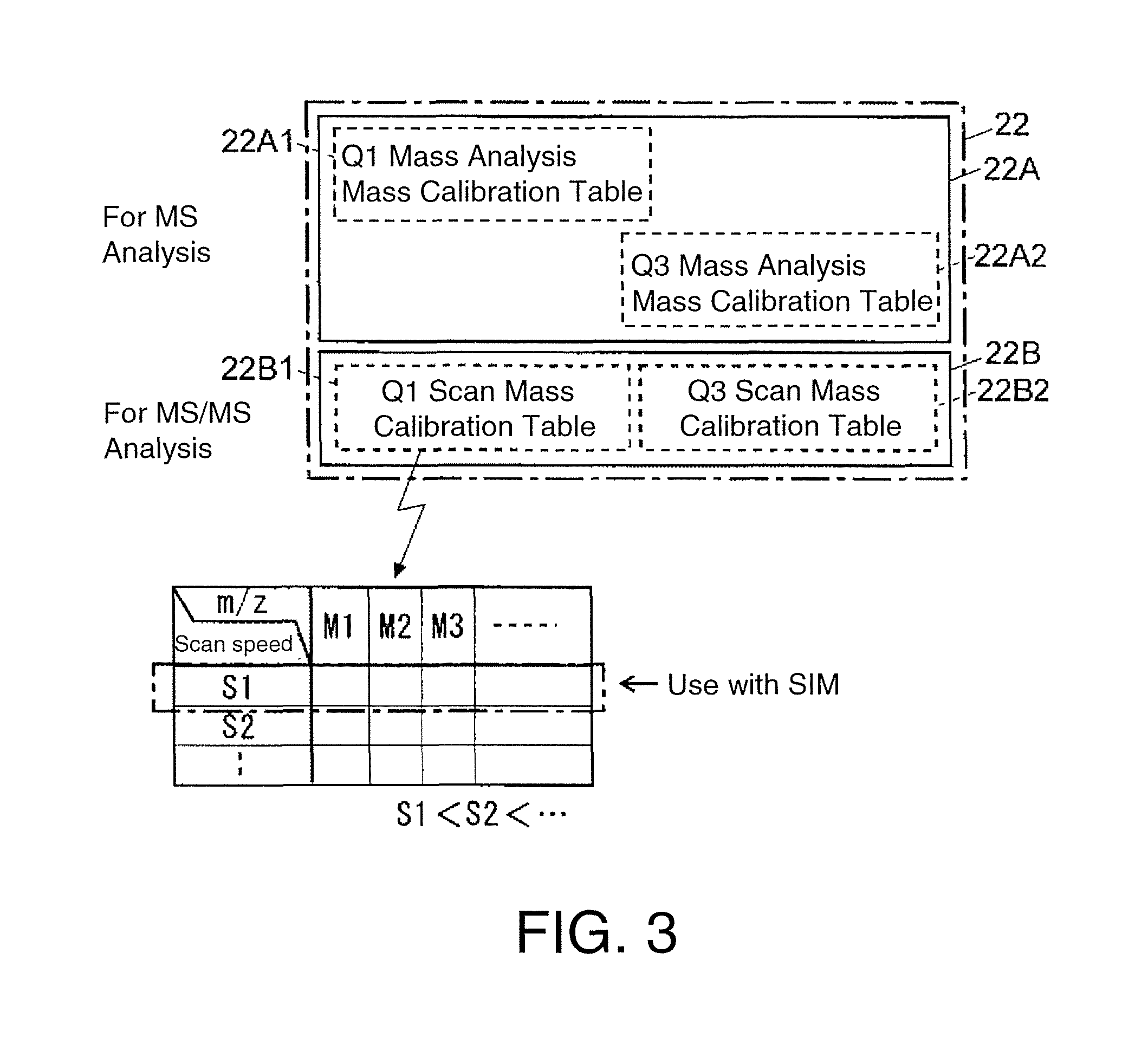
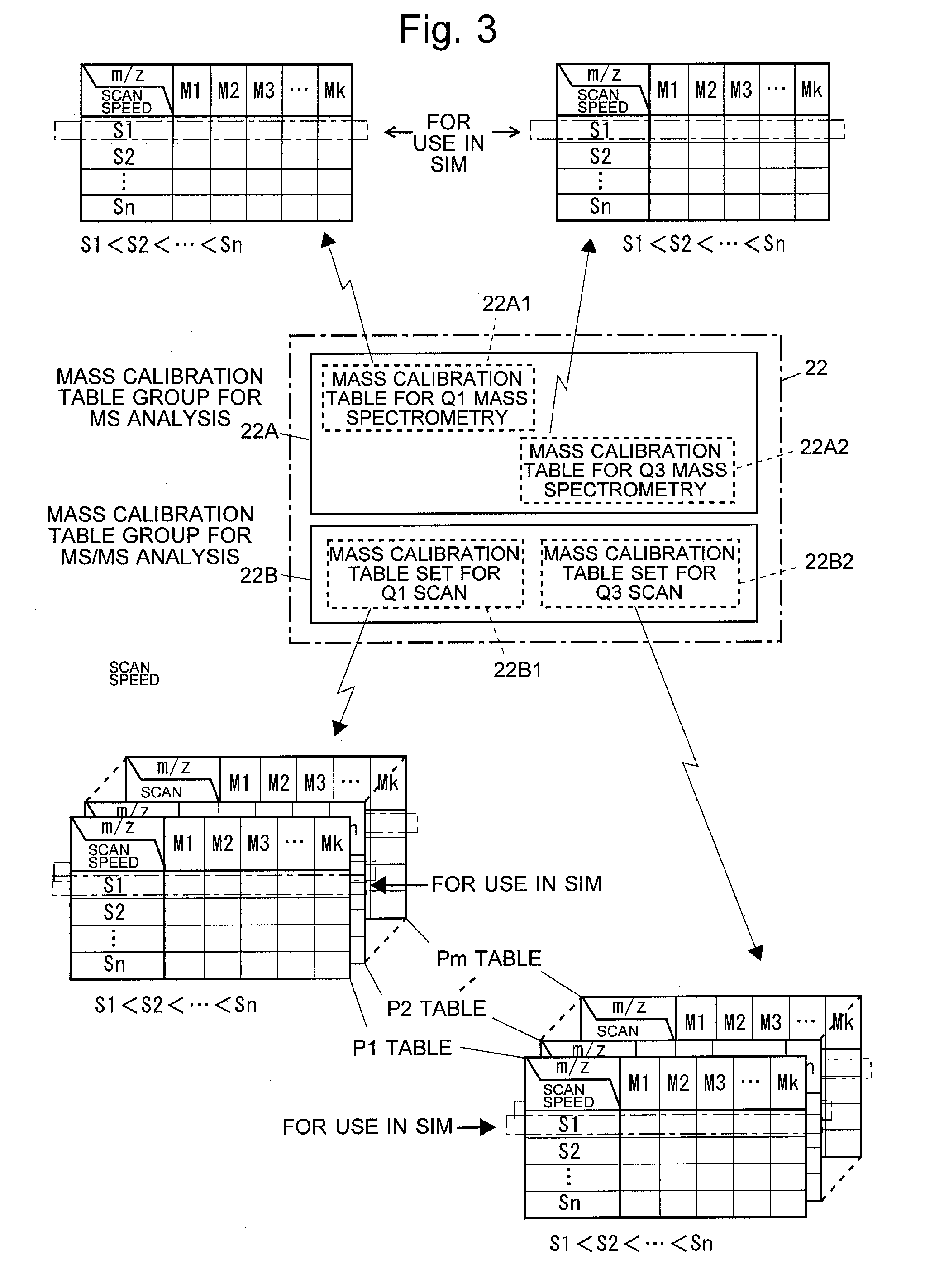
Triple quadrupole mass spectrometer
ActiveUS8698072B2Scan speedSuppression ratioStability-of-path spectrometersIsotope separationTriple quadrupole mass spectrometryQuadrupole
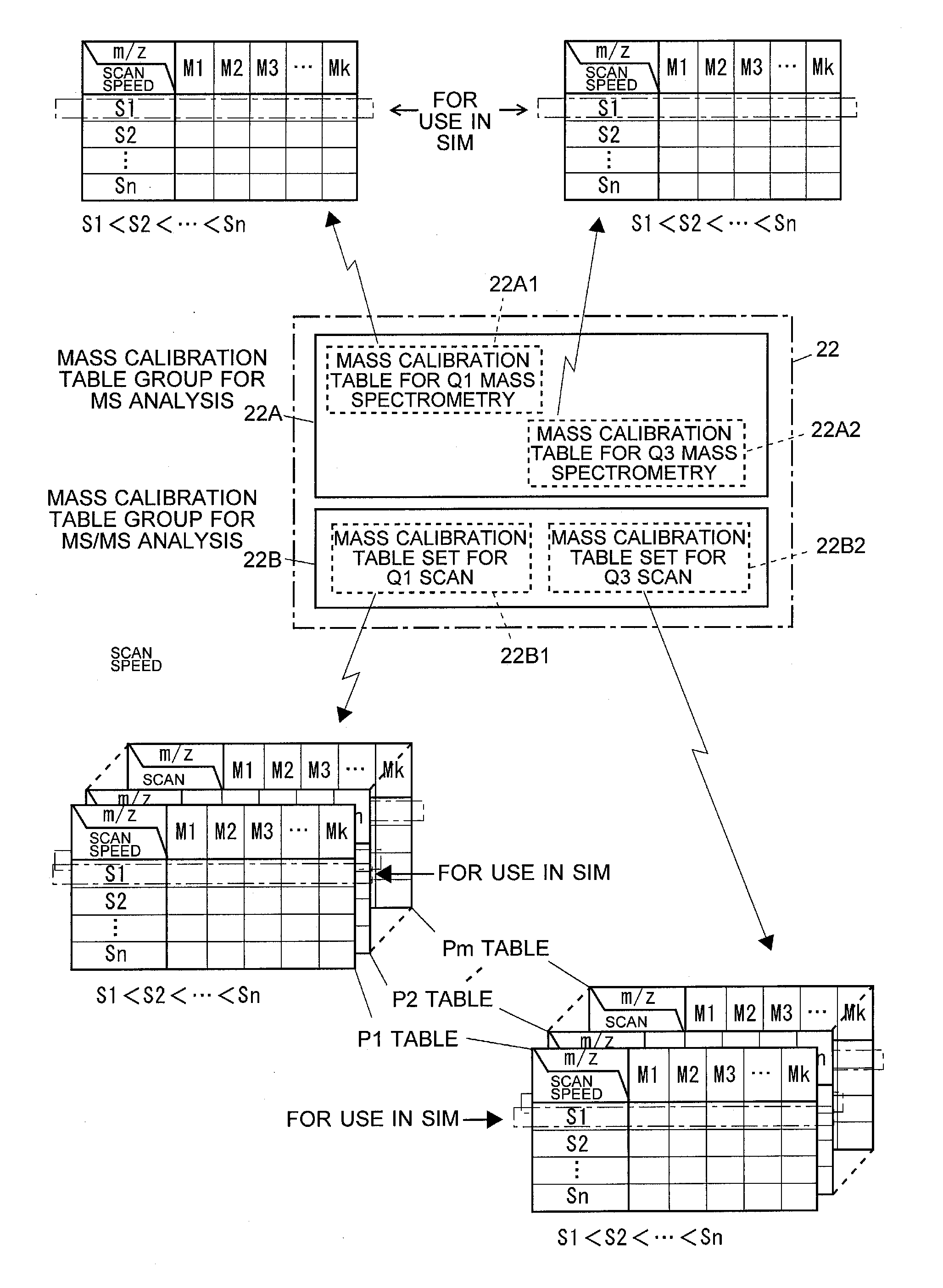
A high-quality mass spectrum is provided with alleviated mass / charge axis deviation in a triple quadrupole mass spectrometer even when executing a high-speed mass scan with MS / MS analysis. Mass calibration tables which denote relations between m / z and a mass deviation value which scan speed is a parameter are prepared separately for use in MS analyses without involving dissociation operations and MS / MS analyses with involving dissociation operations. According to a measuring mode, such as a product ion scan measurement or a neutral loss scan measurement, when performing MS / MS analysis, a mass deviation value for the minimum scan speed in a table is used for a quadrupole where the selected m / z is fixed, and a mass deviation value for a designated scan speed in a table is used for a quadrupole where the mass scan is performed, thus controlling the operations of each of a pre-stage and a post-stage quadrupoles.
Owner:SHIMADZU CORP
Analysis method of glucose energy metabolism-related important metabolites in serum samples
ActiveCN106855551AThe pre-processing process is simpleHigh sensitivityComponent separationMetaboliteFreeze-drying
The invention discloses an analysis method of glucose energy metabolism-related important metabolites in serum samples. According to the analysis method, methanol is adopted for extraction, samples to be detected are subjected to freeze-drying and two-step derivatization, and then are subjected to analysis using a gas chromatography-triple quadrupole mass spectrometer; multi-reaction monitoring mode is adopted for detection, for low-content metabolites, ion pairs with the optimal ion abundance are obtained via optimization and are taken as quantification ion pairs so as to increase sensitivity; for high-content metabolites, ion pairs low in abundance obtained via optimization are selected as quantification ion pairs so as to widen the linear range and realize simultaneous analysis of high and low abundance metabolites. Pretreatment of the analysis method is simple; repeatability is high; sensitivity is high; and linear range is wide.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Detection and analysis method of short-chain chlorinated paraffin
InactiveCN105588889AEase of detectionEasy to implement analysisComponent separationFiltrationChlorinated paraffins
The invention relates to the technical field of chemical detection. A detection and analysis method of short-chain chlorinated paraffin includes the following steps of firstly, processing a sample, wherein the sample is put in acetonitrile to be shaken after being subjected to ultrasonic extraction through tetrahydrofuran, and a processed extraction solution is obtained after sedimentation and filtration; secondly, conducting quanlitative analysis on the processed extraction solution through a high performance liquid chromatography-tandem triple quadrupole mass spectrometer; thirdly, conducting quantitative analysis on the processed extraction solution through the high performance liquid chromatography-tandem triple quadrupole mass spectrometer. According to the method, by optimizing a traditional detection and analysis method of short-chain chlorinated paraffin, pretreatment extraction is improved under the condition of ensuring consistency of extraction effects, and therefore the extraction solution can be suitable for liquid-phase analysis. In addition, by means of the high performance liquid chromatography-tandem triple quadrupole mass spectrometer and through the combination with the advantages of the phase liquid and mass spectrums, detection and analysis on short-chain chlorinated paraffin are conveniently achieved, and a lower method detection limit is obtained.
Owner:上海英格尔认证有限公司 +1
Method for quick detection of content of citrinin in traditional Chinese medicinal materials
InactiveCN105954422AOmit the water removal stepSimple experimentComponent separationCitrininQuechers
The invention belongs to the technical field of analytical testing and relates to a citrinin detection method, in particular to a method for quick detection of the content of citrinin in traditional Chinese medicinal materials. The method comprises the steps of extraction with an improved QuEChERS method, detection of liquid to be detected by means of an ultra-high performance liquid chromatography-triple quadrupole mass spectrometer, preparation of citrinin standard solution, and establishment of a citrinin quality and quantity determination method. The sample to be detected is subjected to QuEChERS extraction and ultra-high performance liquid chromatography-triple quadrupole mass spectrometer (UPLC-MS / MS) analysis, and then quantitative determination is conducted with an external standard method under the condition of electrospray ionization and multi-reaction monitoring mode. Experiment results show that the method is high in sensitivity and stability, and is suitable for accurate and quick detection of citrinin residues in a large number of samples and detection of citrinin residues in traditional Chinese medicinal materials.
Owner:ZHAOQING UNIV
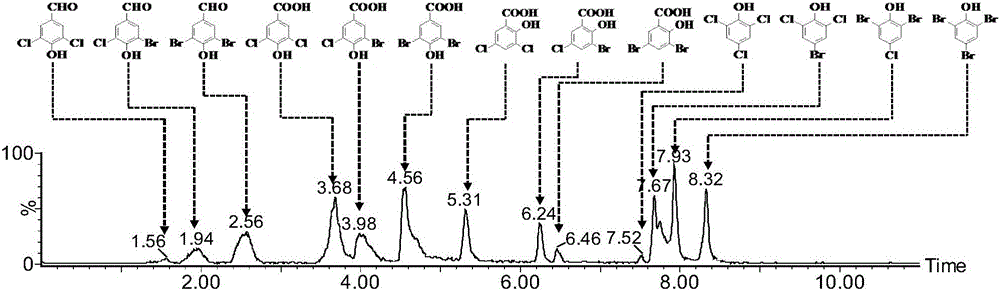
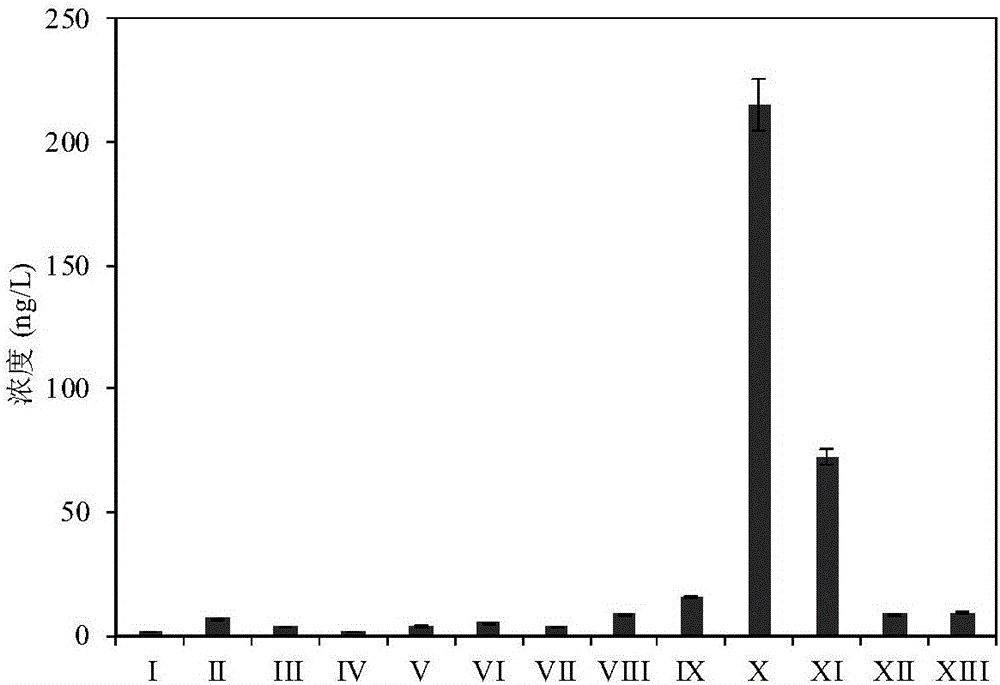
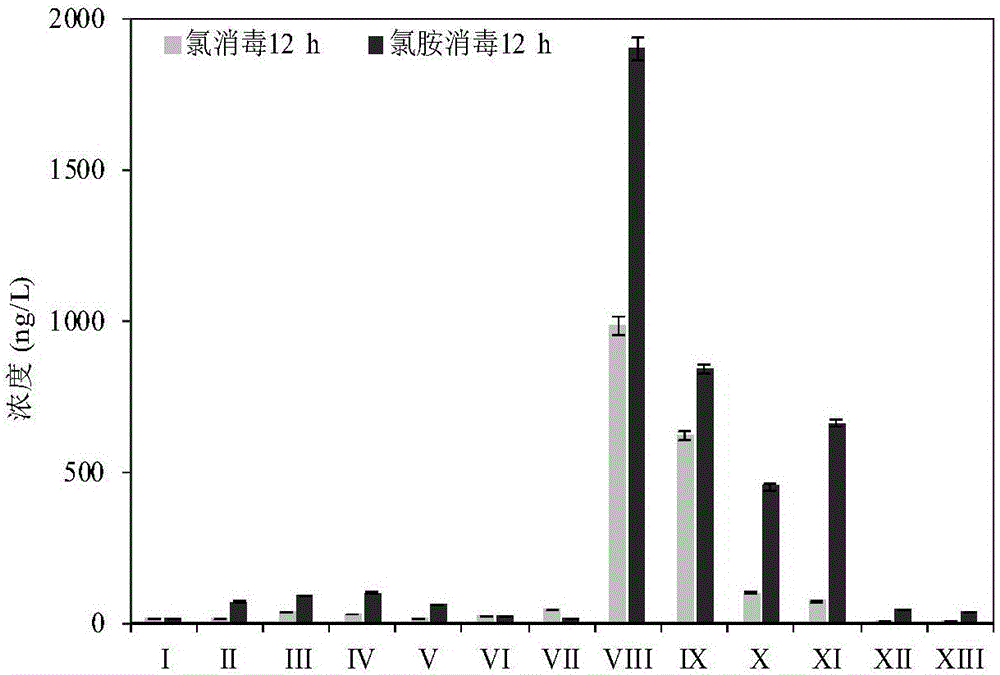
Method for detecting polar phenol type chlorinated/brominated disinfection by-products in water
Owner:NANJING UNIV
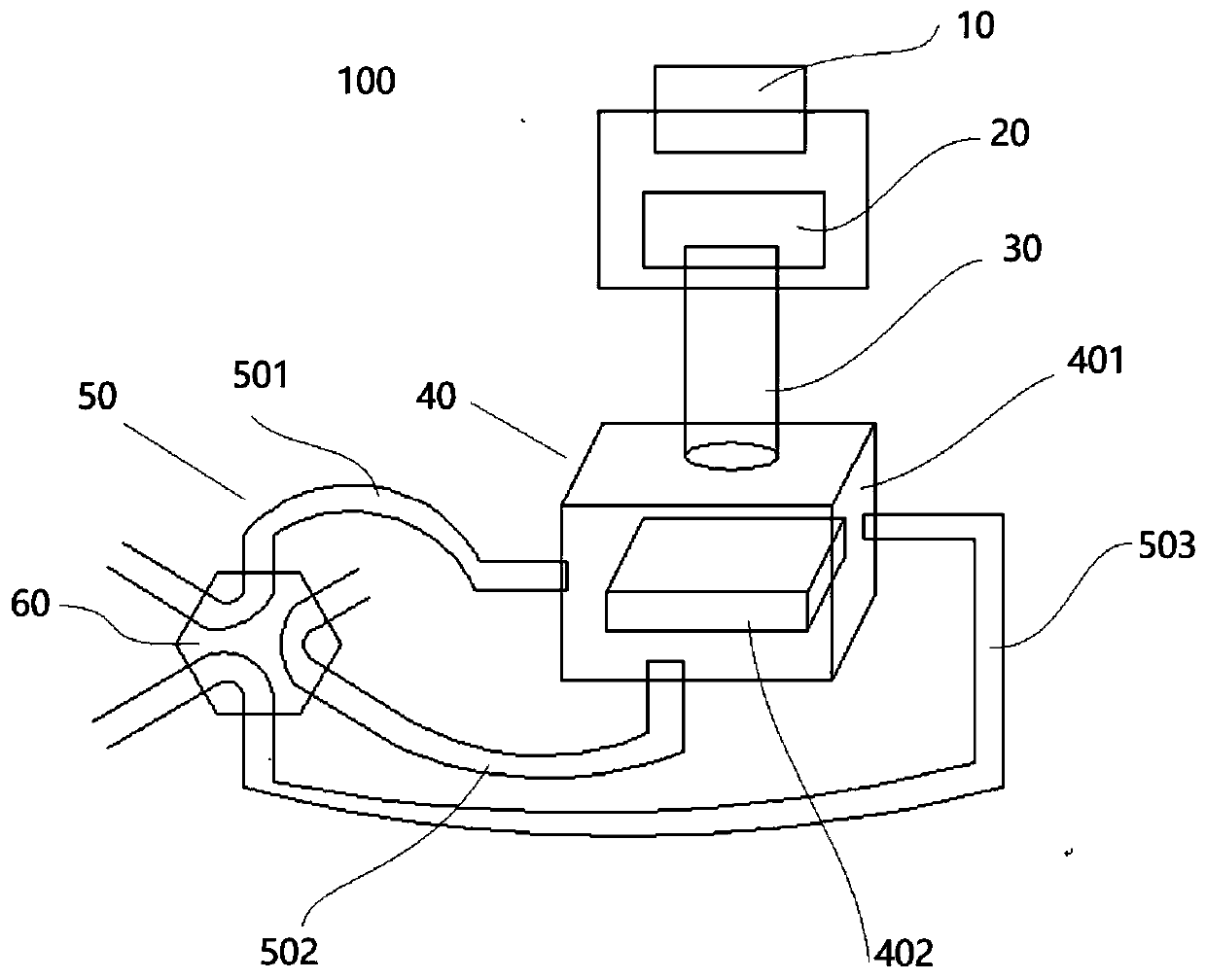
Triple quadrupole mass spectrometer and non-transitory computer-readable medium recording a program for triple quadrupole mass spectrometer
ActiveUS20150262800A1Improve the level ofImprove accuracyStability-of-path spectrometersCalibration apparatusTriple quadrupole mass spectrometryQuadrupole
A triple quadrupole mass spectrometer provided with: a calibration information storage section for storing mass calibration information showing the relationship between the mass-to-charge ratio and a calibration value, with a CID gas pressure as a parameter, for each measurement mode of an MS / MS analysis including a dissociating operation using a collision cell; and a controller for calibrating the mass-to-charge ratio of the ion to be detected by a detector, by reading, from the calibration information storage section, the mass calibration information corresponding to the measurement mode to be performed and a specified CID gas pressure and by driving each the front and rear quadrupoles and using that information.
Owner:SHIMADZU CORP
Online pretreatment device for hair sample injection
InactiveCN109900780ARapid qualitative and quantitativeThe detection process is fastMaterial analysis by electric/magnetic meansImage detectionImaging spectrometer
The embodiments of the present invention provide an online pretreatment device for hair sample injection. The device is used for transferring hair samples during a pre-treatment process. The online pretreatment device for hair sample injection includes an image detector, a mass spectrometer, an objective lens and a sample cabin assembly; the sample cabin assembly includes a sample cavity and a plate drawer disposed within the sample cavity; the plate drawer slidably enters and withdraws from the sample cavity, the objective lens is connected between the mass spectrometer and the sample cabin assembly; and an image under the objective lens is shot through an image sensor and is transmitted to an external device. The online pretreatment device for hair sample injection of the invention adopted, the rapid qualitative and quantitative measurement of drugs or metabolic components thereof in hairs can be realized; samples are directly injected into a time of flight mass spectrometer; the time of flight mass spectrometer is connected in series with a triple-quadrupole mass spectrometer for quantification; and therefore, the online pretreatment device has the advantages of improved sensitivity and high detection speed.
Owner:XIAMEN LIST TECH SERVICE CO LTD
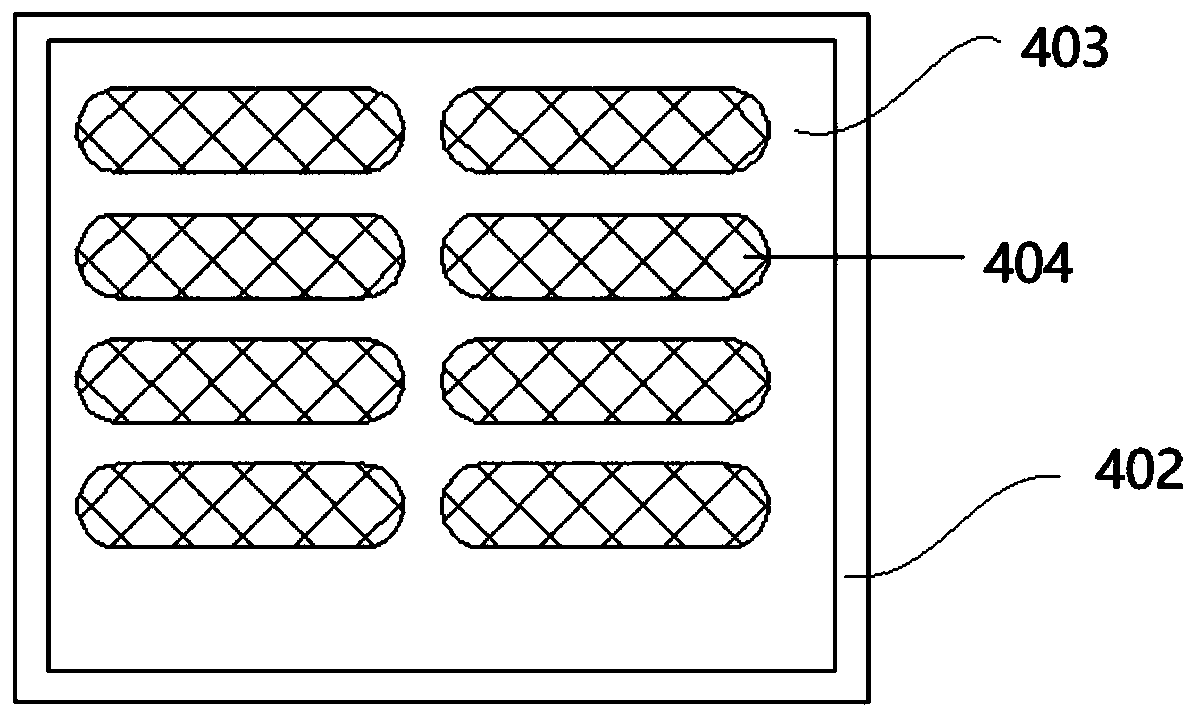
Multichannel triple quadrupole mass spectrum array system
InactiveCN103811268AImprove throughputHigh sensitivityStability-of-path spectrometersGuidance systemIon current
The invention discloses a multichannel triple quadrupole mass spectrum array system. The multichannel triple quadrupole mass spectrum array system comprises an ion guidance system which is used to guide an ion source to a triple quadrupole mass spectrometer, and further comprises the triple quadrupole mass spectrometer which comprises a plurality of groups of triple quadrupole rods arranged in parallel and evenly distributed on peripheries of circles of the same diameter, and is used to dissociate ion currents output by the ion source into characteristic fragments after performing quality selection on the ion currents, and an ion detection system which comprises a plurality of detectors used to detect select the characteristic fragments from each group of the triple quadrupole rods. The multichannel triple quadrupole mass spectrum array system can perform synchronous detection analysis on a plurality of target molecules or a plurality of dissociation galleries of a same target molecule, which are synchronously eluted by liquid chromatography, and accordingly improve analysis flux and sensitivity and enlarges dynamic ranges in multiples for the triple quadrupole mass spectrometer.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for detecting concentration of infliximab in serum and application thereof
InactiveCN110531015AEliminate distractionsSimplified processing stepsComponent separationEnzymatic digestionTriple quadrupole mass spectrometry
The invention discloses a method for detecting concentration of infliximab in serum by high-performance liquid chromatography tandem mass spectrometry and application thereof. The method specificallycomprises the following steps of preparation of standard products, enzymatic digestion reaction, high-performance liquid chromatography separation, tandem mass spectrometry detection, and detection ofinfliximab in serum. The invention also discloses application of the method for detecting concentration of infliximab in serum by high-performance liquid chromatography tandem mass spectrometry. Theinvention uses acetonitrile for protein precipitation, and uses chromatographic tandem triple quadrupole mass spectrometer for detection after the extraction, which is simple in pretreatment steps, can effectively remove serum matrix interference, and has good specificity. In addition, the detection is performed by high performance liquid chromatography tandem mass spectrometer, and infliximab inserum is qualitatively and accurately quantified at the same time, thereby having short detection time, high throughput, high detection sensitivity, good specificity and low cost.
Owner:江苏锐博医疗科技有限公司
Method for quantitative determination of uronic acid-containing polysaccharide
ActiveCN106770871AEasy to operateShort timeComponent separationMulti responseTriple quadrupole mass spectrometry
The invention provides a method for quantitative determination of uronic acid-containing polysaccharide. The method comprises the following specific steps of preparing a uronic acid-containing polysaccharide standard substance solution from trifluoroacetic acid, wherein the uronic acid-containing polysaccharide is the polysaccharide of which a main chain is formed by a hexuronic acid-hexosamine or hexuronic acid-hexose disaccharide repeated disaccharide fragment; carrying out acid hydrolysis and deacidification on a standard substance to obtain a to-be-tested sample acid hydrolysis residue; dissolving a standard sample acid hydrolysis residue, and carrying out analysis by using a high-performance liquid chromatography-tandem triple quadrupole mass spectrometer to obtain a multi-response monitoring chromatogram and a standard curve; processing and detecting the to-be-tested sample acid hydrolysis residue by adopting the same method; and identifying the variety of the uronic acid-containing polysaccharide and obtaining the content of the uronic acid-containing polysaccharide in the to-be-tested sample. The method is simple in operation step, low in reagent price and relatively short in elapsed time, and polysaccharide formed by repeated disaccharide fragments of uronic acid and hexosamine or hexose can be accurately quantified at the same time.
Owner:DALIAN POLYTECHNIC UNIVERSITY
Method for measuring contents of multiple mycotoxins in fresh and raw milk samples
The invention relates to a method for measuring contents of multiple mycotoxins in fresh and raw milk samples. The method comprises the following steps: S1, extracting extracts of the mycotoxins from the fresh and raw milk samples; S2, carrying out liquid chromatographic separation on the extracts of the mycotoxins, so as to obtain elution fractions; S3, carrying out mass spectrum identification on the elution fractions by using a triple-quadrupole mass spectrometer, so as to determine the types and contents of the mycotoxins. After the method is adopted, the fourteen mycotoxins are fully extracted; compared with a single-quadrupole mass spectrometer, the triple-quadrupole mass spectrometer adopted by the method is higher in accuracy, so that a target compound is better separated from interference impurities; therefore, reliable and accurate data can be provided.
Owner:四川省农业科学院分析测试中心
Detection method of gelatin medicinal material
ActiveCN112098577AQuickly and accurately judge the authenticityStrong specificityComponent separationMedicinal herbsEnzyme digestion
The invention discloses a detection method of gelatin medicinal materials. According to the detection method, trypsin is adopted for enzyme digestion of the gelatin medicinal material, so that specific polypeptide is released from collagen; then a liquid chromatography triple quadrupole mass spectrometer is adopted for detection, and if it is detected that the sample is corresponded to the specific polypeptide retentiontime, the parent ions and the daughter ions, determining that the species source of the gelatin medicinal material sample is consistent with the species corresponding to the specific polypeptide; and determining the adulteration proportion of the doped sample according to the peak area ratio of the specific polypeptide. The method has the advantages of strong specificity, high sensitivity, simple operation and the like, and can be used for identification and quality control of the gelatin medicinal materials.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Liquid chromatography tandem mass spectrometry quantification method used for simultaneous detection of plurality of effective components in gelsemium elegans
ActiveCN107894475AEfficient separationRaise quality standardsComponent separationReference sampleGelsemium elegans
The invention discloses a liquid chromatography tandem mass spectrometry quantification method used for simultaneous detection of a plurality of effective components in gelsemium elegans. According tothe liquid chromatography tandem mass spectrometry quantification method, a gelsemine methanol solution, a koumine methanol solution, a gelsenicine methanol solution, and a koumidine methanol solution are taken as reference sample solutions, a gelsemium elegans extracted solution is taken as a sample solution to be measured, a high performance liquid chromatography triple quadrupole mass spectrometer is adopted for detection, and the contents of the plurality of effective components in the sample solution to be measured are calculated based on detection results. The liquid chromatography tandem mass spectrometry quantification method is stable and reliable, is simple, is high in efficiency, sensitivity, and accuracy, possesses wide practicability, and can be used for detection and quantitative analysis of any components or derivative products from gelsemium elegans.
Owner:HUNAN AGRICULTURAL UNIV
Method for testing tetracycline antibiotic residue in cattle pulmonary surfactant extract
ActiveCN107703228AObvious lesionsLow arterial oxygen partial pressureComponent separationReference sampleDecreased surfactant
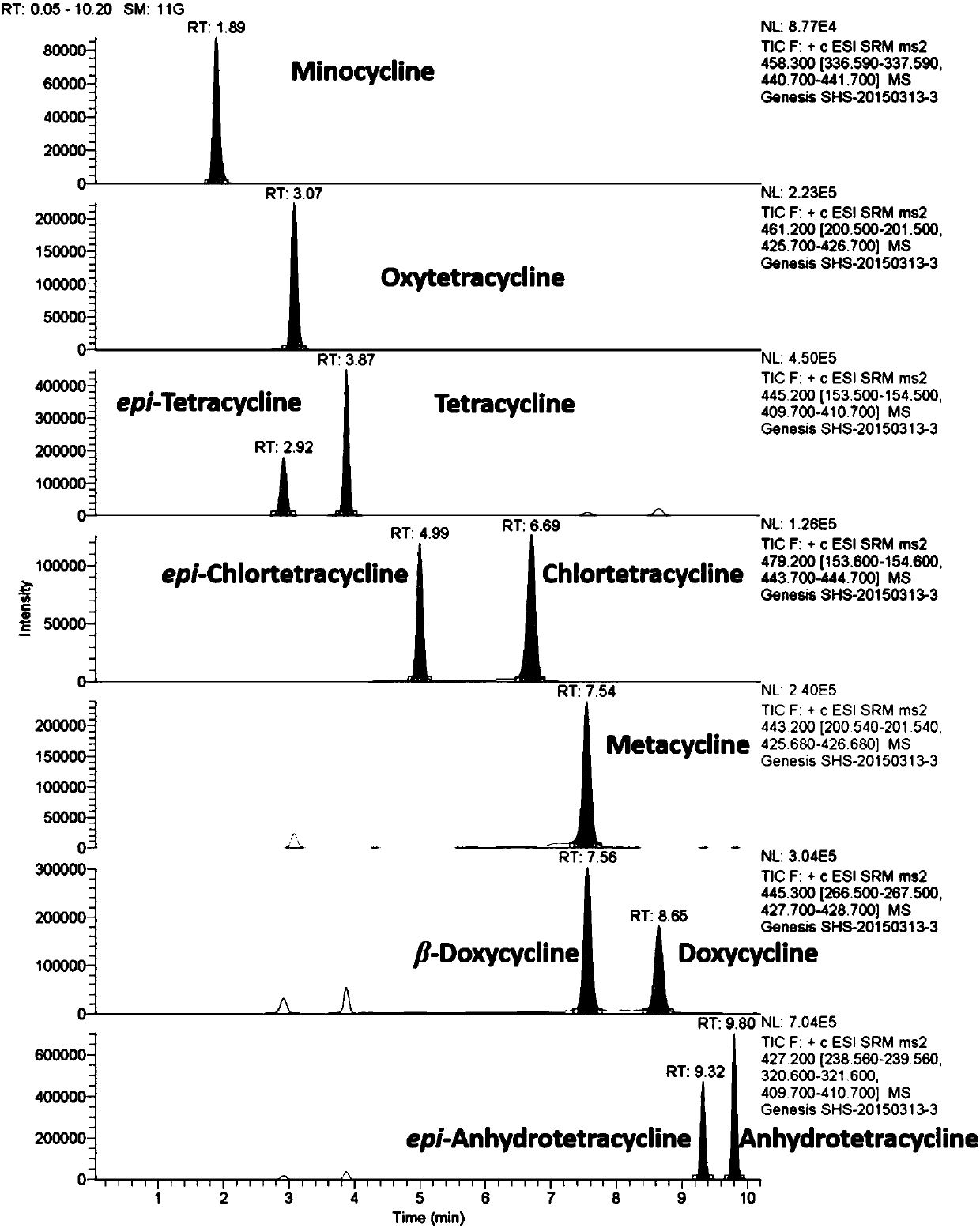
The invention relates to a method for testing tetracycline antibiotic residue in cattle pulmonary surfactant extract. The method adopts ultrahigh-performance liquid chromatography-tandem mass spectrometry for testing and includes following steps: providing an ultrahigh-performance liquid chromatograph, a triple quadrupole mass spectrometer, the cattle pulmonary surfactant extract and a reference sample; determining chromatographic conditions and mass spectrometric conditions; performing liquid preparation, testing and result processing. By using the method, 11 antibiotics including tetracycline, chlorotetracycline, terramycin, doxycycline, metacycline, minocycline, beta-doxycycline, epitetracycline, epichlorotetracycline, anhydrotetracycline and epianhydrotetracycline and known impuritiescan be tested at the same time. Linear range, sensitivity, precision and recovery rate of the method all meet analysis requirements.
Owner:BEIJING INST FOR DRUG CONTROL
Method for determining residual quantity of dazomet and metabolite methyl isothiocyanate thereof in plant-derived food
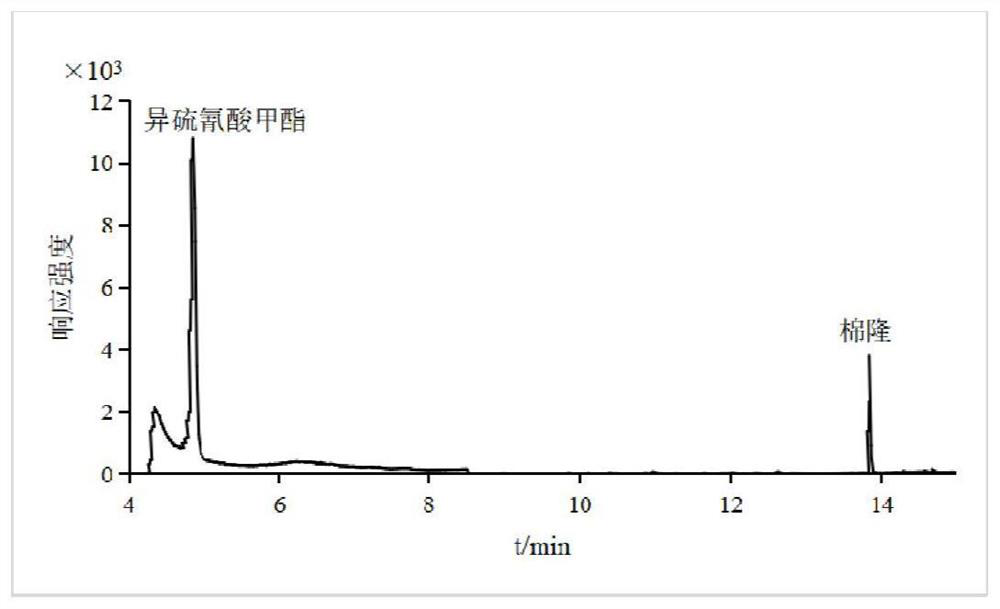
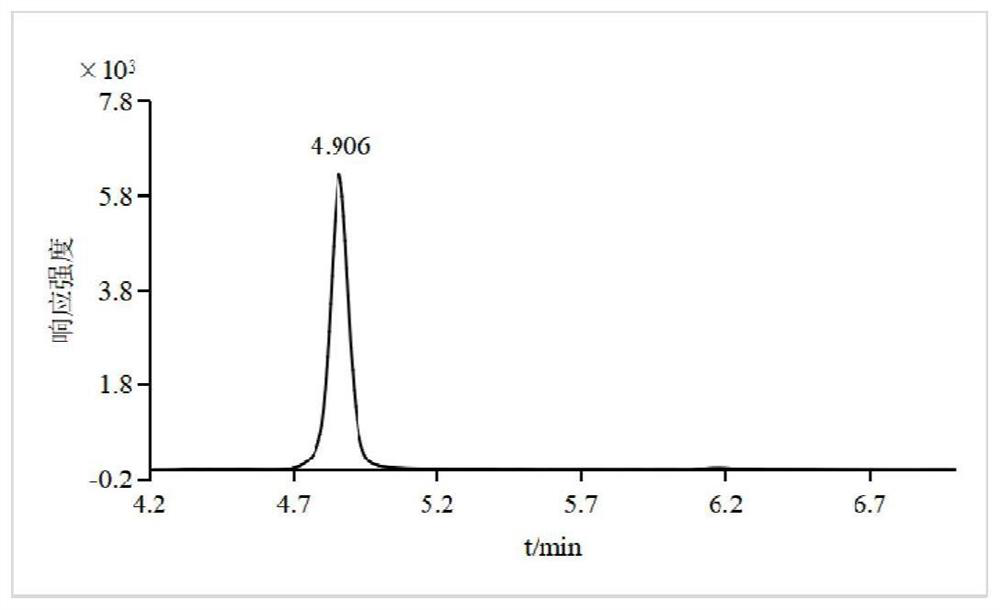
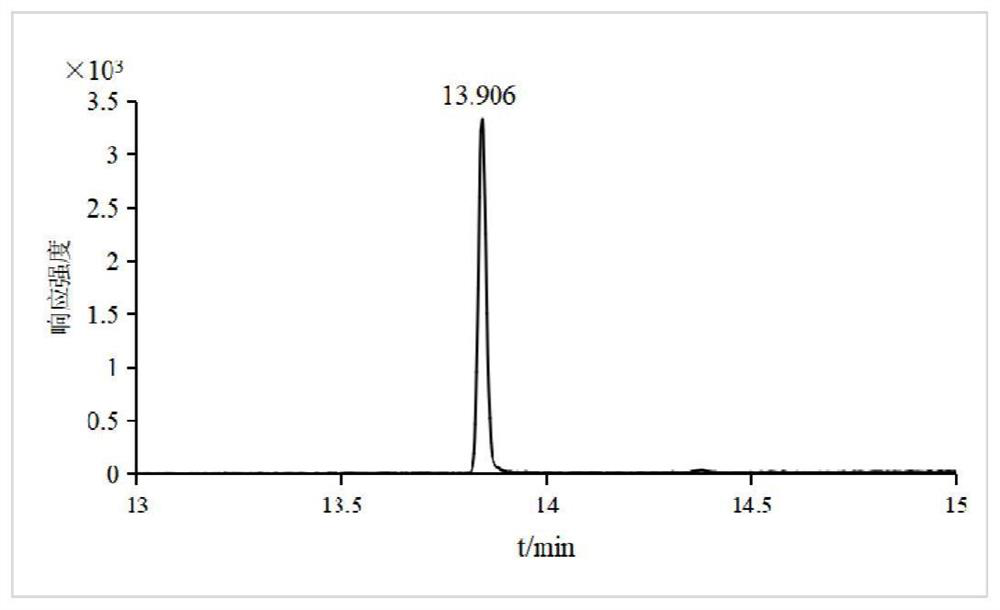
PendingCN114384168AMeet testing needsEasy to operateComponent separationMetaboliteGas liquid chromatographic
The invention relates to a method for measuring the residual quantity of dazomet and metabolite methyl isothiocyanate thereof in plant-derived food, which comprises the following steps: pretreatment: crushing a sample to be measured, adding ethyl acetate, performing vortex oscillation for extraction, and then centrifuging; sucking the supernate, adding ethylenediamine-N-propyl silanized silica gel, graphitized carbon black, C18 and anhydrous magnesium sulfate for purification, carrying out vortex mixing, centrifuging, taking the supernate, and filtering a membrane to obtain an extracting solution; detecting the extracting solution by a gas chromatography-triple quadrupole mass spectrometer, and quantifying by a matrix matching external standard method. According to the method, the linear relation of the target compound in the range of 0.01-0.10 mg / kg is good, and correlation coefficients are all larger than 0.99. The average recovery rate of a blank sample at low, medium and high addition levels is 81.5%-109.4%, the relative standard deviation (n = 6) is 2.8%-9.0%, and the limit of quantitation of the method is 0.01 mg / kg. The method is simple, rapid and sensitive to operate, and can meet the detection requirements of dazomet and metabolite methyl isothiocyanate thereof in plant-derived food.
Owner:厦门海关技术中心
Real-time direct analysis method for rapidly determining free formaldehyde in water-soaked products
InactiveCN104502441AExclude false positive resultsFalse positive results are reliableMaterial analysis by electric/magnetic meansChromatographic separationMass analyzer
The invention discloses a real-time direct analysis method for rapidly determining free formaldehyde in water-soaked products. The method comprises the following steps: enabling 2,4-dinitrophenylhydrazone as a reaction reagent to react with formaldehyde, taking an appropriate amount of reaction liquid to be dripped on a screen so as to be naturally dried in air to obtain a product, placing the product between a real-time direct analysis mass spectrum ion source gas outlet and a triple quadrupole mass spectrometer ion inlet, and determining, wherein DART ion source conditions are as follows: a negative ion mode is adopted and an ion source temperature is 300 DEG C, and triple quadrupole mass spectrometer conditions are as follows: the mass analyzer voltage is 3000V, and the mass-to-charge ratio of parent ions to quantitative ions to qualitative ions is 209 to 151 to 133 in a second-grade scanning mode; and by comparing second-grade mass spectrograms of a sample and a control sample, analyzing the free formaldehyde in the water-soaked products and quantifying by adopting an external standard method. The real-time direct analysis method provided by the invention has the advantages that the complex pretreatment and chromatographic separation process is not needed, the detection limit is up to 1ppm, and the detection method is sensitive, simple and convenient, and can be used for rapidly detecting the free formaldehyde in the water-soaked products.
Owner:NANJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com