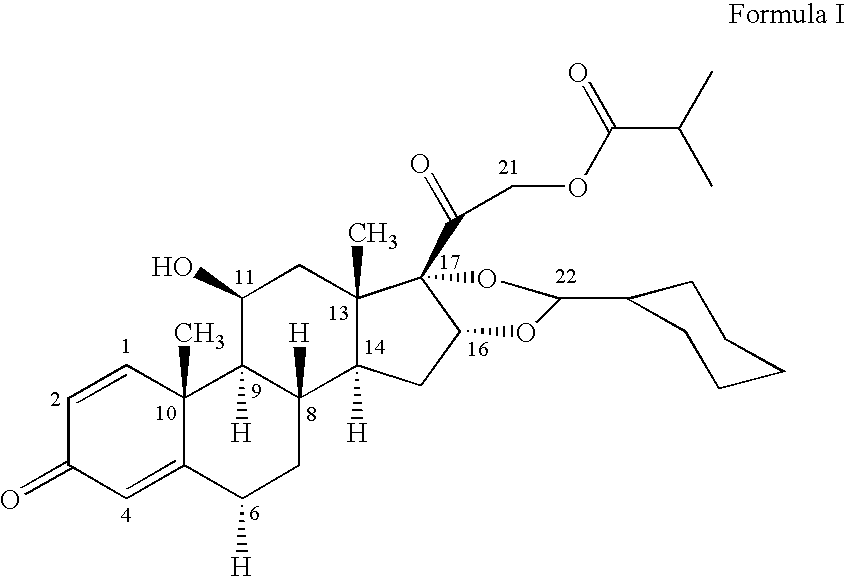
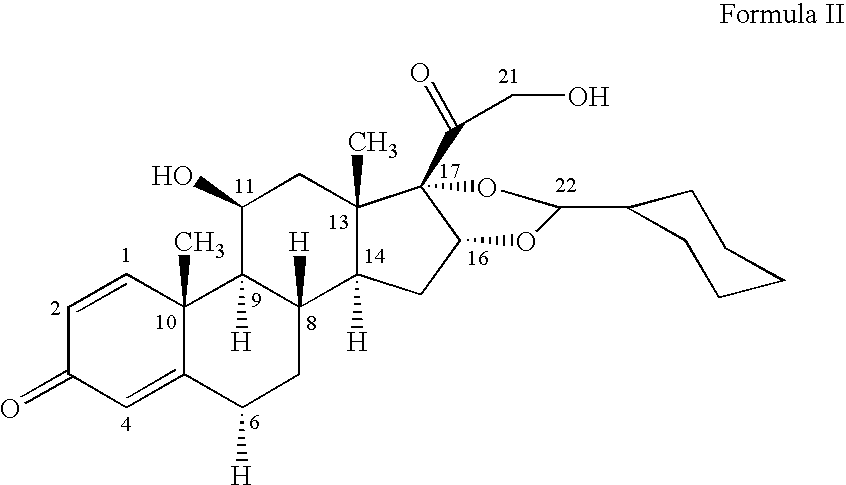
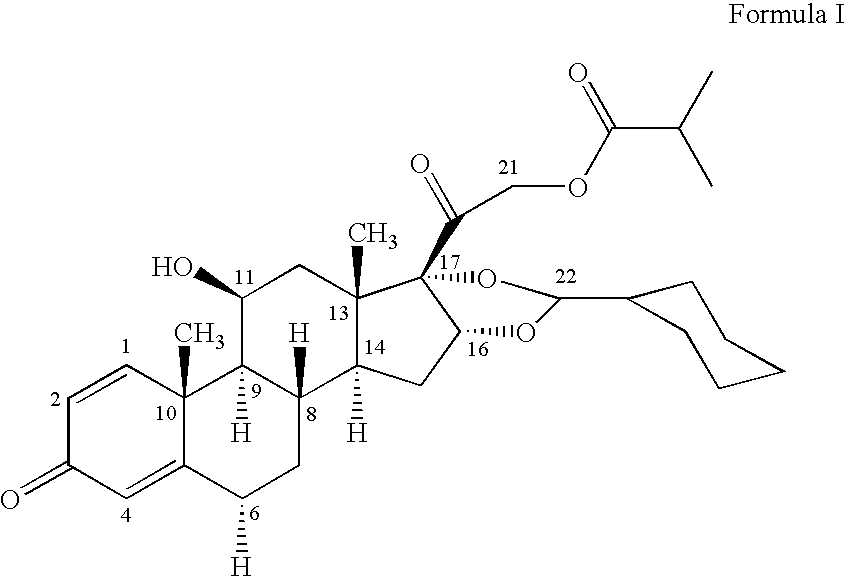
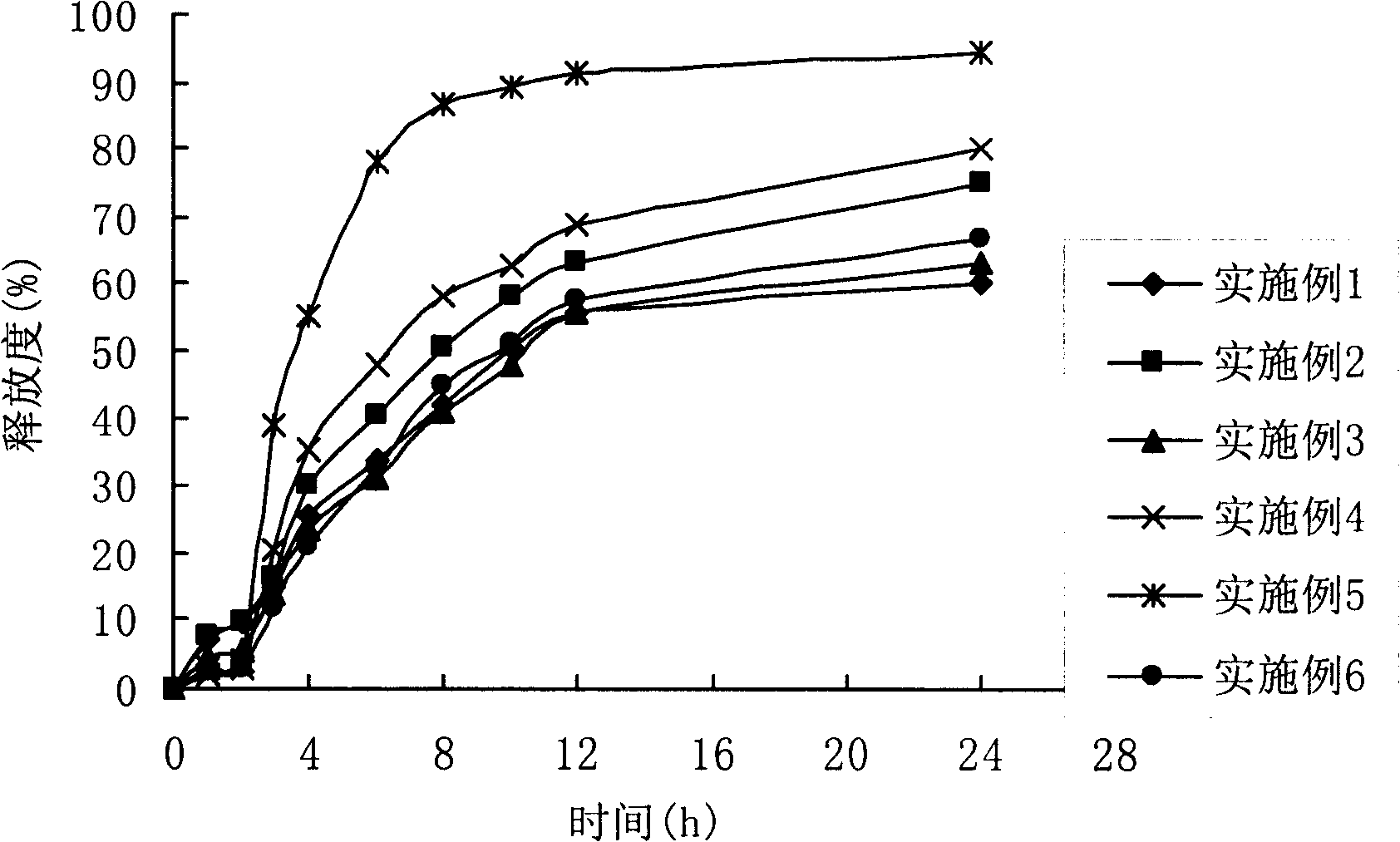
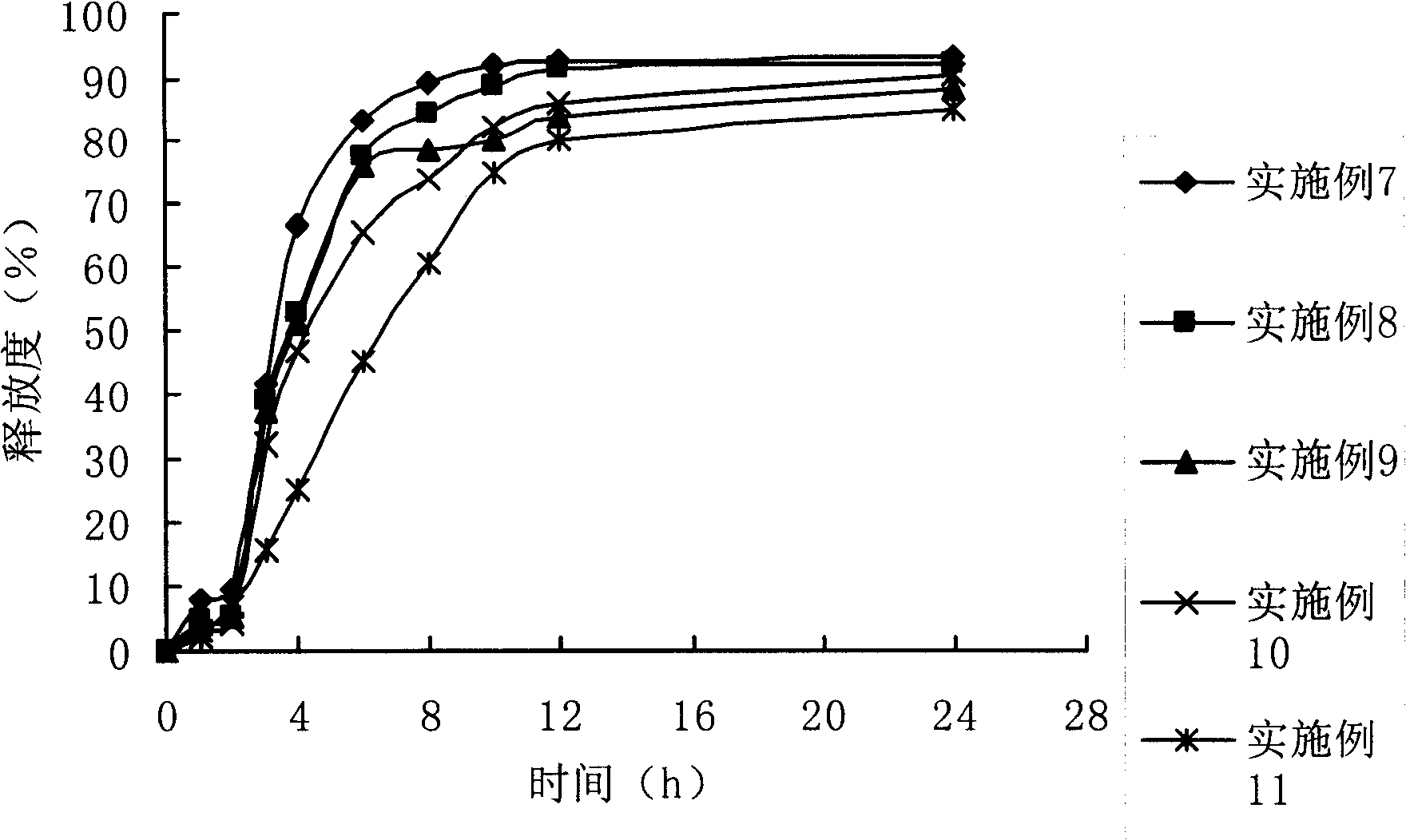
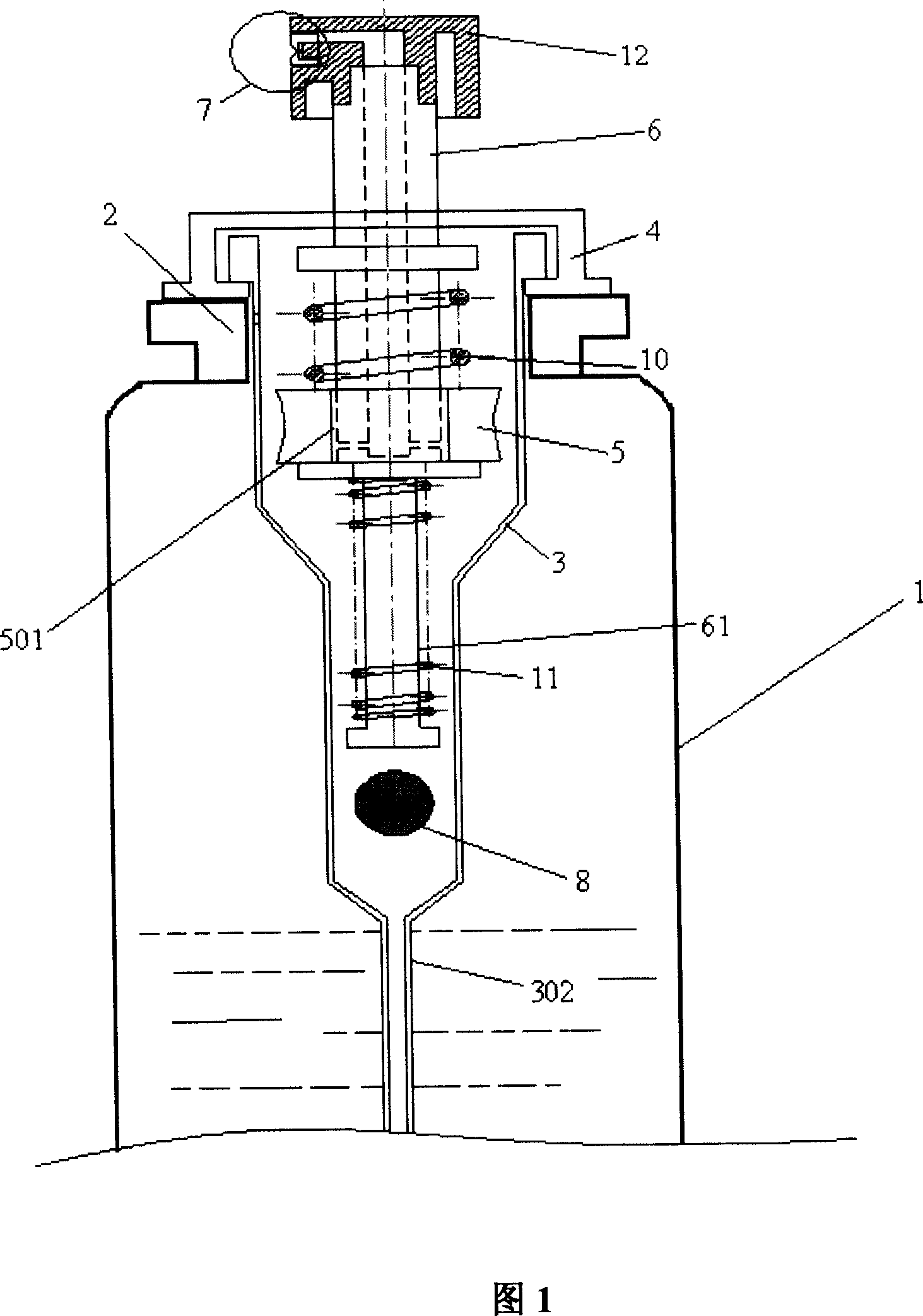
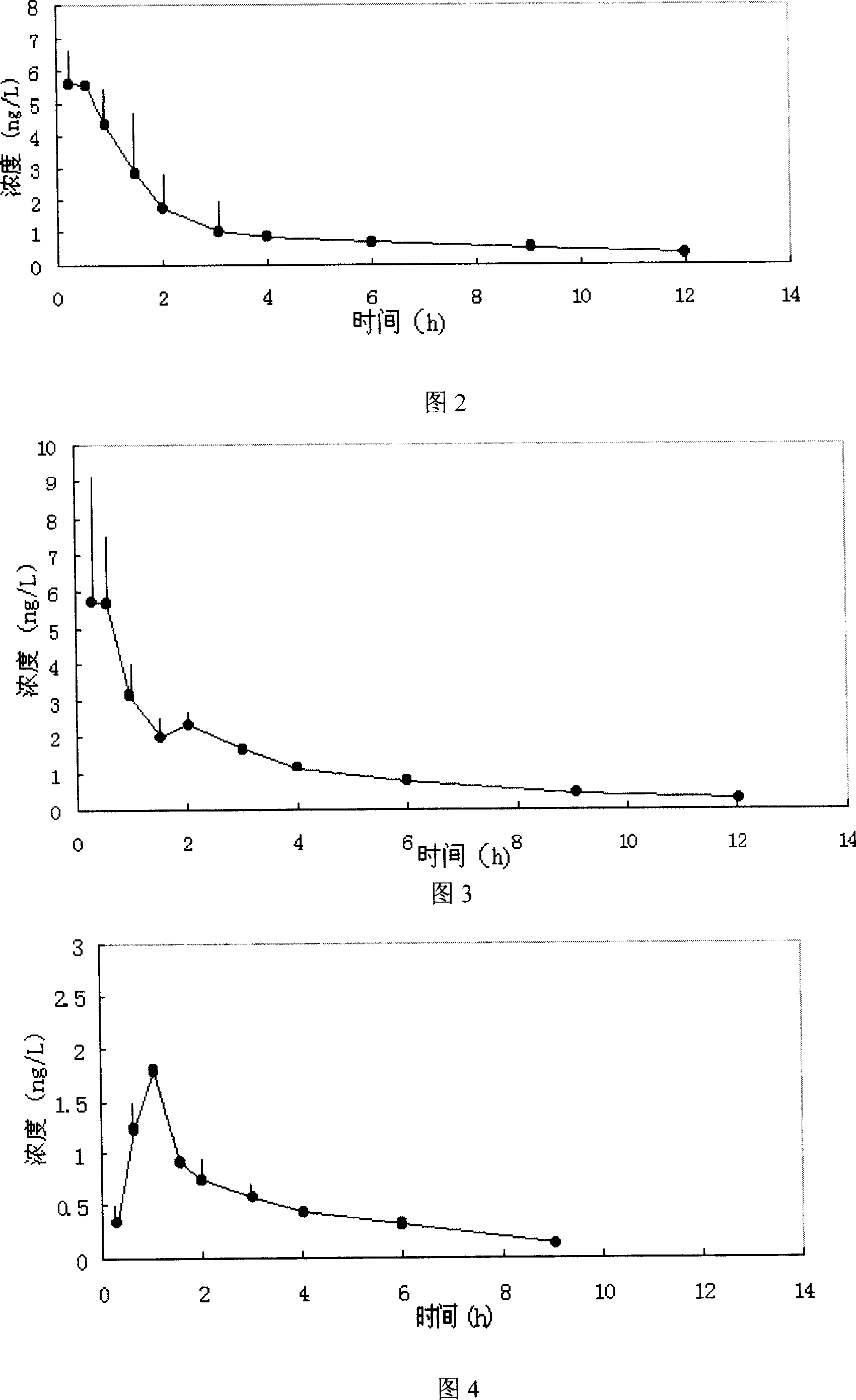
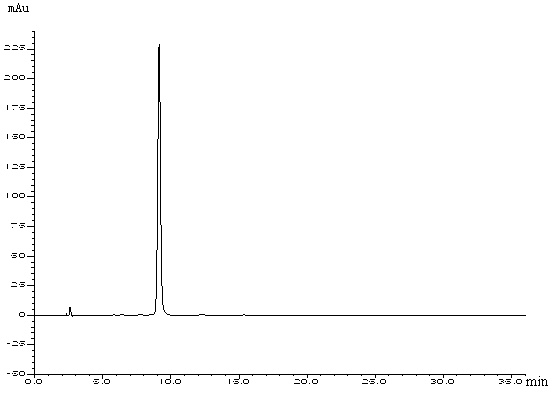
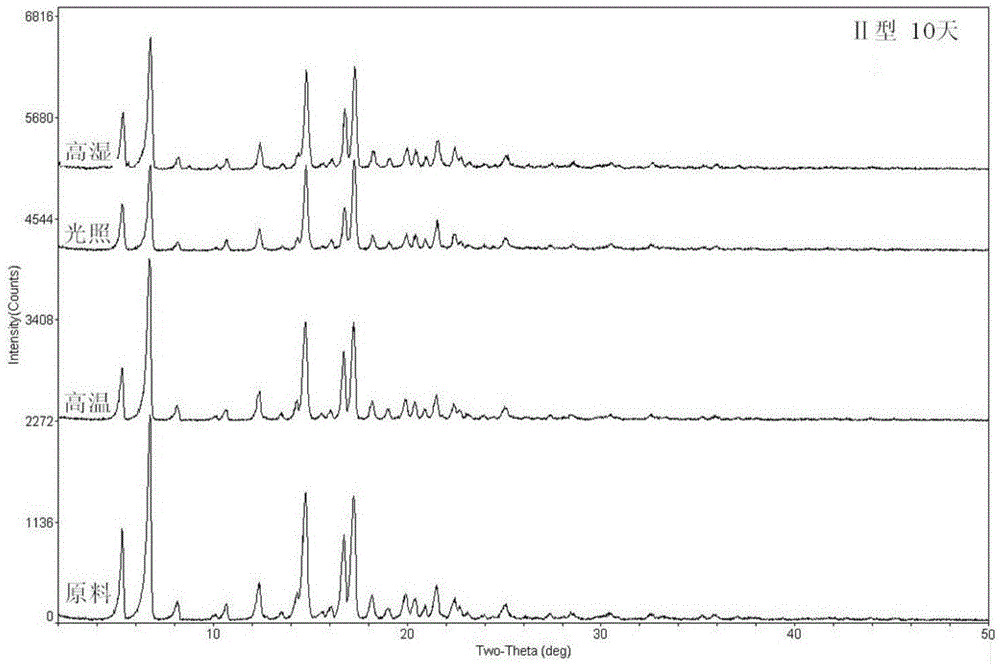
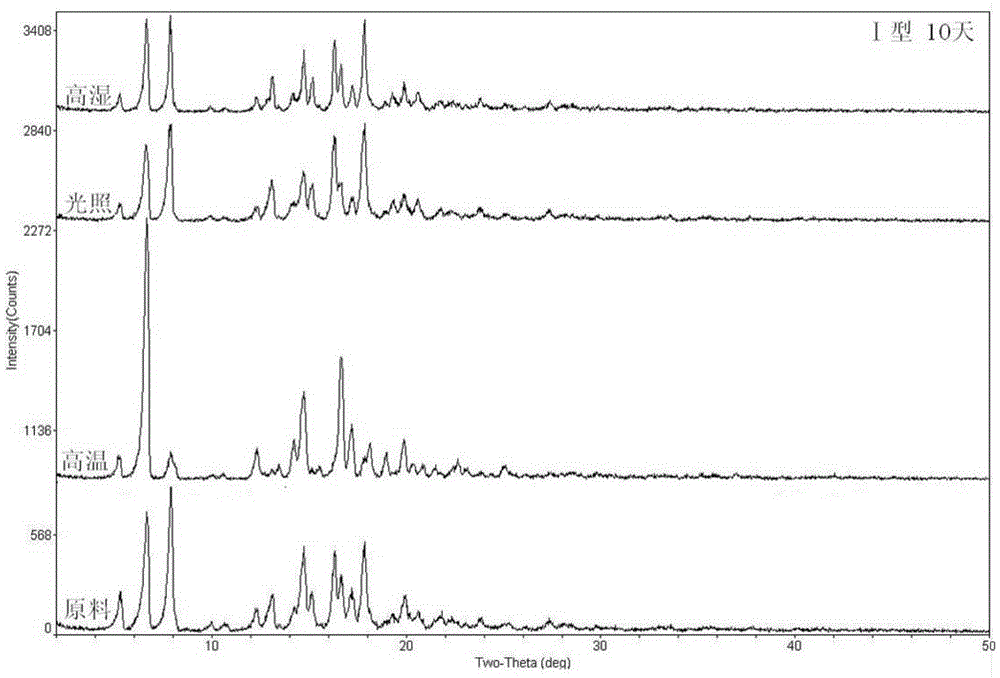
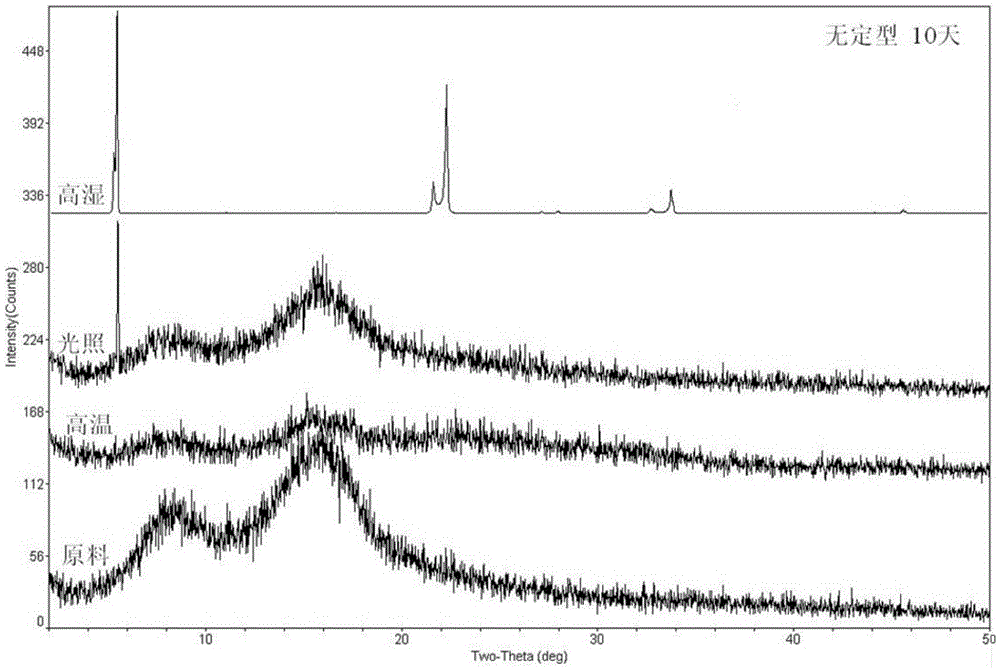
Patents
Literature
70 results about "Ciclesonide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
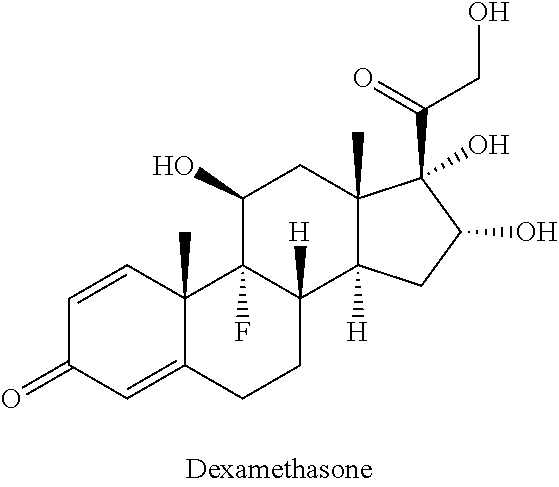
Ciclesonide is used to relieve seasonal and year-round allergy symptoms of the nose such as stuffy/runny nose, itching, and sneezing.
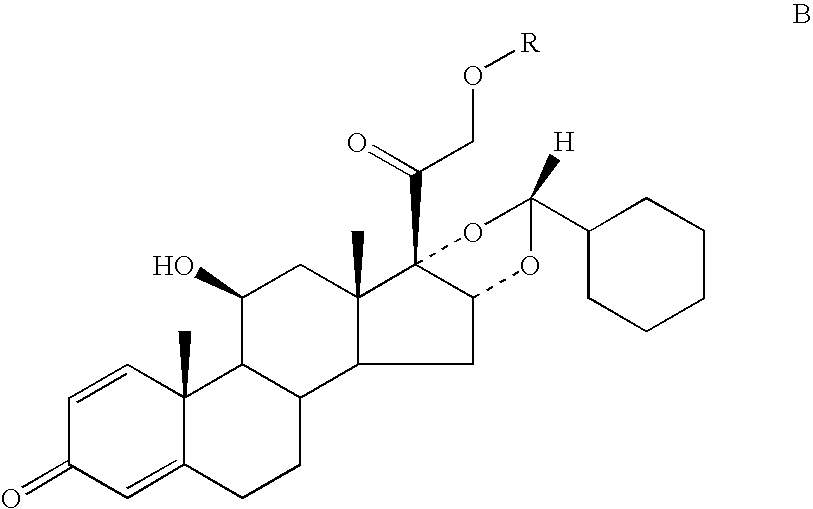
Ciclesonide and Syk Inhibitor Combination and Method of Use Thereof
InactiveUS20080027034A1Unexpected therapeutic benefitGood treatment effectAntibacterial agentsBiocideDiseaseRespiratory disease
The invention relates to pharmaceutical formulations containing combinations of ciclesonide and a syk inhibitor and the use of such pharmaceutical compositions in medicine, in particular in the prophylaxis and treatment of respiratory disease.
Owner:NYCOMED GMBH
Ciclesonide-containing sterile aqueous suspension
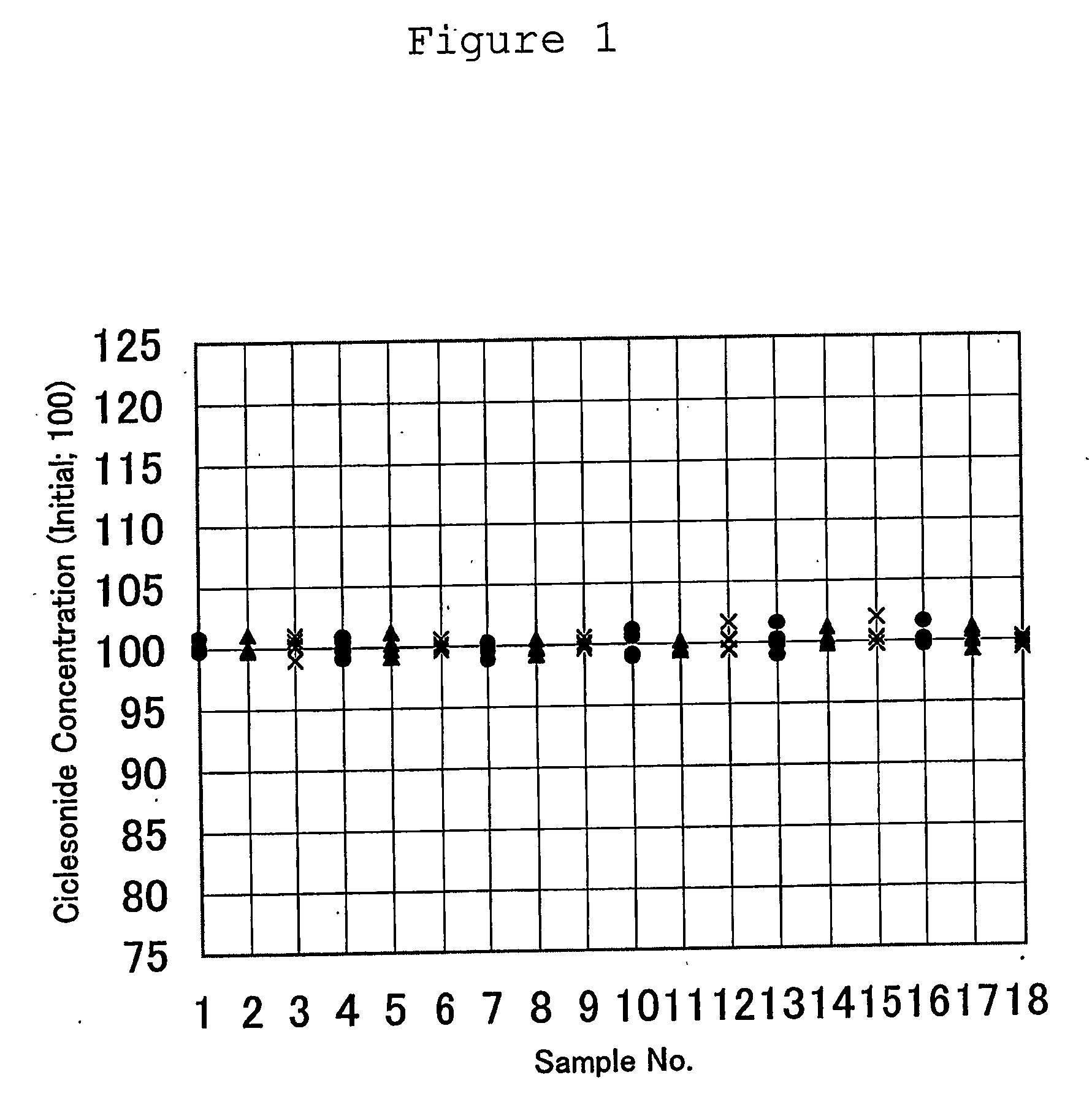
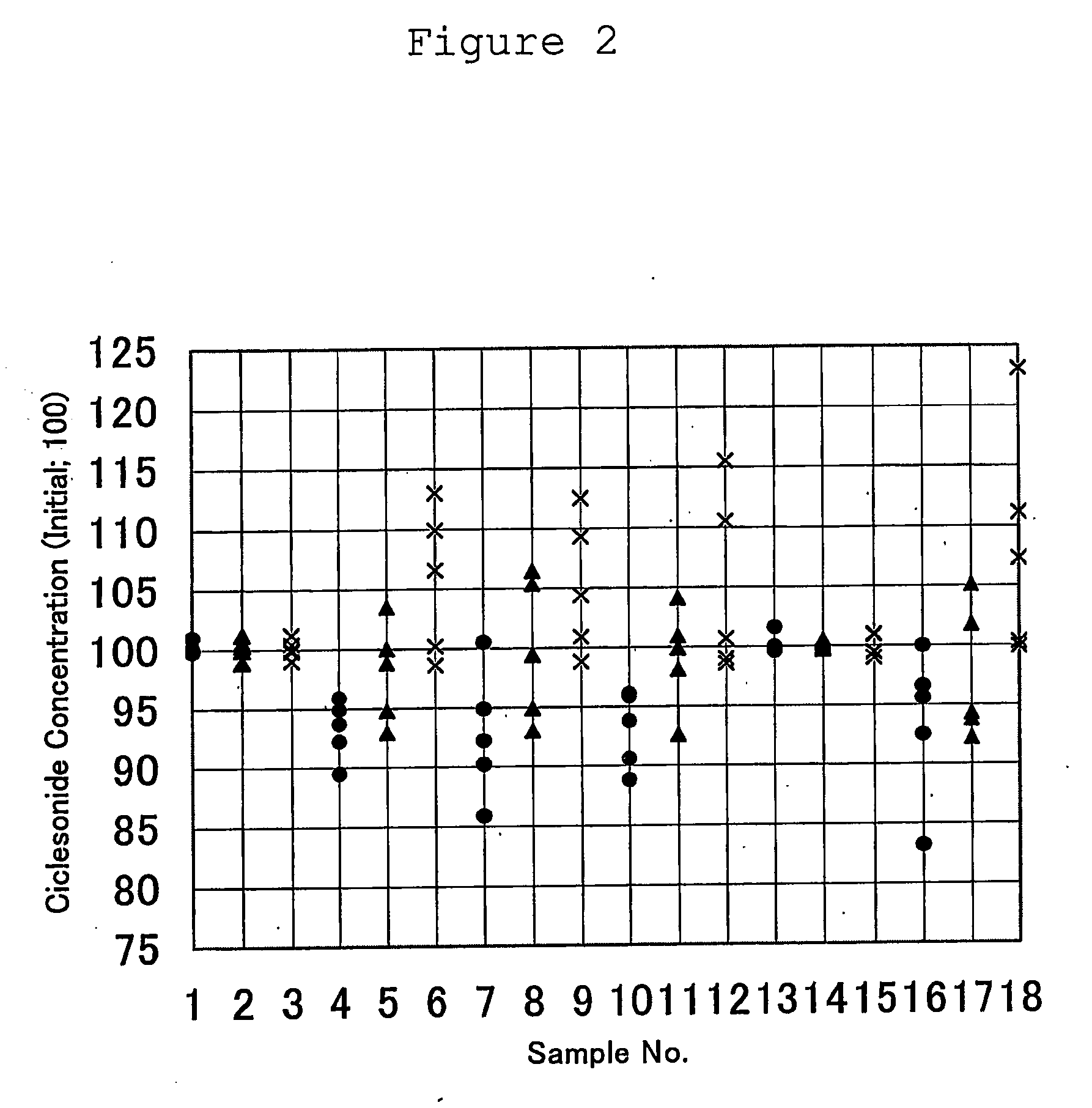
ActiveUS20060166953A1Maintain consistencyMaintain uniformityOrganic active ingredientsLavatory sanitoryChemistryAqueous suspension
Owner:COVIS PHARM GMBH
Use of the combination of ciclesonide and antihistamines for the treatment of allergic rhinitis
Owner:COVIS PHARM GMBH
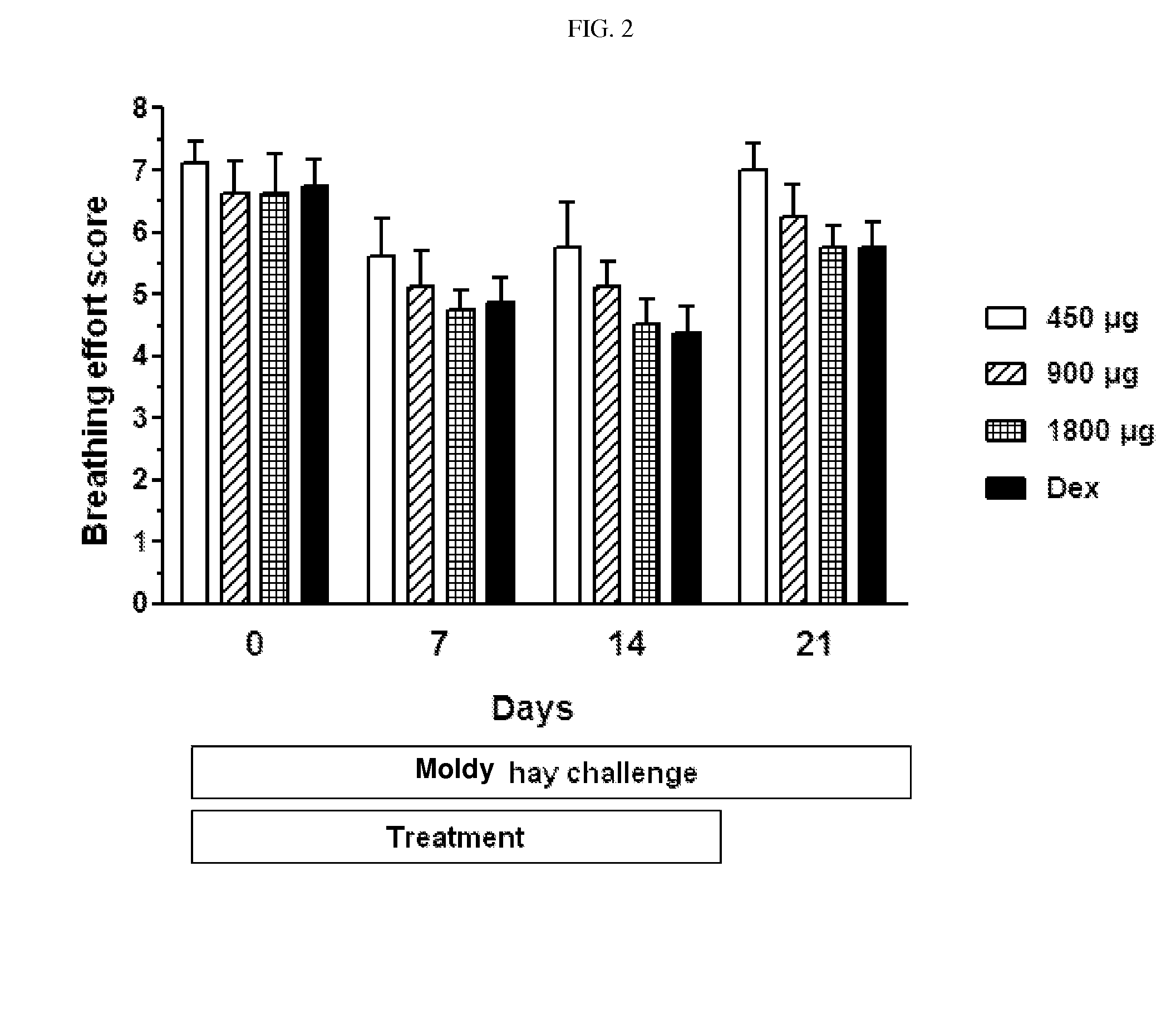
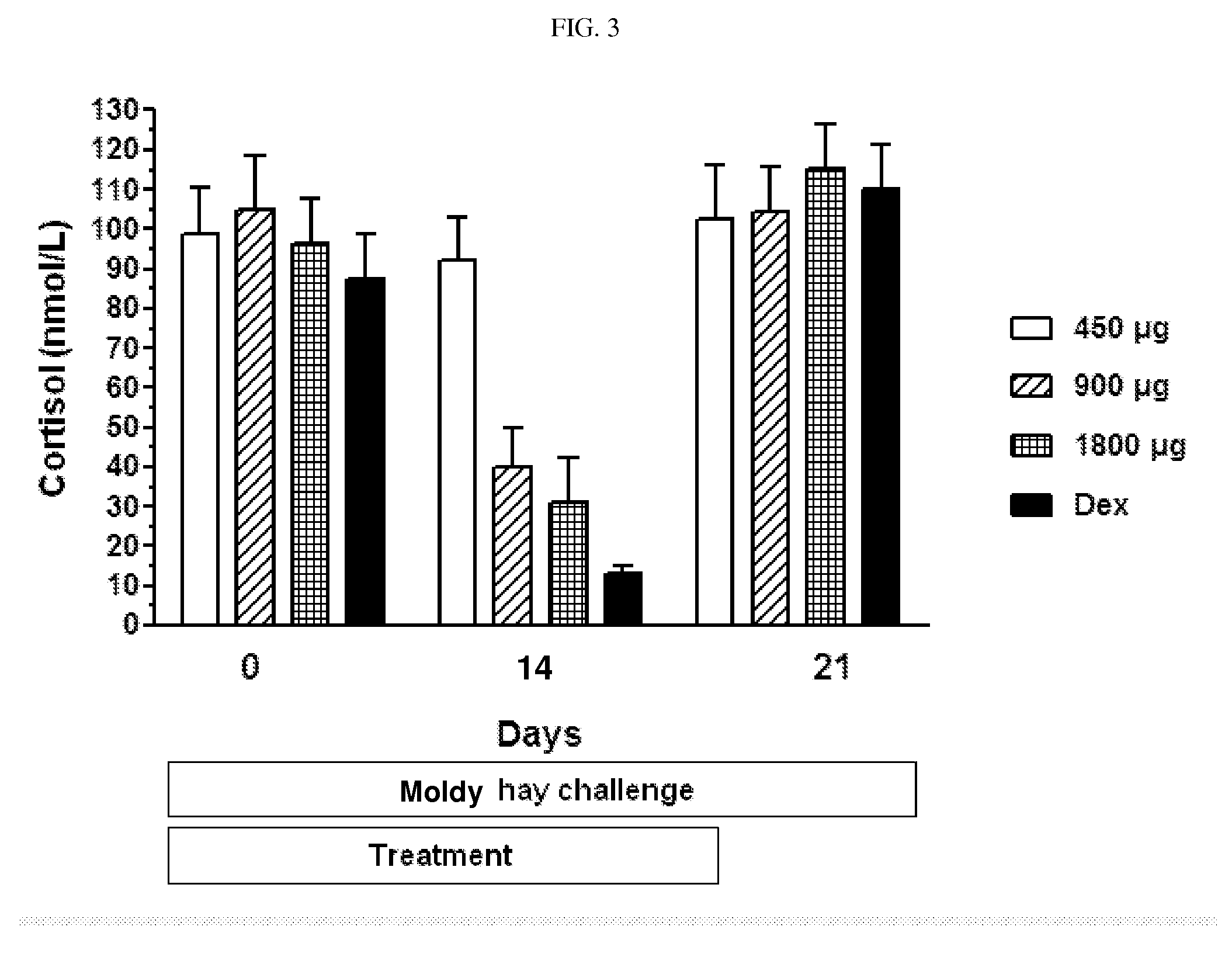
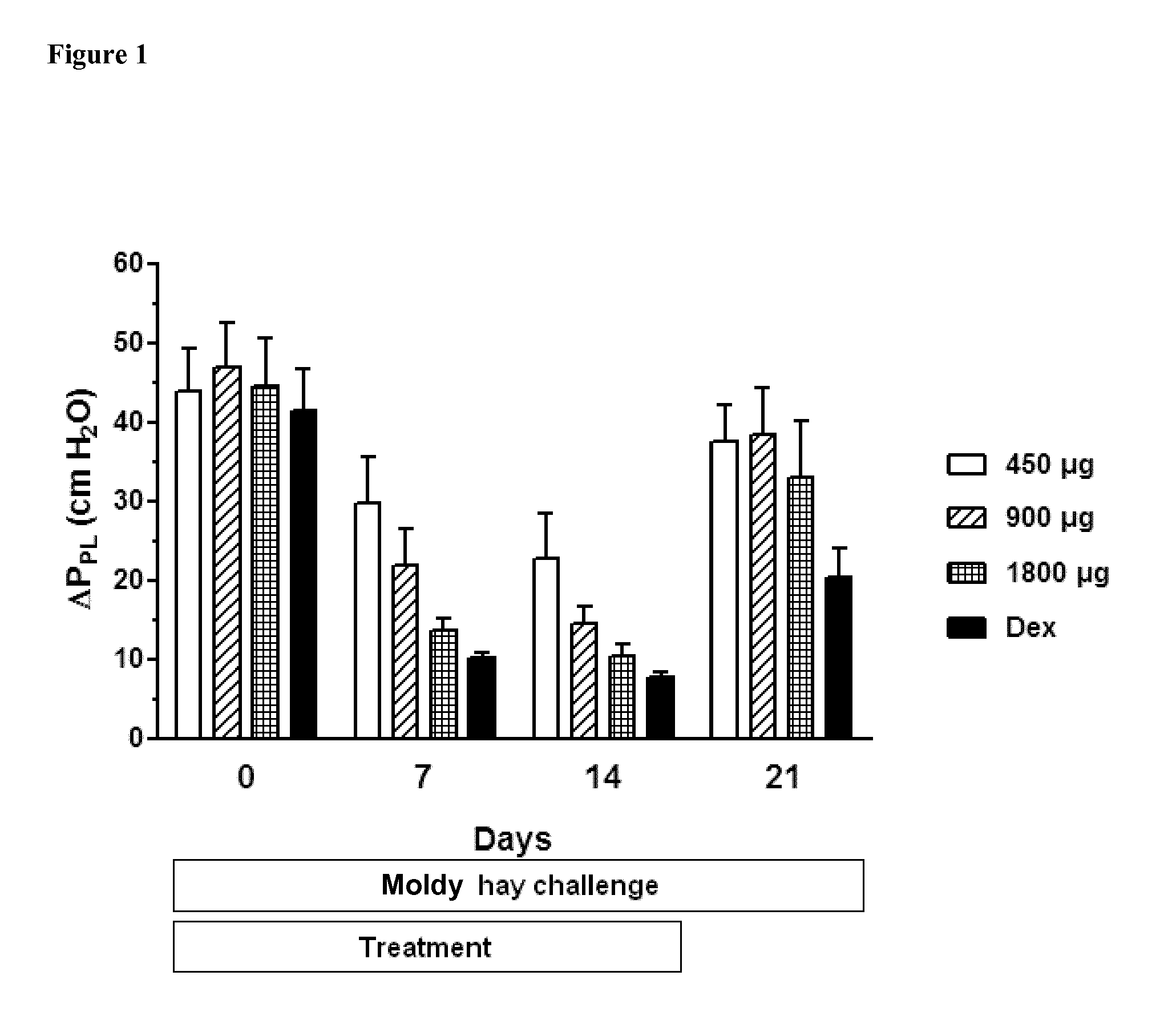
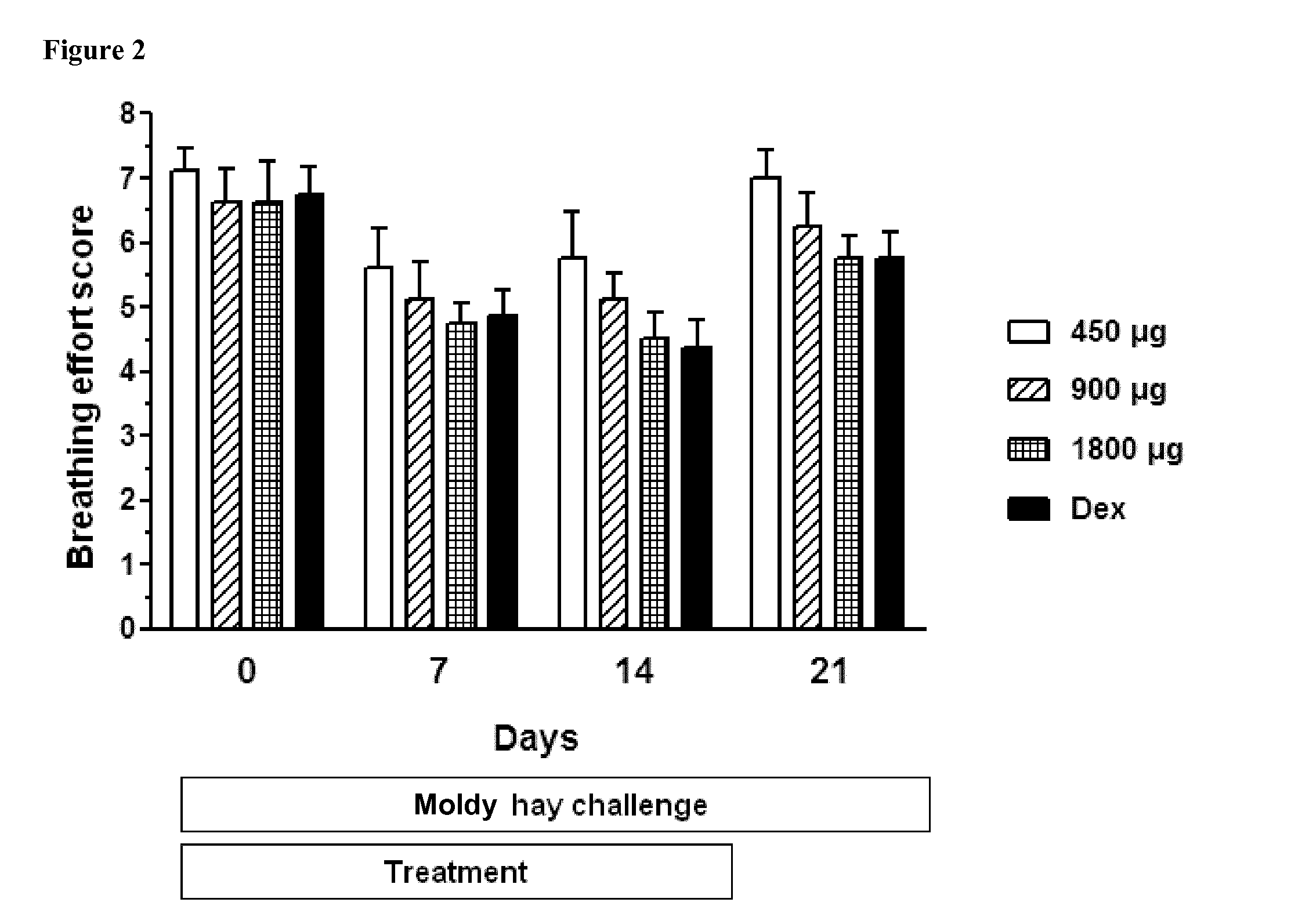
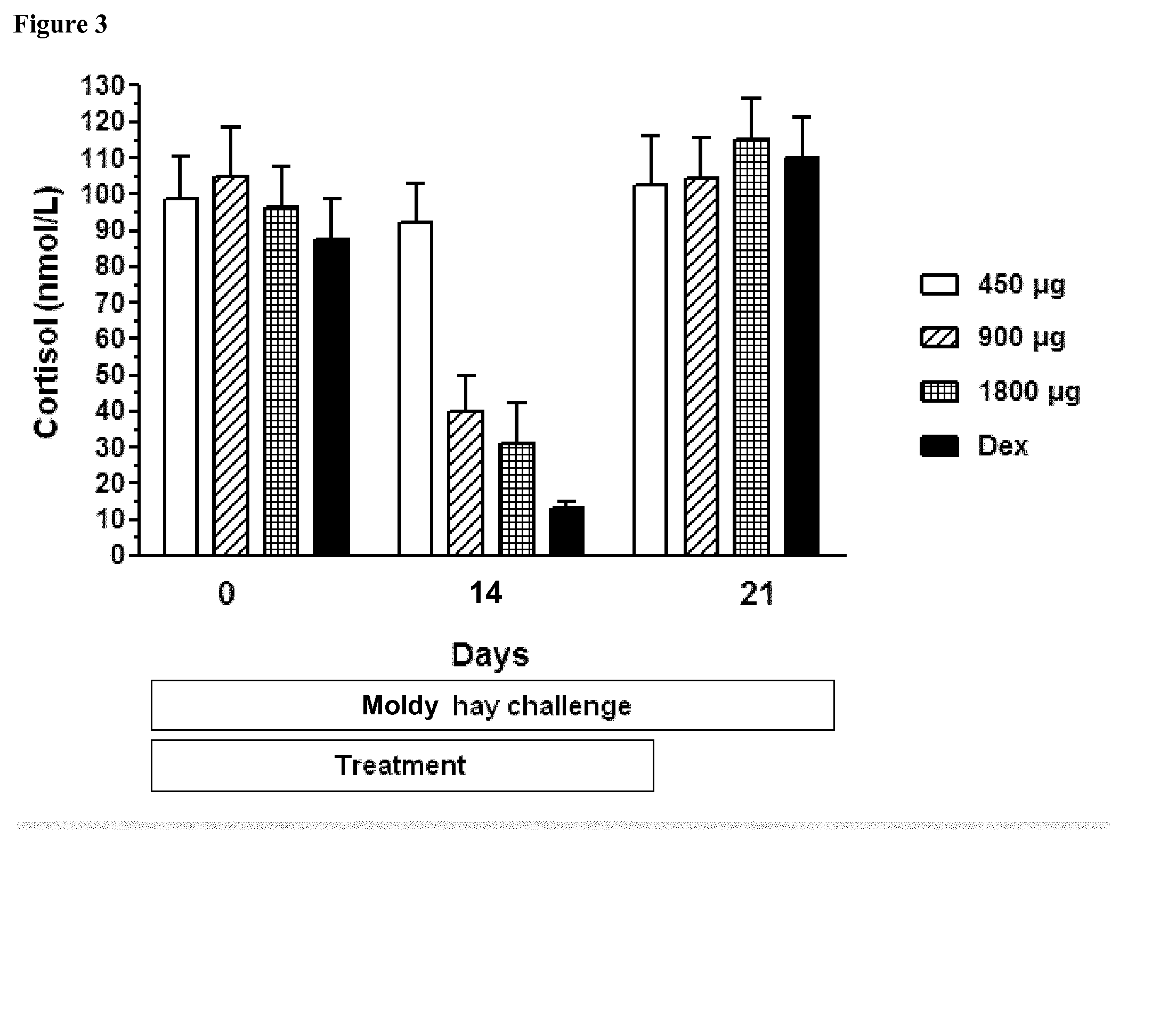
Ciclesonide for the treatment of airway disease in horses
InactiveUS20140179651A1Risk minimizationIncreased susceptibilityPowder deliveryOrganic active ingredientsDiseaseGlucocorticoid
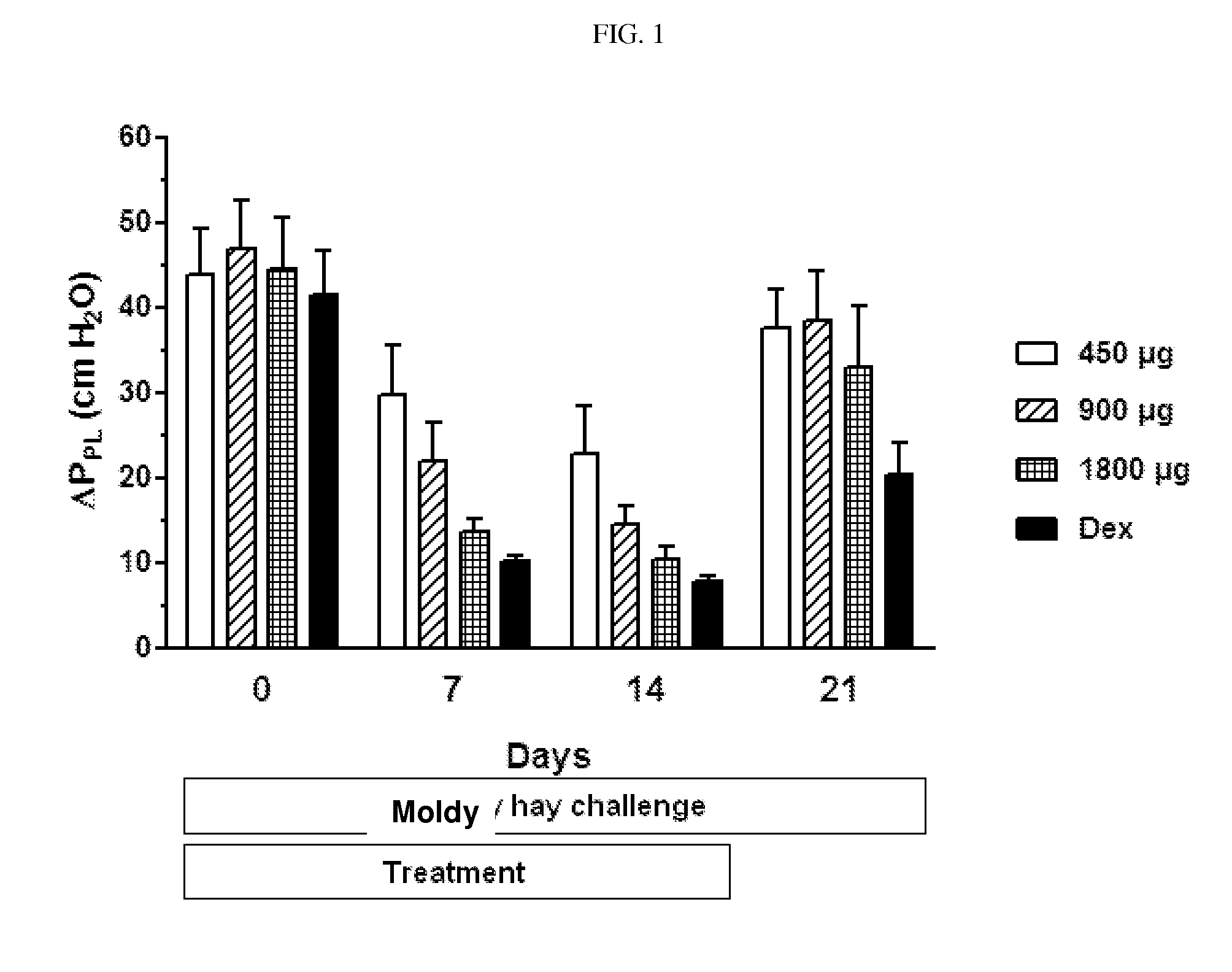
The invention relates to the field of medicine, in particular to the field of veterinary medicine. The invention relates to glucocorticoids, especially ciclesonide or a pharmaceutically acceptable derivative or salt thereof, for the treatment of airway disease in horses, such as pulmonary disease, preferably recurrent airway obstruction (RAO), Summer Pasture Associated Obstructive Pulmonary disease (SPAOPD), and inflammatory airway disease (IAD).
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Pharmaceutical formulation comprising ciclesonide
ActiveUS20140179650A1Present inventionSimple processRespiratorsPowder deliveryGlucocorticoidPharmaceutical formulation
The invention relates to the field of medicine, in particular to the field of veterinary medicine. The invention relates to a pharmaceutical (medicament) formulation of glucocorticoids, especially ciclesonide or a pharmaceutically acceptable derivative thereof.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Process for the preparation of ciclesonide
InactiveUS20070135398A1Increasing epidemic ratioOrganic active ingredientsSteroidsChemistryCiclesonide
Owner:SICOR SOC ITAL CORTICOSTEROIDI SPA
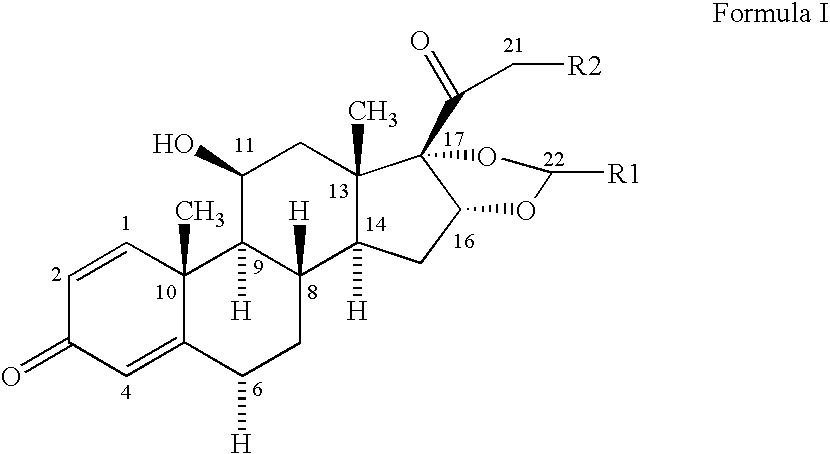
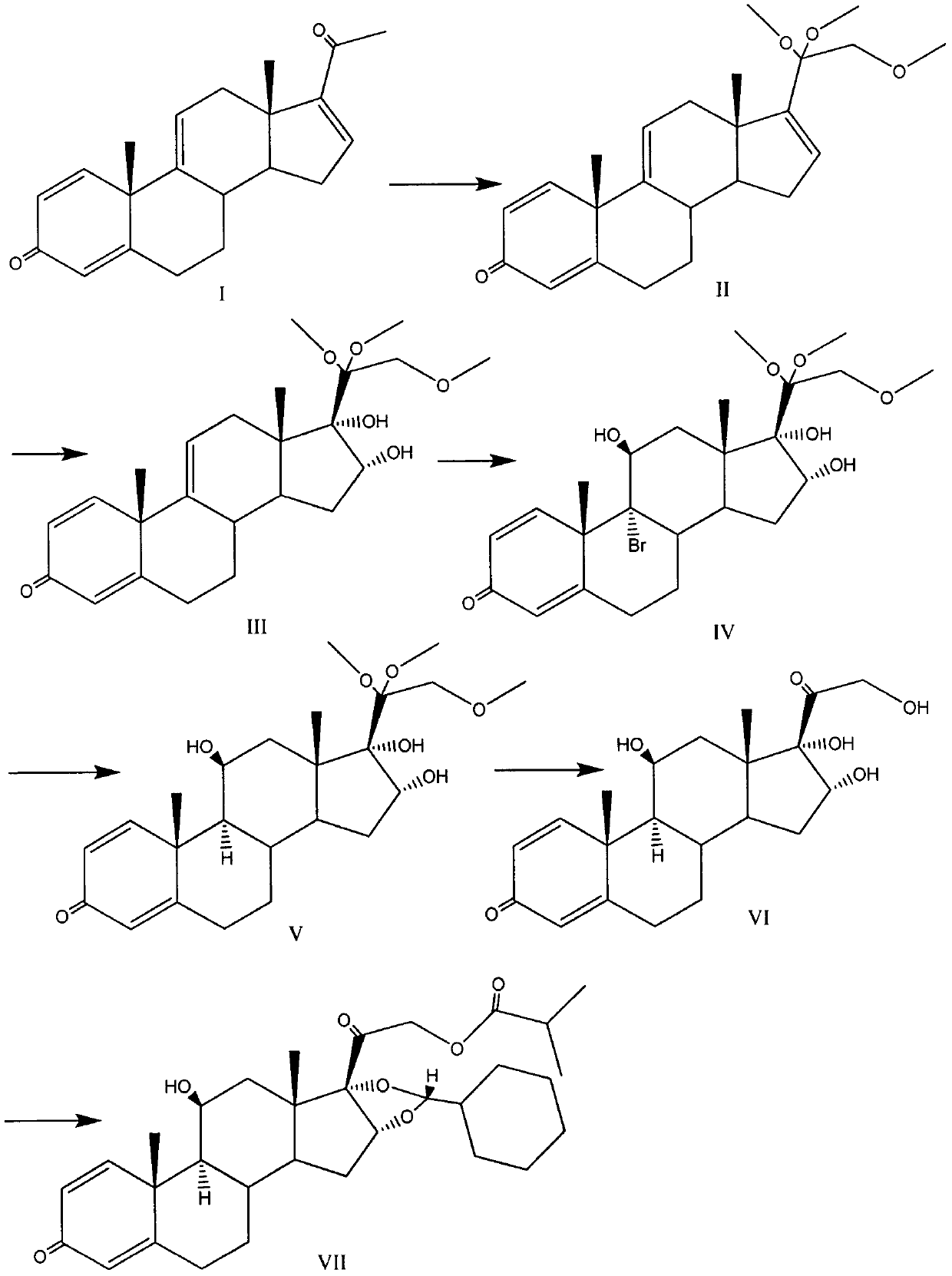
Novel 16,17-ketal intermediate for preparing ciclesonide
ActiveCN102477064AAtrophy improvedNo reduction in efficacyOrganic active ingredientsSenses disorderCombinatorial chemistryCiclesonide
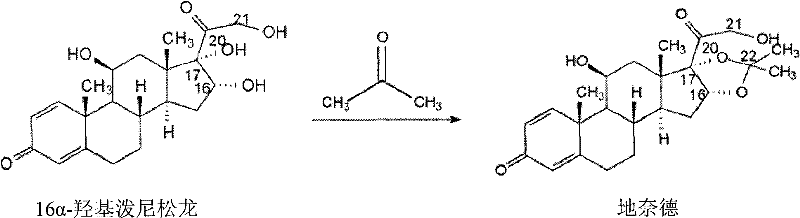
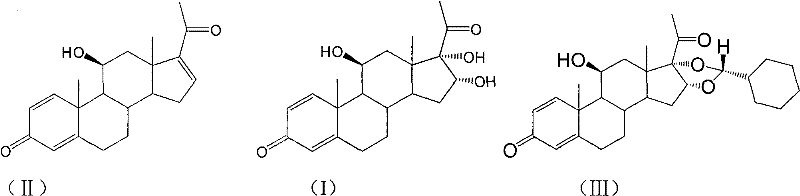
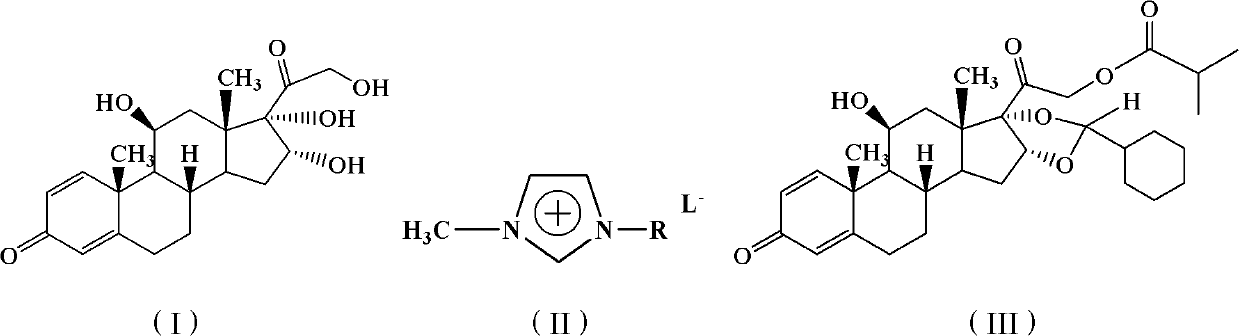
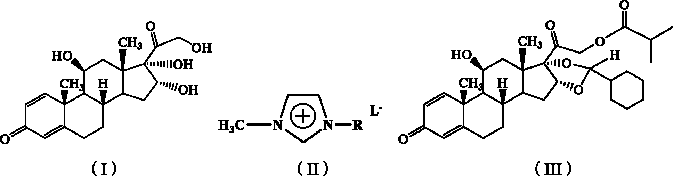
The invention relates to a novel 16,17-ketal intermediate for preparing ciclesonide, which discloses a compound of formula (III), a method for synthesizing the compound of formula (III) from a compound of formula (I), and a method for synthesizing ciclesonide from the compound of formula (III).
Owner:TIANJIN JINYAO GRP
Ciclesonide suspension composition for inhalation
The invention discloses a ciclesonide suspension composition for inhalation. The composition contains a ciclesonide hydrate and a pharmaceutically acceptable carrier, wherein the ciclesonide hydrate exists in a form of crystal.
Owner:TIANJIN JINYAO GRP
Ciclesonide oral ulcer paster and its preparing method
The present invention relates to a sticking tablet for curing stomatocace. Its adhesive layer contains 0.01-2mg of cyclosonide. Said oral sticking tablet can be general single-layer tablet, also can be double-layer tablet including medicine adhesive layer and water-proof protective layer, its adhesive material mainly is selected from hydroxypropyl methyl cellulose, carbopul, carboxymethylcellulose sodium, hydroxypropyl cellulose and polyvinylpyrrolidone.
Owner:CHONGQING PHARMA RES INST +1
Ciclesonide for the treatment of airway disease in horses
ActiveUS20170079988A1Reduce productionLower Level RequirementsPowder deliveryOrganic active ingredientsDiseaseGlucocorticoid
The invention relates to the field of medicine, in particular to the field of veterinary medicine. The invention relates to glucocorticoids, especially ciclesonide or a pharmaceutically acceptable derivative or salt thereof, for the treatment of airway disease in horses, such as pulmonary disease, preferably recurrent airway obstruction (RAO), Summer Pasture Associated Obstructive Pulmonary disease (SPAOPD), and inflammatory airway disease (IAD).
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Process for preparing crystalline ciclesonide with defined particle size
The invention relates to a novel process for preparing crystalline ciclesonide with an advantageous particle size and to the use for producing pharmaceutical preparations, in particular for topical use. The crystalline ciclesonide obtained by the novel process has advantageous aerodynamic properties, and can be further processed to inhalable or nasally administered pharmaceutical preparations without further mechanical micronization.
Owner:COVIS PHARM GMBH
Ciclesonide capsule type inhalation aerosol powder and preparation method thereof
InactiveCN101766586AOvercome the problem of prone to electrostatic accumulationLittle side effectsPowder deliveryOrganic active ingredientsActive componentMedicine
The invention discloses ciclesonide capsule type inhalation aerosol powder and a preparation method thereof. The ciclesonide capsule type inhalation aerosol powder can improve the deposition rate of ciclesonide in the inhalation aerosol powder in lung, avoid the deposition of carrier micropowder in the lung, improve the stability of a composition and consists of an active component and micropowder of a carrier and is characterized in that the average particle diameter of the micropowder as the active component is 0.5-10mu m and the average particle diameter of the micropowder of the carrier is 20-45mu m.
Owner:TIANJIN JINYAO GRP
Pharmaceutical Composition
Described herein is a pharmaceutical composition that includes a beta2-agonist selected from indacaterol and formoterol in combination with a corticosteroid selected from fluticasone and ciclesonide, and, optionally, one or more pharmaceutically acceptable excipients.
Owner:CIPLA LTD
Ciclesonide for the treatment of airway disease in horses
ActiveUS20150313918A1Reduce productionLower Level RequirementsOrganic active ingredientsPowder deliveryDiseaseGlucocorticoid
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
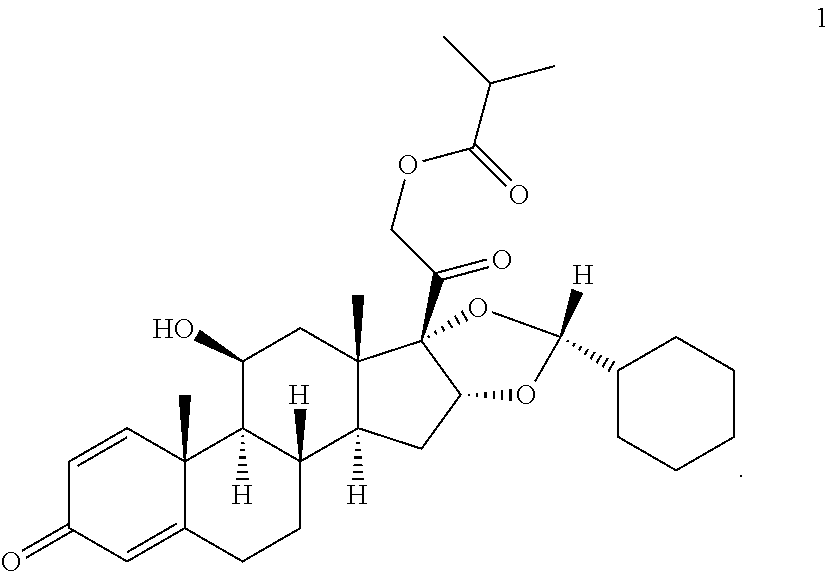
Method for manufacturing of ciclesonide
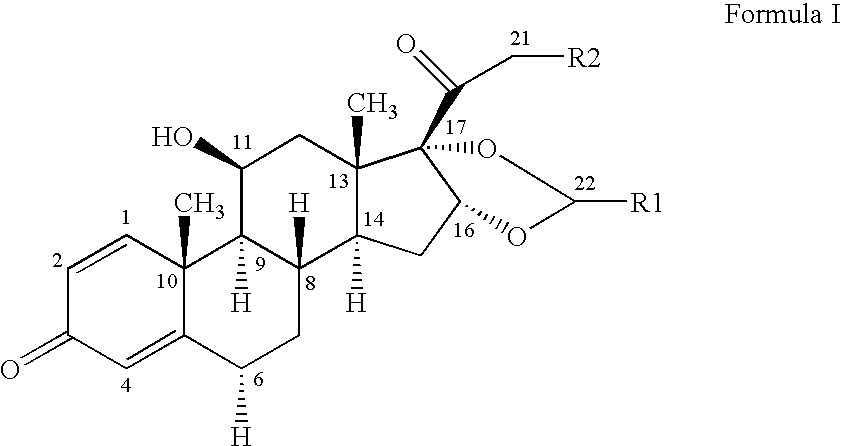
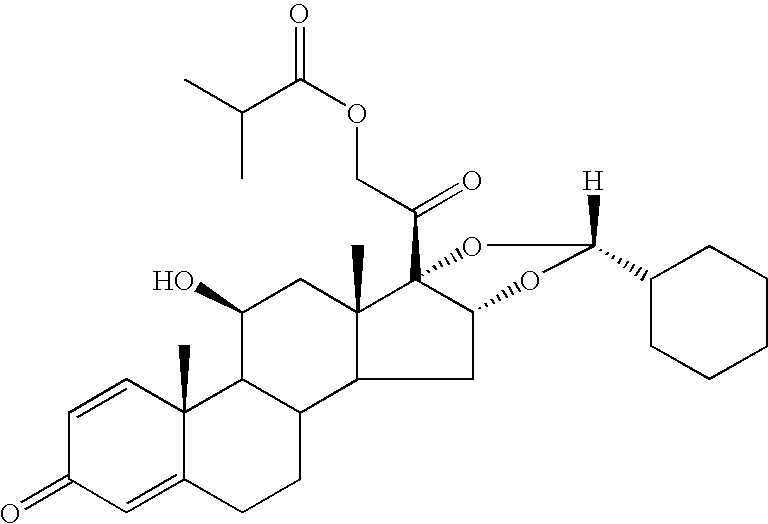
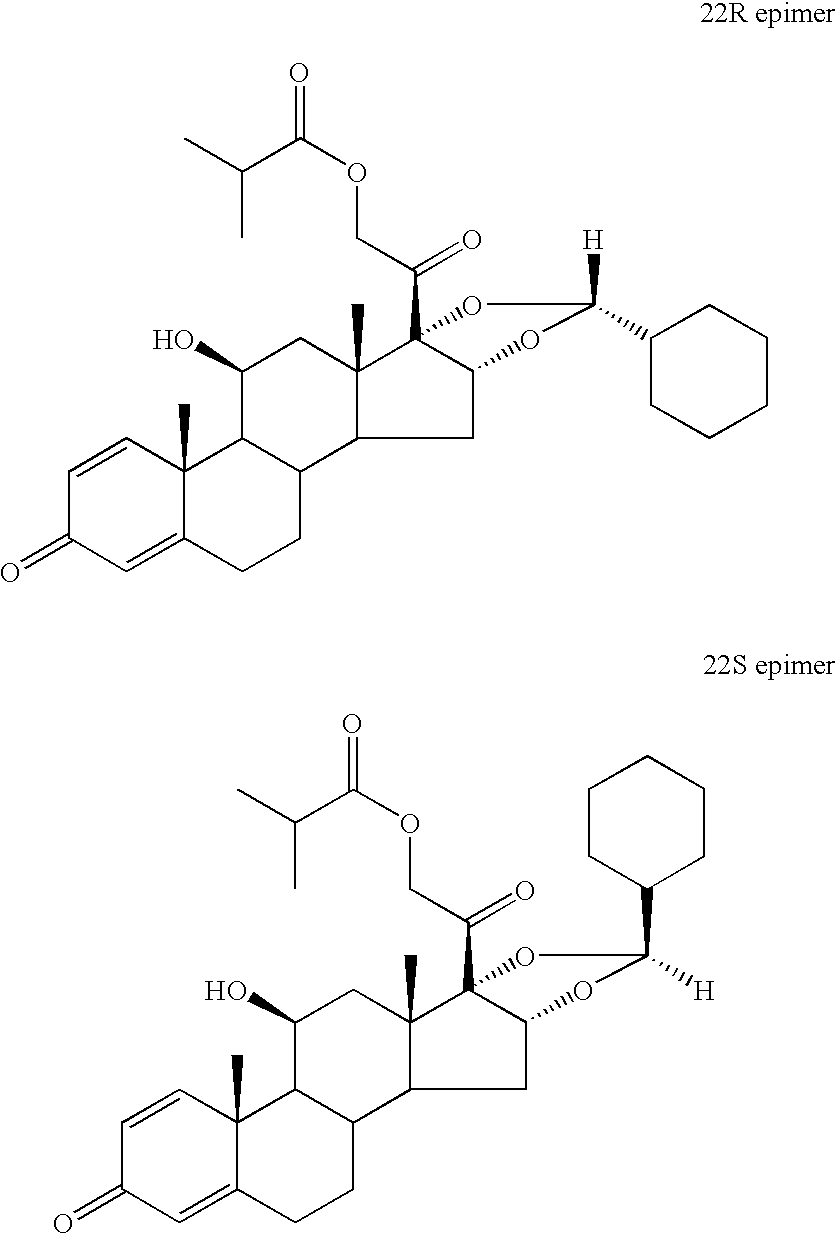
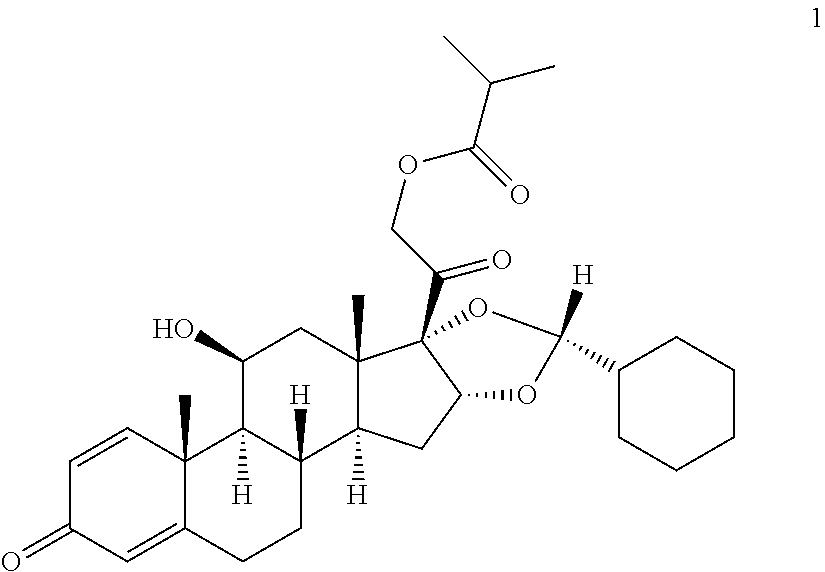
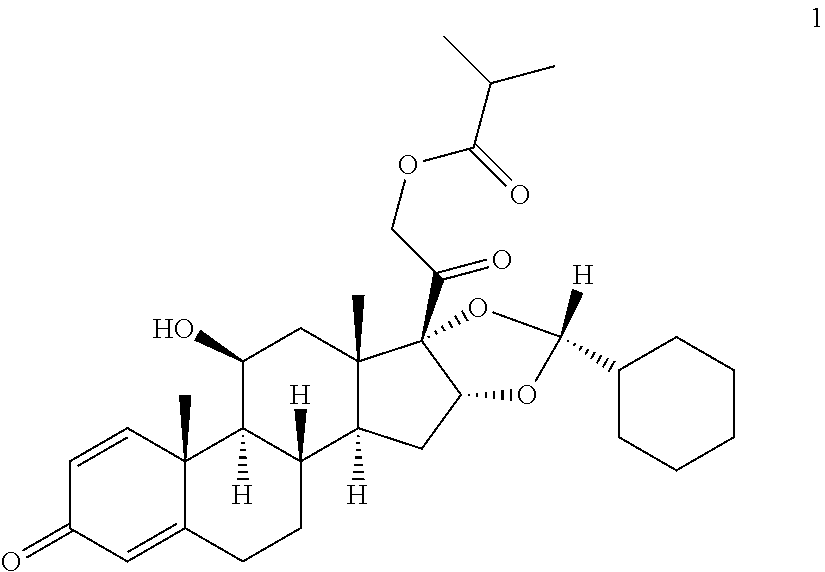
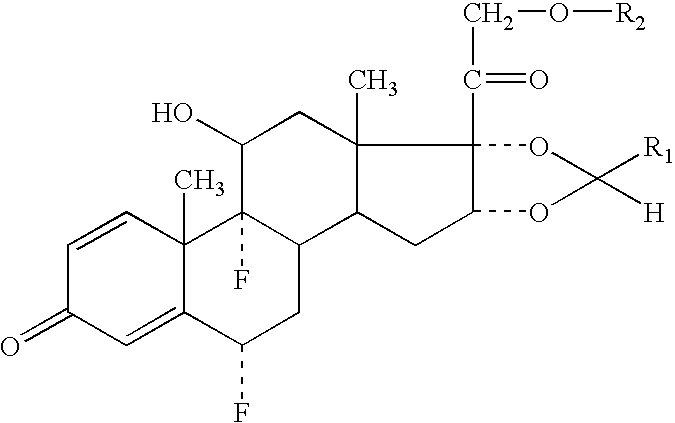
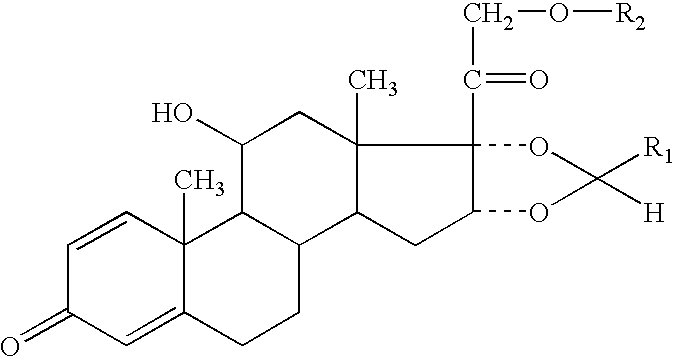
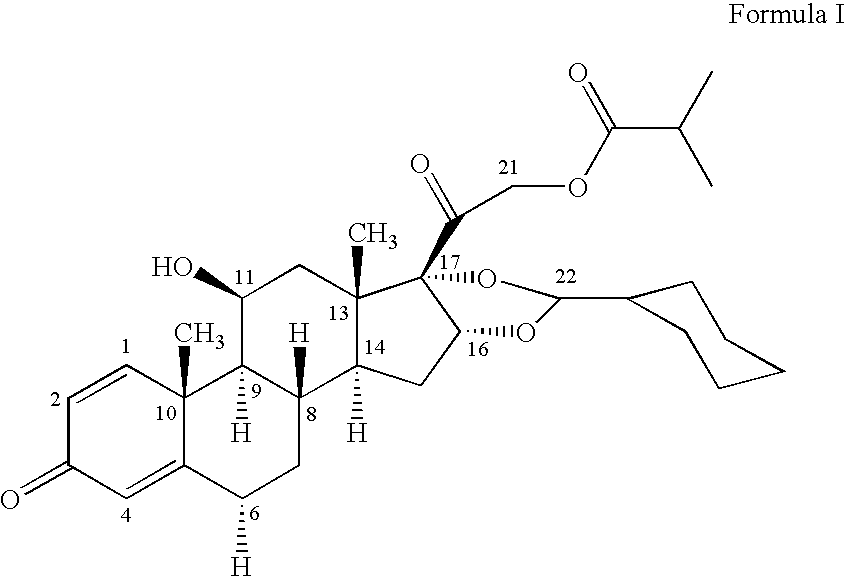
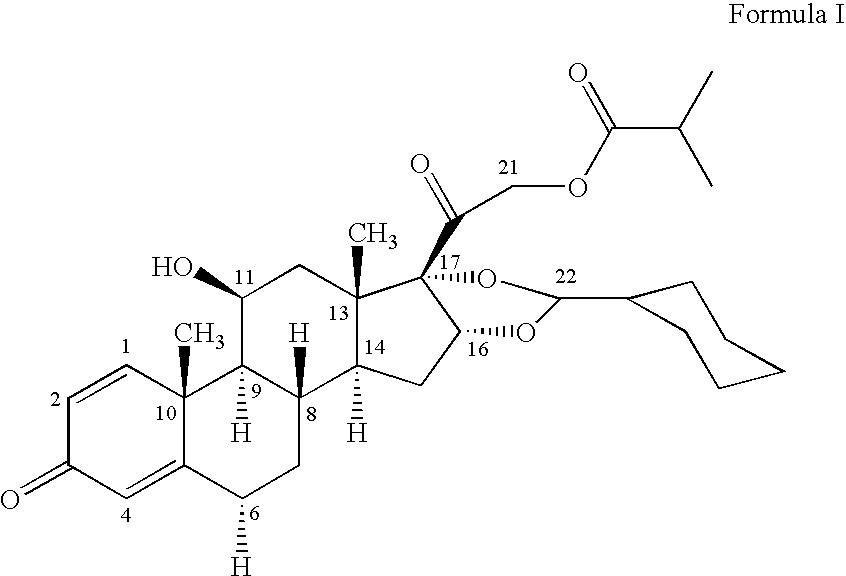
The invention relates to a process for preparing the corticosteroid ciclesonide, used for the treatment of respiratory complaints, in epimerically pure form of formula 1:
Owner:BOEHRINGER INGELHEIM INT GMBH
Processes for the preparation of ciclesonide and its crystal modification
InactiveUS8158780B2Reduce inflammationOrganic active ingredientsAntipyreticKetoneCombinatorial chemistry
Owner:CIPLA LTD
Ciclesonide nanometer freeze-dried powder and preparation method thereof
InactiveCN103070836ASmall particle sizeGood dispersionPowder deliveryOrganic active ingredientsSolubilityFreeze-drying
The invention relates to ciclesonide nanometer freeze-dried powder and a preparation method thereof and belongs to the technical field of medicinal preparation. The preparation method comprises the following steps: adding 2400-3900 weight parts of freeze-drying protecting agents into a ciclesonide nanometer grain colloidal solution, dissolving and filtering, and then pre-freezing and freeze-drying, thereby obtaining the ciclesonide freeze-dried powder, wherein the ciclesonide nanometer grain colloidal solution comprises 1 weight part of ciclesonide, 5.0-12 weight parts of carrier materials and 25-35 weight parts of hydrophilic surface active agents. The obtained ciclesonide nanometer freeze-dried powder is excellent in stability and solubility and high in safety and has the advantage of excellent appearance; after re-dissolving, the grain size of the solution is below 800nm and the medicine encapsulation efficiency is above 70%; and the technology provided by the invention has the advantages of simplicity and easiness in operation.
Owner:台州百牛生物技术有限公司
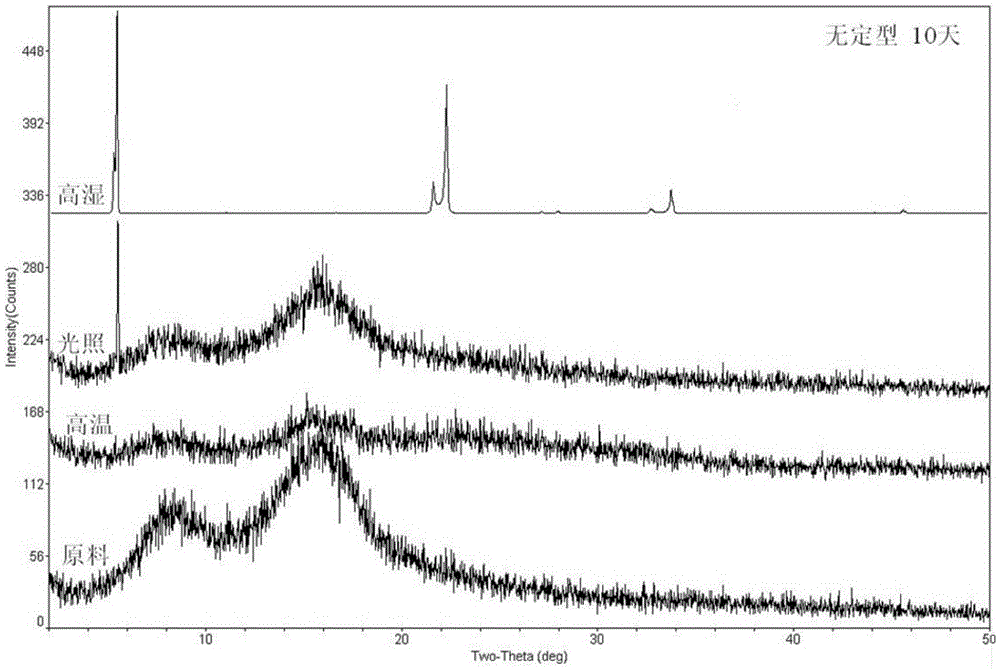
Ciclesonide suspension nasal spray composition
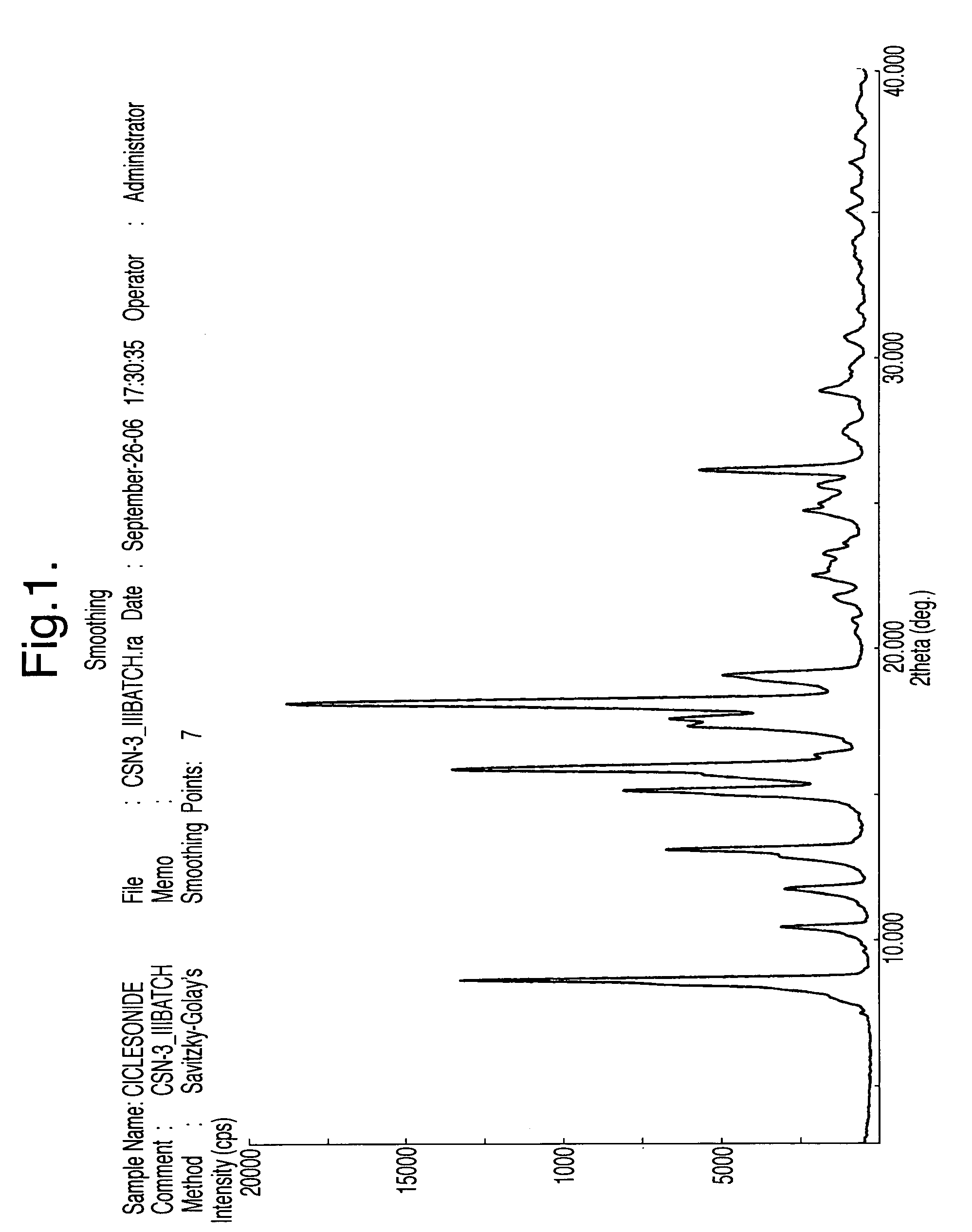
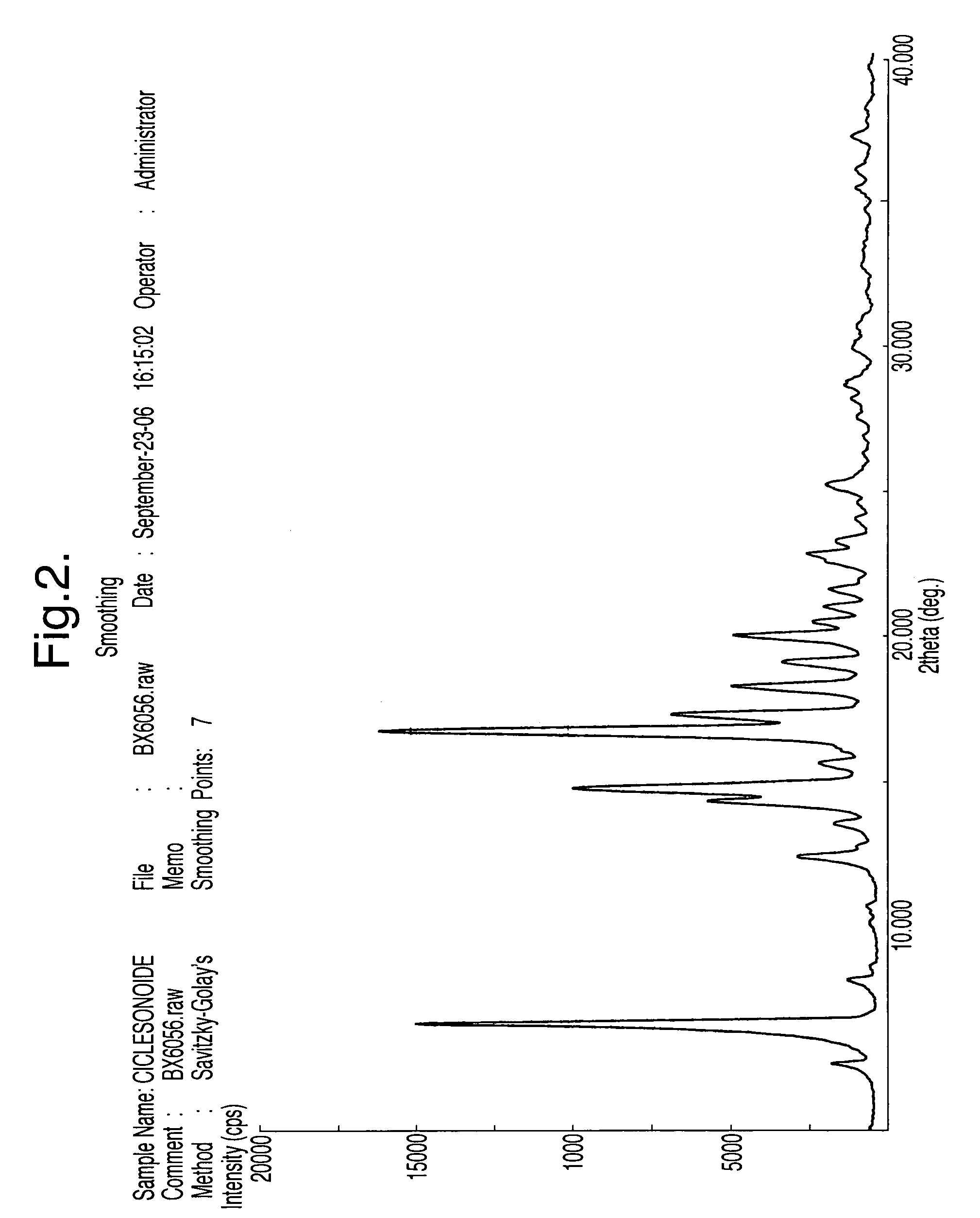
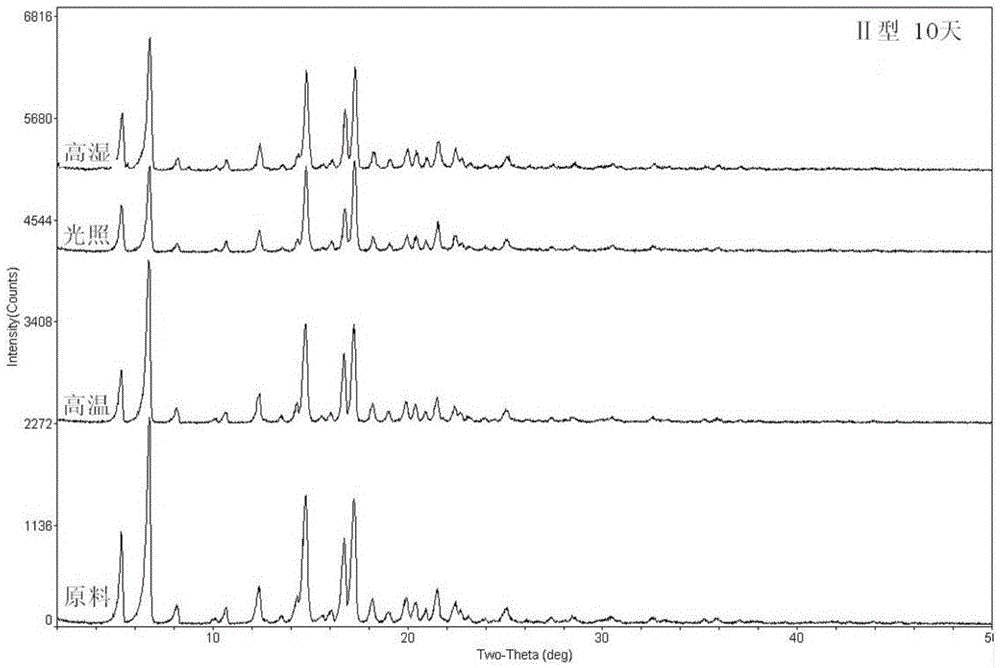
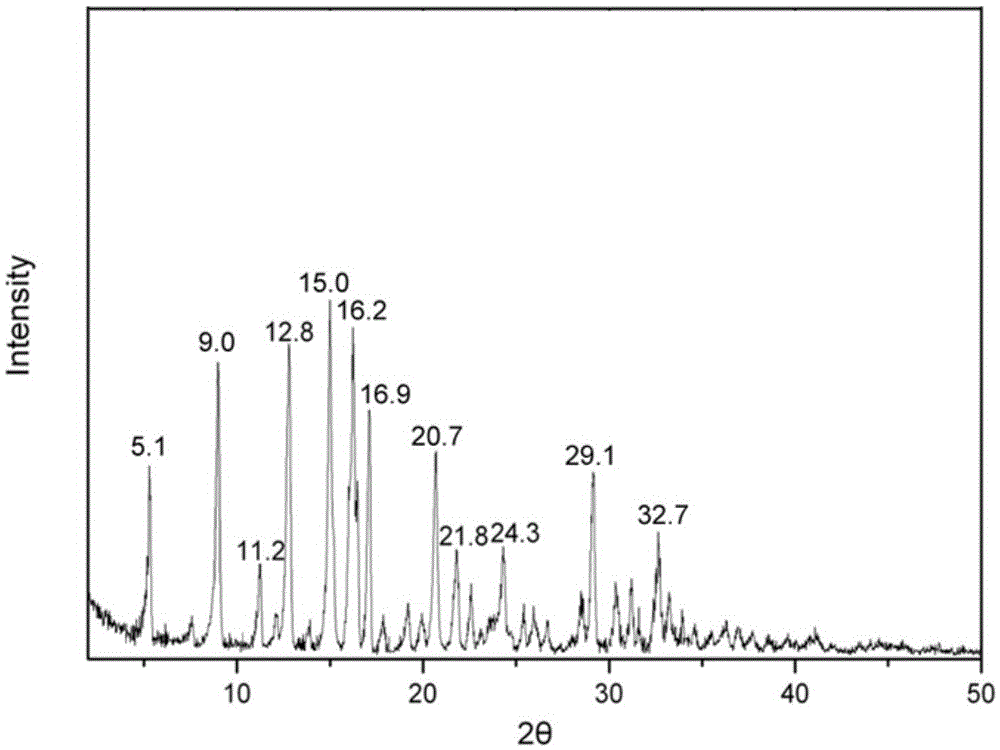
A ciclesonide suspension nasal spray composition is disclosed, and the ciclesonide suspension nasal spray composition comprises ciclesonide monohydrate and a pharmaceutically acceptable carrier. The ciclesonide monohydrate is existed in a crystal form, and characteristic peaks are shown at diffraction angle 2*theta of 5.1 degrees, 9.0 degrees, 11.2 degrees, 12.8 degrees, 15.0 degrees, 16.2 degrees, 16.9 degrees and 20.7 degrees in X ray powder diffraction.
Owner:TIANJIN JINYAO GRP
Medicament for treating inflammatory bowel disease
The invention relates to a colon-targeted pellet and a preparation method thereof, in particular to a colon-targeted pellet with ciclesonide and a preparation method thereof. The invention also discloses a pharmaceutical composition for treating inflammatory bowel diseases in mammals, especially in human; and the pharmaceutical composition consists of an active component and a pharmaceutically acceptable adjuvant for oral administration, which is characterized in that the active component contains the ciclesonide.
Owner:TIANJIN PHARMA GROUP CORP
Ciclesonide medicinal spraying agent and its preparation method
InactiveCN101007008AFast inhalationAbsorb moreOrganic active ingredientsAerosol deliveryMedicineWater spray
The invention discloses a Ciclesonide medicinal water spray agant and its preparing process, wherein the constituents include (by weight portions): Ciclesonide 1 part, solvent 0.2-4 parts, emulsifying agent 0.04-4 parts, water 0.2-0.4 part. The process requires no casting agents such as CFC and HFC, thereby no pressure containers are needed.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
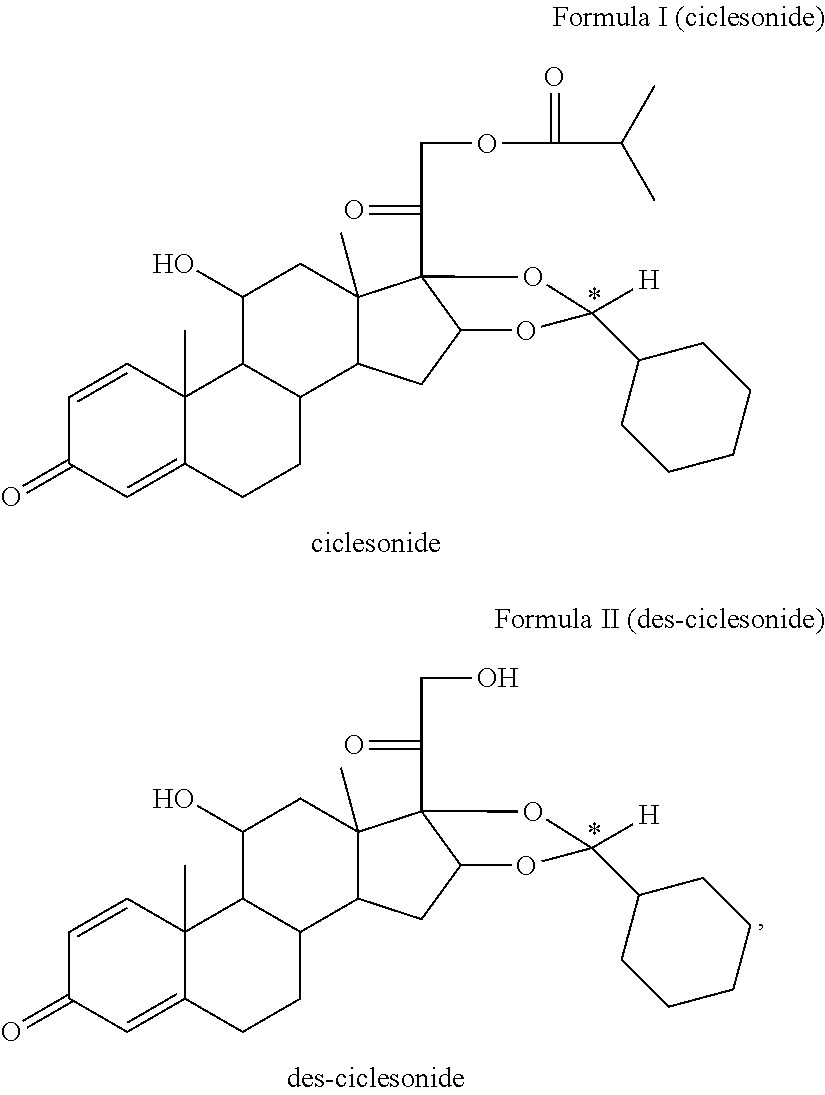
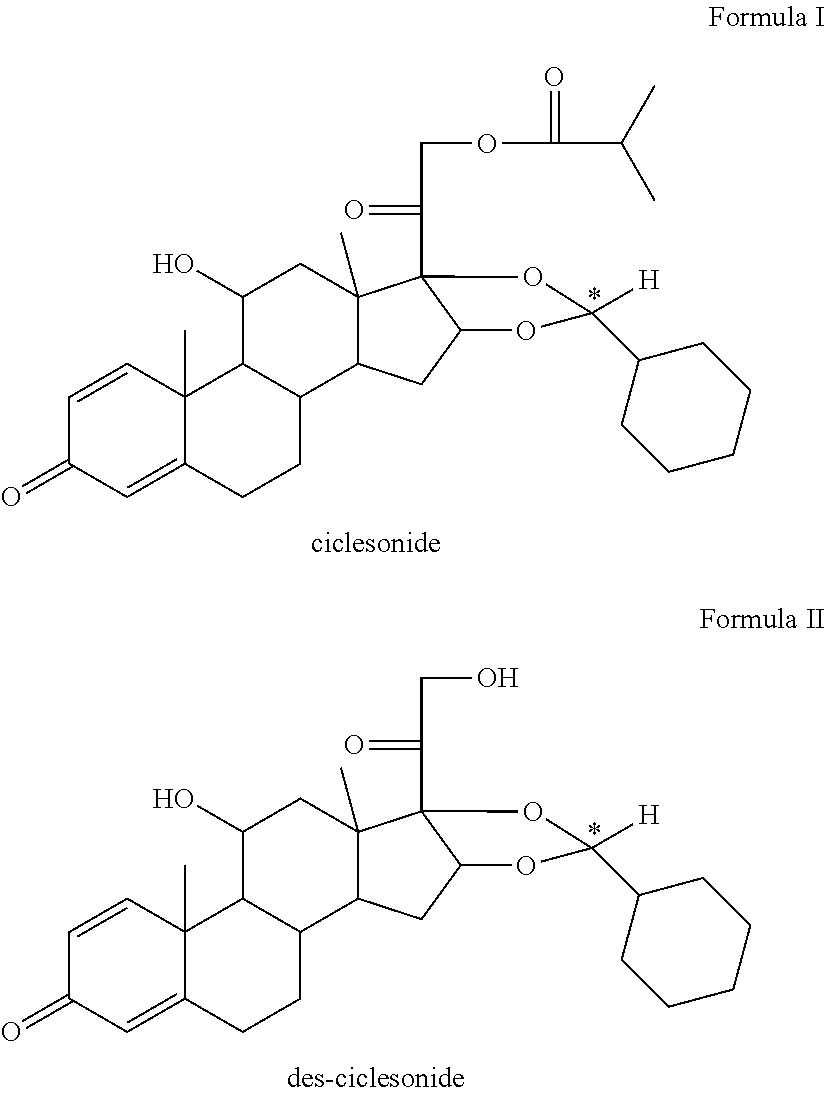
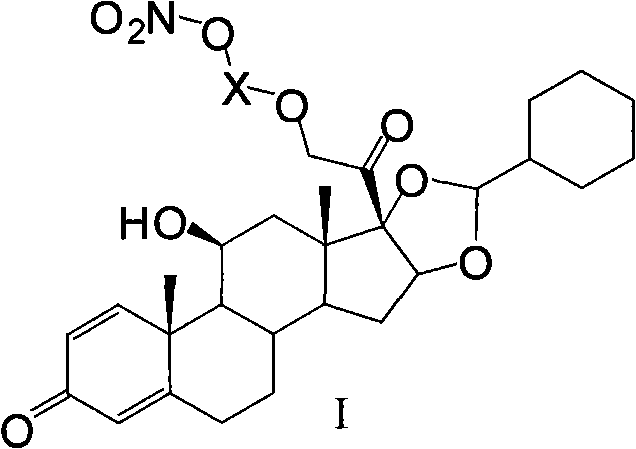
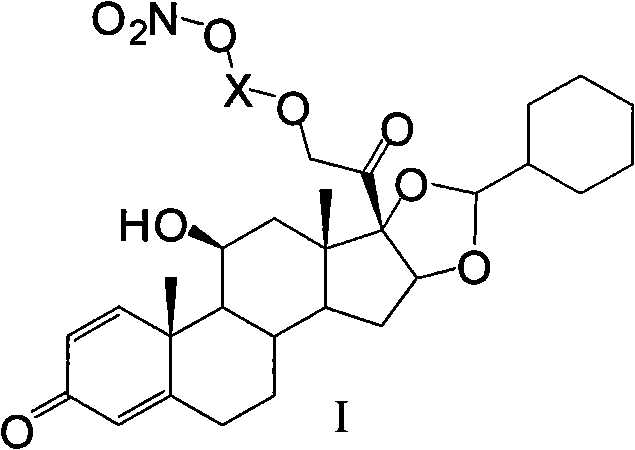
Ciclesonide nitrate derivative
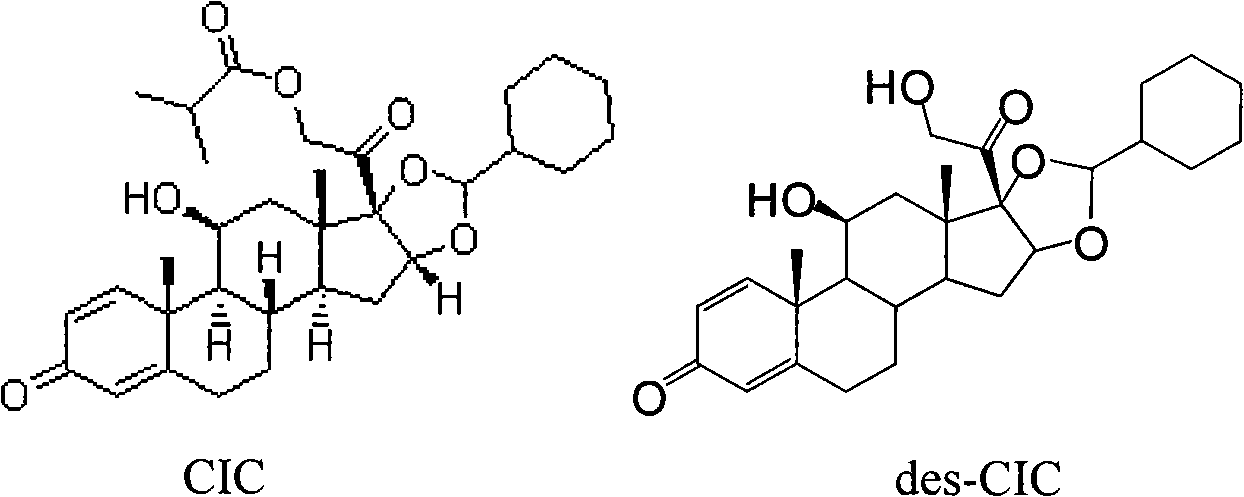
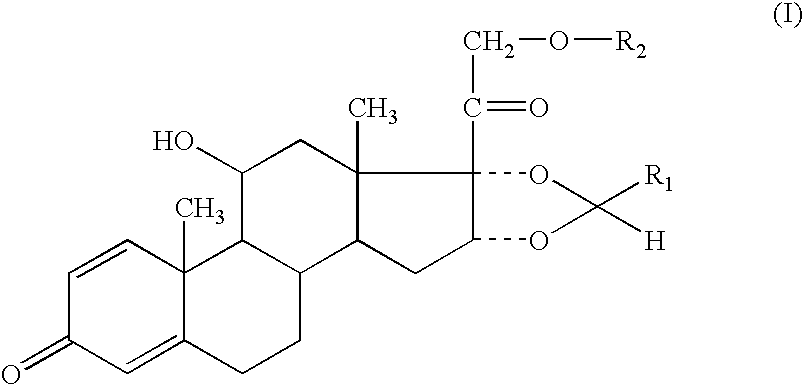
The invention relates to a ciclesonide nitrate derivative (I) shown in the specification, wherein X is -CO(O)a(CR4R5)b' D(CR4R5)b''-, a is 0 or 1, b' and b'' can be same or different and are integers from 0 to 6, R4 and R5 are same or different and are selected from H and hydrocarbon compounds with 1-6 carbons, D can be nonexistent and also can be a hydrocarbon compound with 1-8 carbons containing 0-2 heteroatoms, the heteroatom is one or two of N, O and S, and X and 21 hydroxyl groups of des-ciclesonide (des-CIC) are connected through ester bonds.
Owner:TIANJIN JINYAO GRP
Synthesis method for ciclesonide
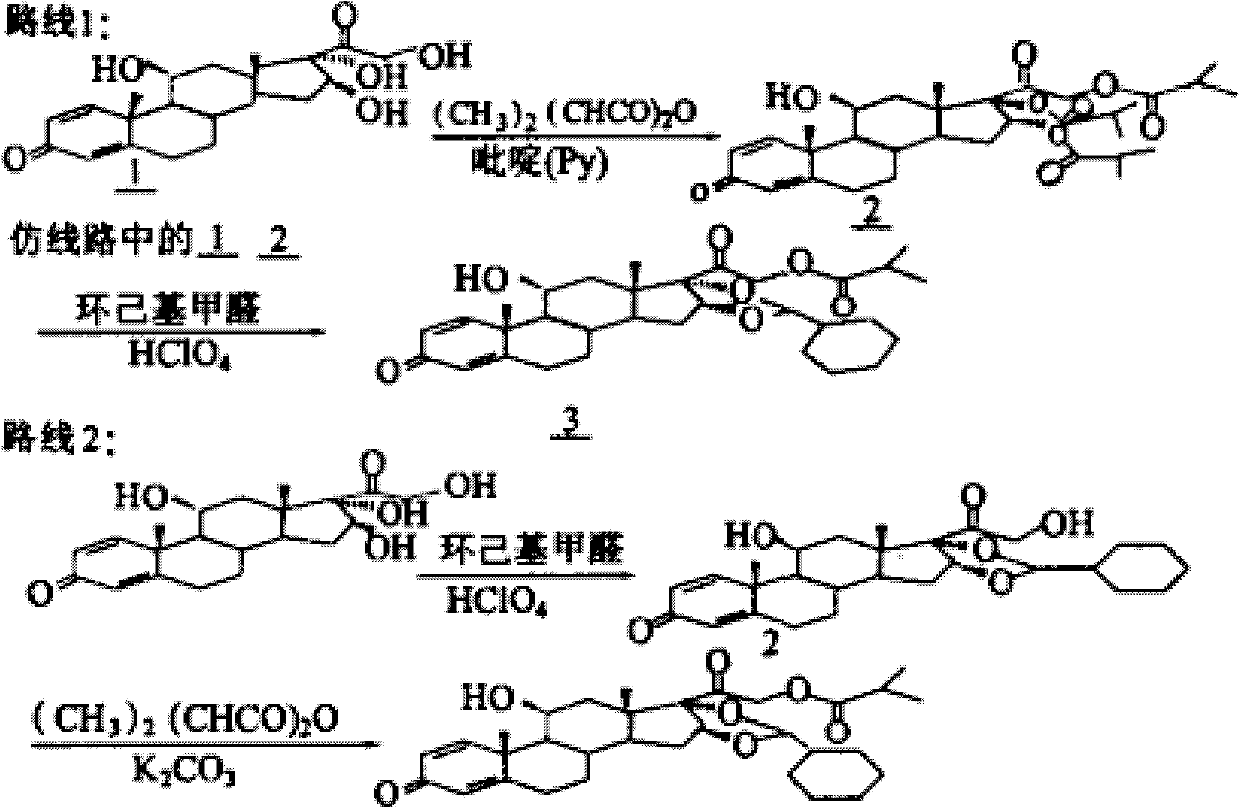
The invention discloses a synthesis method for ciclesonide with a structure shown as the formula (III). The synthesis method adopts 16 Alpha-hydroxyprednisolone as material with a structure shown as the formula (I) and acidic ionic liquid as solvent and catalyst with a structure shown as the formula (II), and includes the following specific steps that: the 16Alpha-hydroxyprednisolone, isobutyricanhydride, cyclohexanal and the acidic ionic liquid are added into a reaction container together, and react under the temperature condition of 10 DEG C to 100 DEG C, and after sufficient reaction, the obtained reaction liquid is postprocessed, so that the ciclesonide product is obtained. Since the invention adopts the acidic ionic liquid as solvent and catalyst and a one-pot method for preparation, the technique has the advantages of easy operation, high capacity, high efficiency, less three wastes and convenient postprocessing, the ionic liquid can be reused, and therefore the technique is an economic, practical and environment-friendly technique.
Owner:无棣鑫岳化工集团有限公司
Formoterol and ciclesonide aerosol formulations
InactiveUS20050207984A1Improve homogeneityImprove physical stabilityOrganic active ingredientsDispersion deliveryCompound (substance)Formoterol
A pharmaceutical aerosol formulation comprising particles of formoterol or a pharmaceutically acceptable salt, solvate or physiologically functional derivative thereof, said particles being suspended in the formulation; a compound of the formula (I), in which: R1 is 1-butyl, 2-butyl, cyclohexyl or phenyl and R2 is acetyl or isobutanoyl, said compound of formula (I) being dissolved in the formulation; and a propellant selected from 1,1,1,2-tetrafluoroethane, 1,1,1,2,3,3,3-heptafluoropropane and a mixture thereof.
Owner:3M INNOVATIVE PROPERTIES CO
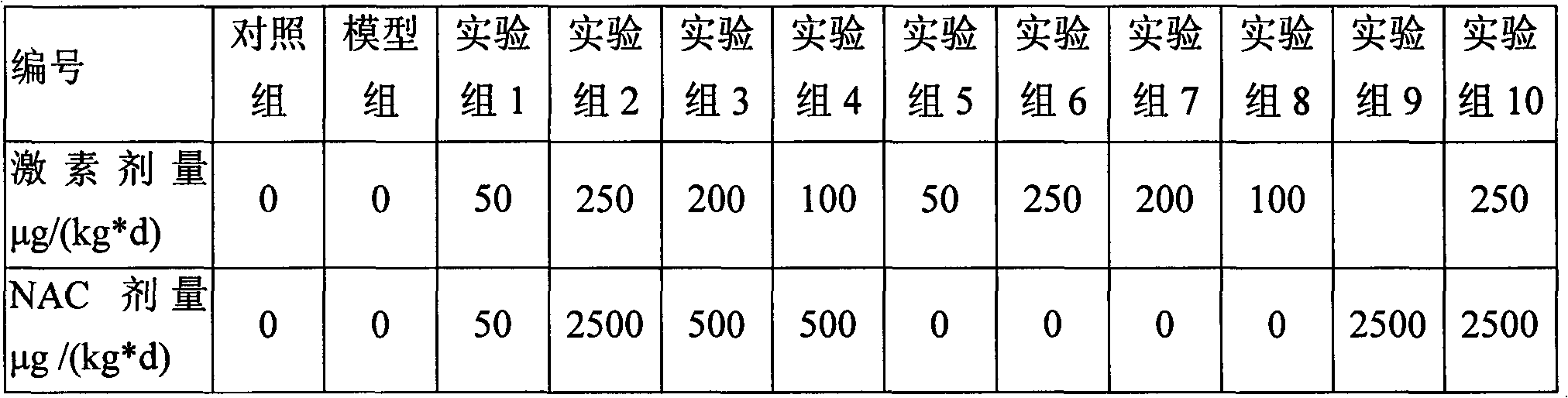
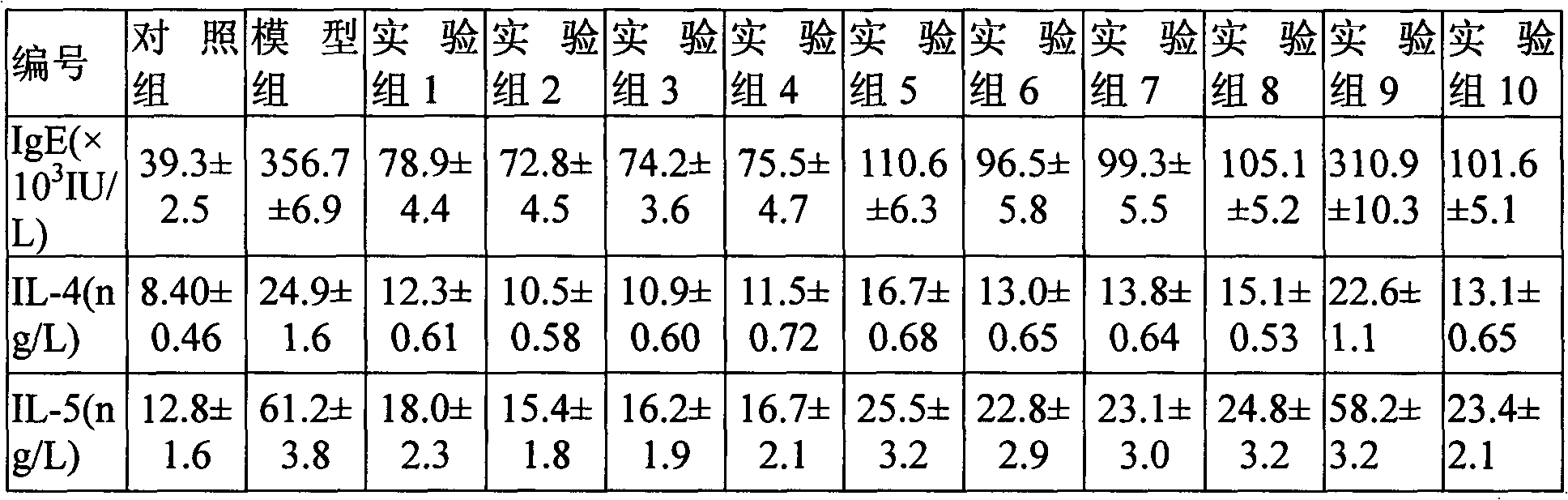
Inhalant containing N-acetyl-L-cysteine and ciclesonide and preparation method thereof
ActiveCN102846633AImprove airway remodelingGood treatment effectOrganic active ingredientsRespiratory disorderCysteic acidBULK ACTIVE INGREDIENT
The invention relates to an inhalant containing N-acetyl-L-cysteine and ciclesonide and preparation method thereof. The inhalant is composed of ciclesonide and N-acetyl-L-cysteine as the active ingredients, and one or more pharmaceutical adjuvants suitable for inhalation drug delivery.
Owner:TIANJIN JINYAO GRP
Process for preparing crystalline ciclesonide with defined particle size
The invention relates to a novel process for preparing crystalline ciclesonide with an advantageous particle size and to the use for producing pharmaceutical preparations, in particular for topical use. The crystalline ciclesonide obtained by the novel process has advantageous aerodynamic properties, and can be further processed to inhalable or nasally administered pharmaceutical preparations without further mechanical micronization.
Owner:COVIS PHARM GMBH
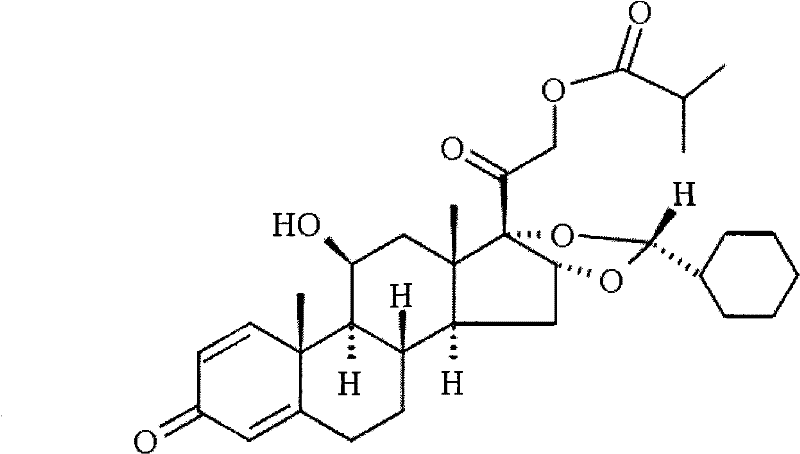
Impurity of ciclesonide and preparation method thereof
The invention relates to a impurity compound ((11beta,16alpha)-16,17-[[(R)-cyclohexyl methylene]bis(oxy)]-11-hydroxy-21-(2-methyl-1-carbonyl propoxy)-1,5-cyclopregnane-3-ene-2,20-dione) of glucocorticoids drug ciclesonide, a preparation method thereof, and an application of the impurity compound as a reference in quality control of the ciclesonide.
Owner:CHONGQING PHARMA RES INST
Emulsion type nasal spray containing ciclesonide, and application thereof
The invention relates to an emulsion type nasal spray containing ciclesonide, and an application thereof, in particular to an emulsion type nasal spray prepared from ciclesonide and pharmaceutically acceptable solvents or surfactants, and an application thereof. The emulsion type nasal spray is prepared from ciclesonide as an active component and pharmaceutically acceptable auxiliary materials by a certain preparation process, and can be used for treating asthma, allergic rhinitis and chronic obstructive pulmonary disease. In the invention, ciclesonide is used as an active component and some specific kinds and proportions of auxiliary materials are added to develop the emulsion type nasal spray according to the preparation process specified by the invention, and the dosage form has very ideal physical and chemical stabilities and excellent administration characteristics.
Owner:FUKANGREN BIO PHARMA
Ciclesonide azelastine compound composition
The invention relates to a ciclesonide azelastine compound composition. The ciclesonide azelastinenasal spray composition contains (A) ciclesonide monohydrate, and (B) azelastine or salt thereof and a pharmaceutically acceptable carrier, wherein the ciclesonide monohydrate exists in a crystal form.
Owner:TIANJIN JINYAO GRP
Formoterol and ciclesonide combination
ActiveUS20060127323A1Reduce riskRapid onsetPowder deliveryBiocideDiseasePulmonary circulation diseases
The invention relates to pharmaceutical compositions containing combinations of formoterol and ciclesonide and the use of such pharmaceutical compositions in medicine, in particular, the prophylaxis and treatment of respiratory disease.
Owner:COVIS PHARM GMBH
Preparation method of ciclesonide
The invention provides a new synthetic route for preparation of ciclesonide. The method comprises the steps that used raw materials are cheaper and available, the reaction raw materials are protectedafter hydroxylation, selective oxidation of a five-membered ring double bond is then performed, then, a six-membered ring double bond bromination is conducted, bromine atom is removed by reduction, acarbonyl group and a hydroxyl protecting group are removed, then, an oxidized dihydroxyl group is protected, the six-membered ring double bond is then epoxidized and reacts with cyclohexanecarboxaldehyde and isobutyryl chloride in turn to obtain a ciclesonide product. A reaction process is easy to operate, yield is higher in each step, purity of the obtained product is also higher, generation of by-products is effectively avoided, production cost is reduced, and the new synthetic route for the preparation of the ciclesonide is advantageous to industrial production.
Owner:TIANJIN PACIFIC PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com