Method for detecting concentration of infliximab in serum and application thereof
A technology of fliximon and liximon, which is applied in the field of mass spectrometry detection, can solve problems such as cross-reaction, and achieve the effects of low cost, high detection sensitivity, and short detection time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1 standard substance preparation
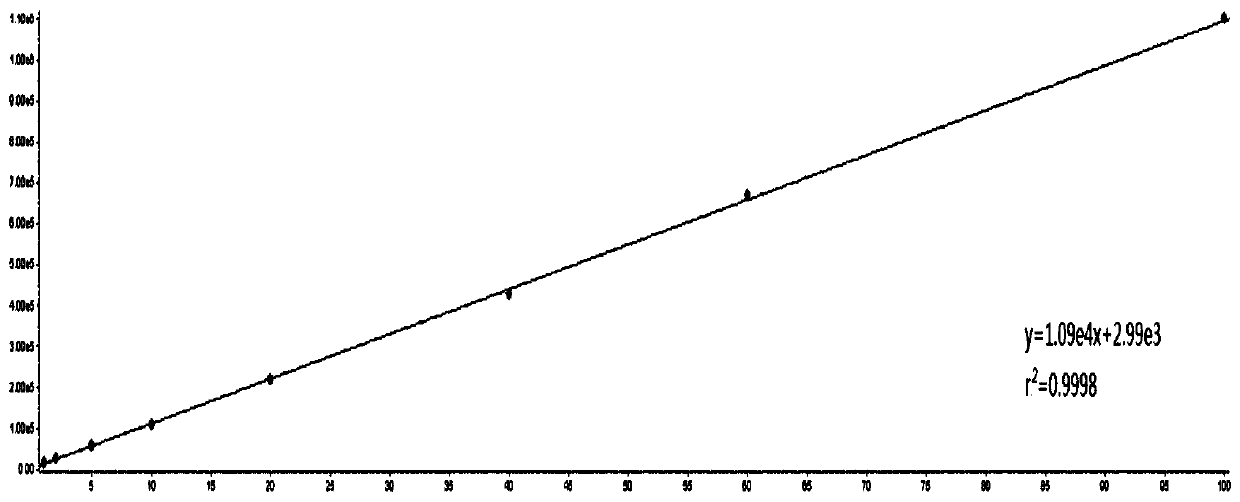
[0039] Take 0.1 mL of infliximab acetonitrile solution with 8 different concentrations (0.1nmol / L, 1nmol / L, 5nmol / L, 10nmol / L, 20nmol / L, 40nmol / L, 60nmol / L, 100nmol / L) To a 1.5mL EP tube, add 0.3mL of the above-mentioned isotope peptide internal standard acetonitrile solution (270nmol / L) and 30mg / mL trypsin 0.5μL, which were stored at low temperature, respectively, mix thoroughly with a vortex mixer, and then use a 1mL pipette Transfer 0.3mL internal standard acetonitrile solution containing isotope peptides to another 1.5mL EP tube with a liquid gun, and then lyophilize with centrifugal concentration and freezing system;
[0040] After lyophilization, add 25 μL of water: acetonitrile: formic acid solution with a volume ratio of 9:1:0.05. The mixed components were centrifuged in a cryogenic centrifuge at 17,000 g at 4°C for 5 min, and the supernatant was taken in a 96-well plate for analysis by liquid chromatography tandem ...
Embodiment 2
[0041] Embodiment 2 high performance liquid chromatography separation
[0042] Using ultra-high pressure liquid chromatography and corresponding mobile phase, the supernatant of the sample prepared in Example 1 was chromatographically eluted and separated on a C18 analytical column, and the desired detection infliximon was separated by controlling the elution conditions anti;
[0043] The separation conditions of high performance liquid chromatography are: Waters ACQUITY UPLC C18Column: pore sizeparticle size 1.7μm, 2.1mm×50mm; cat.#186002350IVD; column temperature: 60℃; injection volume: 10μL; flow rate: 0.3mL / min; Mobile phase composition: A phase is 0.1% formic acid aqueous solution, B phase is 0.1% formic acid acetonitrile solution;
[0044] The elution gradient is shown in Table 1 below:
[0045] Table 1
[0046] serial number time (min) Flow rate (mL / min) A% B% curve value 1 0.00 0.30 99.00 1.00 - 2 2.00 0.30 99.00 1.00 6 3 4.0...
Embodiment 3
[0047] Embodiment 3 mass spectrometry detects and makes standard curve
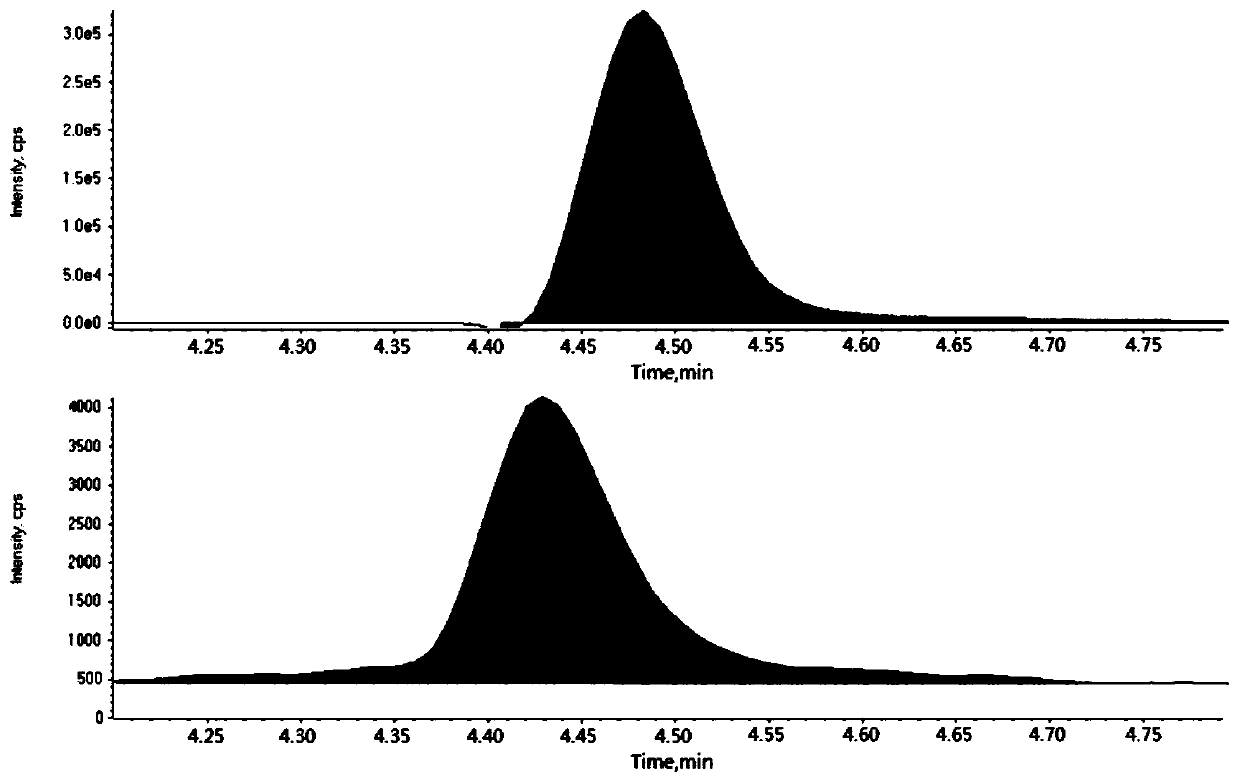
[0048] Infliximab and matrix were separated by liquid chromatography and entered into triple quadrupole mass spectrometer for detection, using the reaction monitoring mode in triple quadrupole mass spectrometer to specifically detect the content of infliximab, and draw a standard curve picture;
[0049] According to different chromatographic elution times, different detection windows and parameters are set. The detection parameters are first optimized with standards, and then the multiple reaction monitoring mode in the triple quadrupole mass spectrometer and the optimized specific mass spectrometry parameters are used to specifically detect Inflix. Ciximab and isotopic peptide internal standards.
[0050] The mass spectrometer is Waters Xevo TQD IVD (Waters, Milford, MA); the mass spectrometry detection conditions are as follows: electrospray needle voltage: 2.5kV, desolvation gas flow rate: 800L / h, des...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com