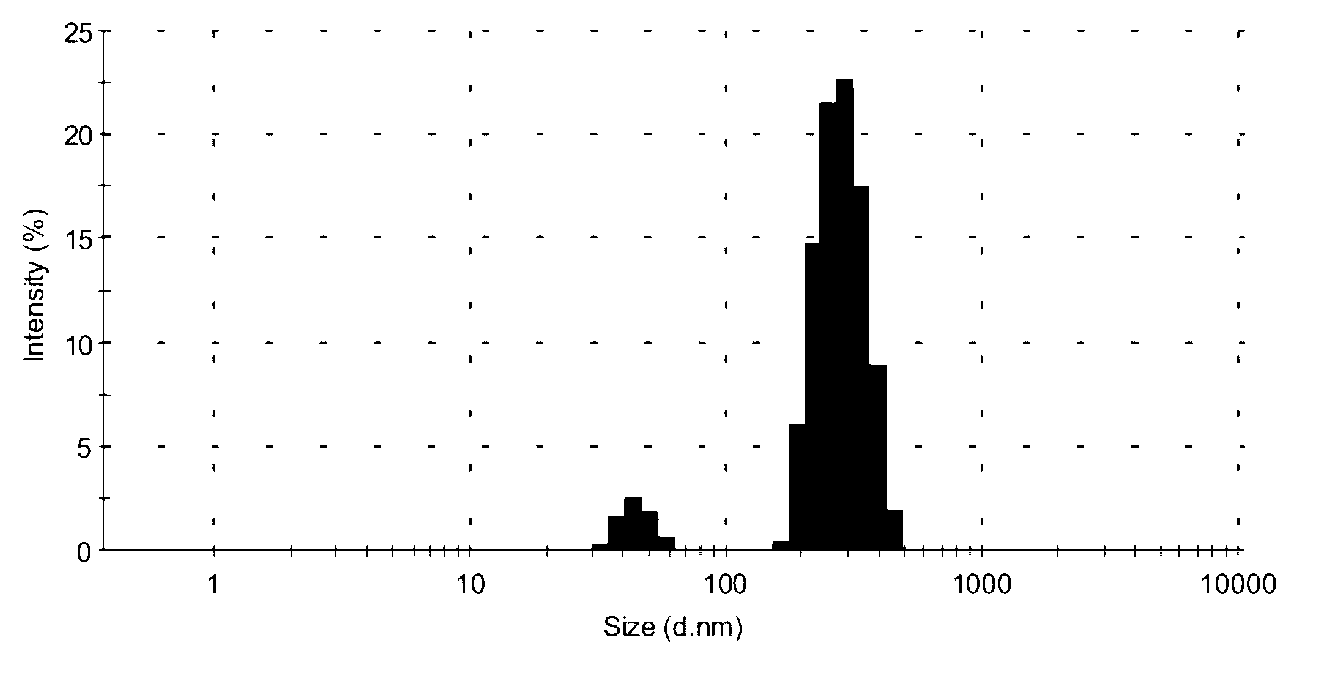Ciclesonide nanometer freeze-dried powder and preparation method thereof
A technology of nano-freeze-dried powder and ciclesonide, which is used in freeze-dried transportation, powder transportation, pharmaceutical formulations, etc., can solve the problems of small particle size and polydispersity coefficient of nano-particle solution, poor stability, and difficulty in long-term storage. , to achieve the effect of improving stability, being conducive to absorption and preservation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
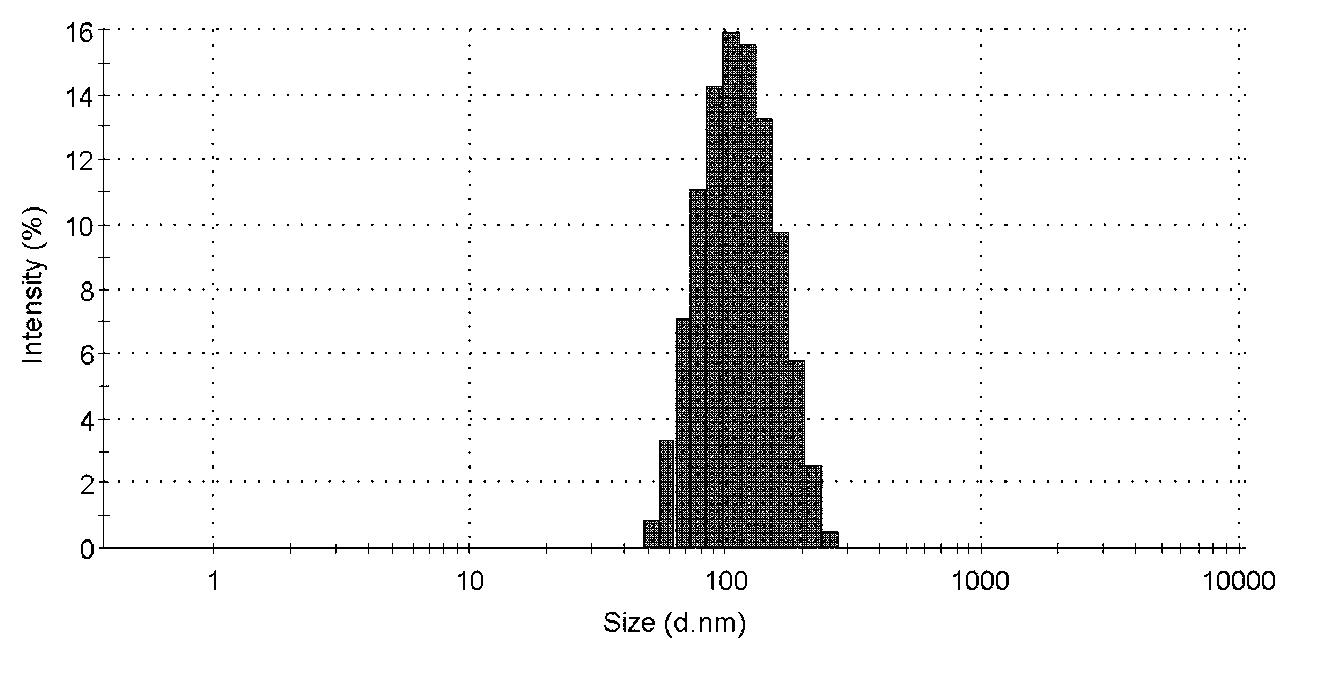
Embodiment 1
[0054] Ciclesonide nano freeze-dried powder, the freeze-dried powder includes the weight of the following ingredients:
[0055] Ciclesonide: 10mg;
[0056] Lecithin: 53mg;
[0057] Glyceryl monostearate: 27mg;
[0058] Tween 40: 250mg;
[0059] Poloxamer 188: 40mg;
[0060] Lyoprotectant lactose: 10.2g;
[0061] Lyoprotectant mannitol: 15.0 g.
[0062] The ciclesonide nano freeze-dried powder of the present embodiment is obtained by the following preparation method:
[0063] Ciclesonide Nanoparticle Colloidal Solution:
[0064] Add 10mg of ciclesonide, 53mg of lecithin and 27mg of glyceryl monostearate into 15mL of ethyl acetate and dissolve to obtain an ethyl acetate solution, which can be accelerated by stirring or ultrasonic instrument;
[0065] Add 250mg of Tween 40 and 40mg of Poloxamer 188 into 300mL of water for injection and dissolve it with stirring or ultrasonic instrument to obtain the corresponding aqueous solution;
[0066] Add the ethyl acetate solution o...
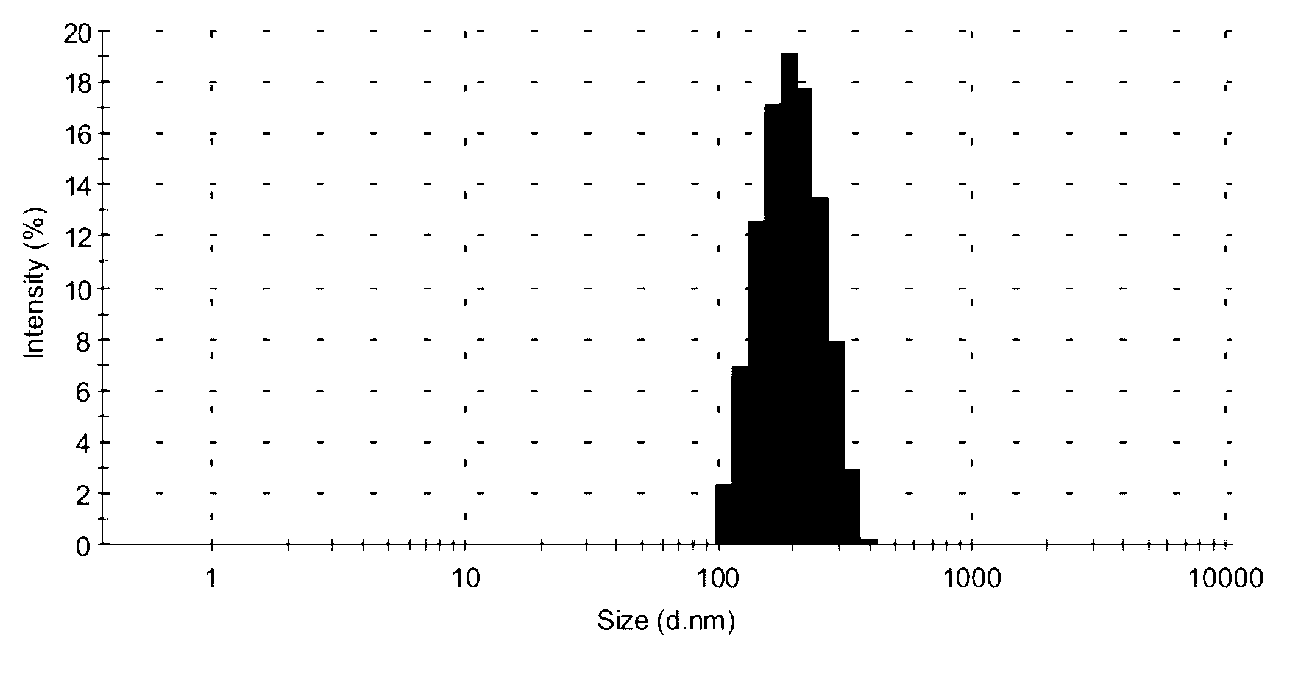
Embodiment 2
[0069] Ciclesonide nano freeze-dried powder, the freeze-dried powder includes the weight of the following ingredients:
[0070] Ciclesonide: 10mg;
[0071] Lecithin: 53mg;
[0072] Glyceryl monostearate: 27mg;
[0073] Tween 40: 250mg;
[0074] Poloxamer 188: 40mg;
[0075] Lyoprotectant lactose: 3.0g;
[0076] Lyoprotectant mannitol: 3.0 g.
[0077] The ciclesonide nano freeze-dried powder of the present embodiment is obtained by the following preparation method:
[0078] The specific preparation method of the ciclesonide nanoparticle colloidal solution is consistent with the method in Example 1, and will not be repeated here.
[0079] Add 3.0g lactose and 3.0g mannitol to the ciclesonide nanoparticle colloidal solution prepared above, stir or ultrasonically dissolve, then use a 0.22 micron hydrophilic microporous membrane to filter, and use a hydrophilic microporous The porous filter membrane is used to better remove large particle impurities in the solution to improv...
Embodiment 3
[0081] Ciclesonide nano freeze-dried powder, the freeze-dried powder includes the weight of the following ingredients:
[0082] Ciclesonide: 10mg;
[0083] Lecithin: 53mg;
[0084] Glyceryl monostearate: 27mg;
[0085] Tween 40: 250mg;
[0086] Poloxamer 188: 40mg;
[0087] Lyoprotectant lactose: 21g;
[0088] Lyoprotectant mannitol: 18g.
[0089] The ciclesonide nano freeze-dried powder of the present embodiment is obtained by the following preparation method:
[0090] The specific preparation method of the ciclesonide nanoparticle colloidal solution is consistent with the method in Example 1, and will not be repeated here.
[0091]Add 21g of lactose and 18g of mannitol to the ciclesonide nanoparticle colloidal solution prepared above, stir or ultrasonically dissolve, then use a 0.22 micron hydrophilic microporous filter to filter. Membrane is in order to remove the big particle impurity in the solution better, to improve product quality, after filtration, obtain corre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com