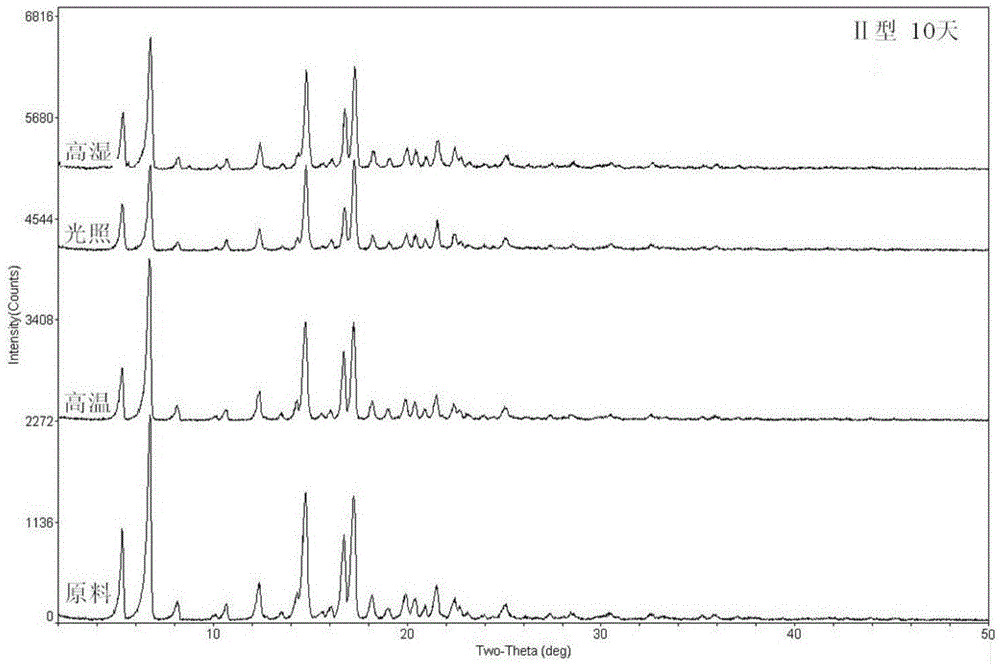
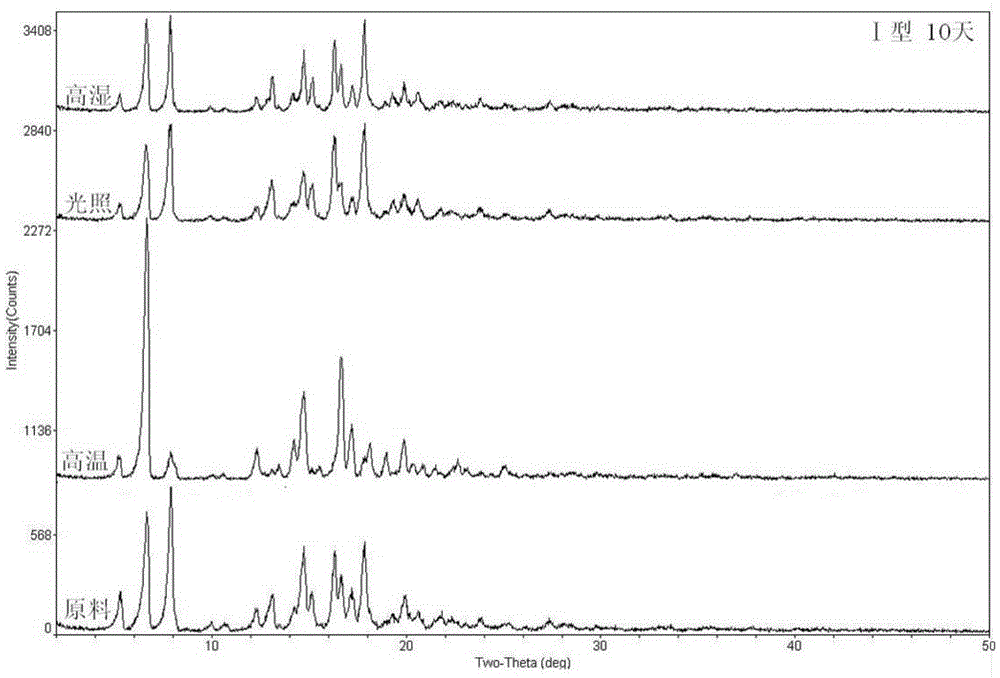
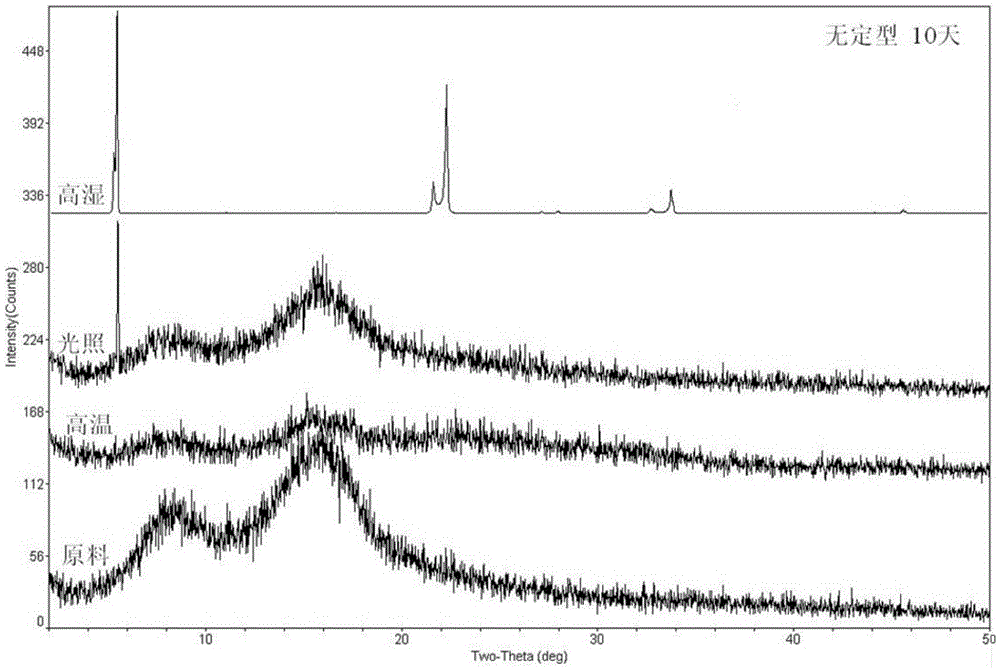
Ciclesonide azelastine compound composition
A technology of azelastine and azelastine hydrochloride, which is applied in the direction of active ingredients of heterocyclic compounds, drug combination, drug delivery, etc., and can solve the problems of not obtaining crystal form III of ciclesonide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Invention Example 1 Preparation of Ciclesonide Monohydrate by Supercritical Method
[0057] (1) Preparation of ciclesonide solution 1: 5 g of ciclesonide was completely dissolved in a mixed solution of 200 ml of acetone and 20 ml of water at 50° C.;
[0058] (2) The ciclesonide solution 1 configured in step (1) is connected to the solution pump 2, and the working pressure is controlled to be 10MPa;
[0059] (3) Carbon dioxide feeding: the CO in the steel cylinder is input into the supercritical fluid anti-solvent equipment system through the booster pump 8, and enters the crystallization kettle 4, the flow rate is controlled at 10ml / min, the starting temperature is controlled at 50°C, and the pressure is 10MPa;
[0060] (4) The ciclesonide solution 1 configured in the above step (1) is rapidly sprayed into the crystallization kettle 4 through the nozzle 3 in the supercritical fluid antisolvent equipment system by the solution pump 2, and the flow control is 1.5ml / min, a...
Embodiment 2
[0065] Invention Example 2 Preparation of Ciclesonide Monohydrate
[0066] Take 5g of ciclesonide and add 100ml of ethanol, 10ml of water, and 10ml of acetonitrile into the mixed solution and heat to 50°C, heat filter to remove insoluble matter, cool to 30°C (if crystals are precipitated, take the supernatant), Then add the seed crystals prepared in Example 1 of the invention, heat and stir for 30 minutes, a large amount of crystals are precipitated, cooled to 0-5°C, filtered, and dried. The dried crystals are analyzed by TG-DTA, and the weight loss is about 3.1%, which is confirmed to be ring crystals. Sonide monohydrate. The obtained crystal was subjected to X-ray powder diffraction measurement, and the measured characteristic peak positions were 2θ=5.1°, 9.0°, 11.2°, 12.8°, 15.0°, 16.2°, 16.9°, 20.7°, 21.8°, 24.3°, 29.1 °, 32.7°.
Embodiment 3
[0067] Invention Example 3 Preparation of Ciclesonide Monohydrate
[0068] Take 5g of ciclesonide and add 100ml of ethanol, 10ml of water, and 15ml of acetonitrile into the mixed solution and heat to 50°C, heat filter to remove insoluble matter, cool to 30°C (if crystals are precipitated, take the supernatant), Then add the seed crystals prepared in Example 1 of the invention, heat and stir for 30 minutes, a large amount of crystals are precipitated, cooled to 0-5°C, filtered, and dried. The dried crystals are analyzed by TG-DTA, and the weight loss is about 3.1%. Sonide monohydrate. The obtained crystals were subjected to X-ray powder diffraction measurement, and the measured characteristic peak positions were 2θ=5.1°, 9.0°, 11.2°, 12.8°, 15.0°, 16.2°, 16.9°, 20.7°, 21.8°, 24.3°, 29.1° °, 32.7°.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com