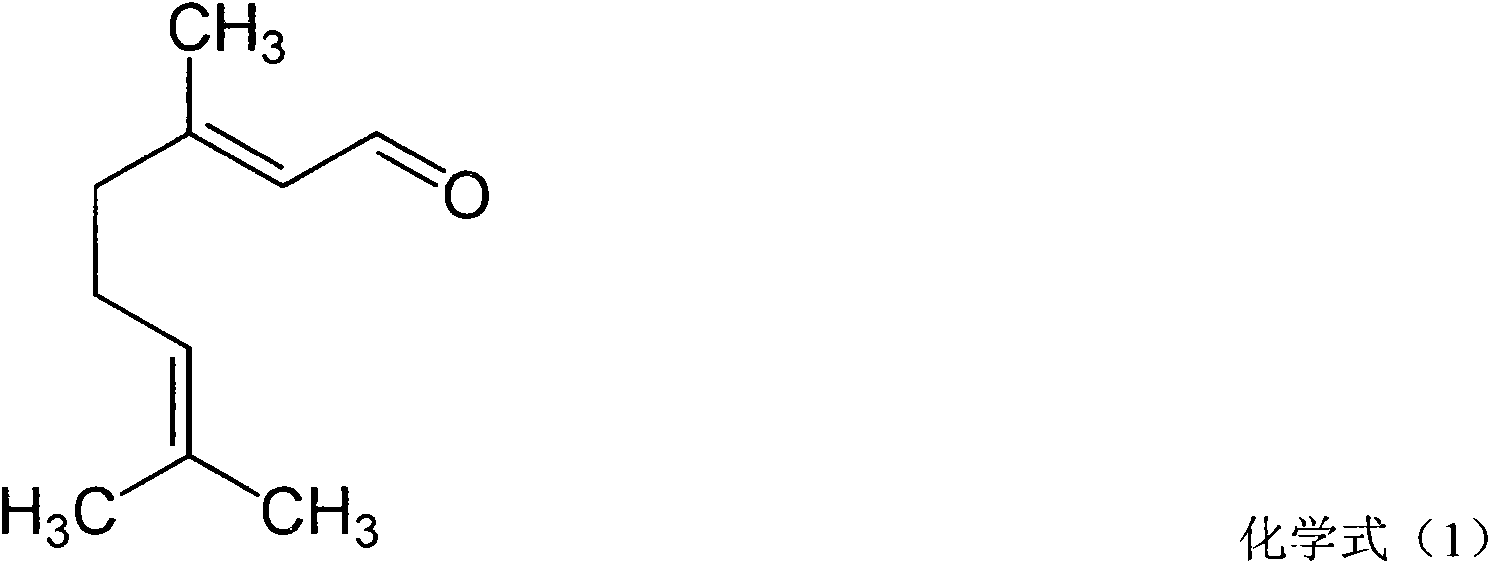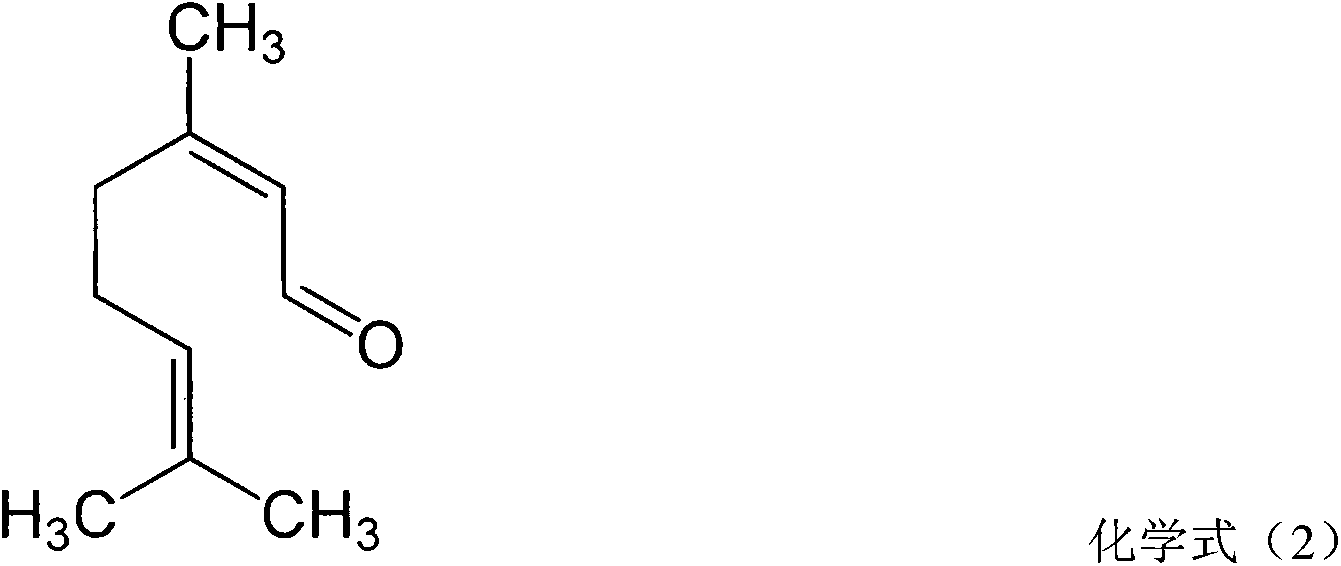Pharmaceutical composition for treating rhinitis
A composition and drug technology, which can be used in drug combinations, active ingredients of heterocyclic compounds, antipyretic drugs, etc., can solve problems such as large side effects and lack of non-specific treatment for successful treatment cases.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0040] Example 2: Preparation of nasal spray or nasal lotion formulation containing citral (1%).
[0041]
[0042] Pour 45kg of pure water into a suitable mixing container with a mixer, and rotate and mix microcrystalline cellulose RC591 at high speed. Then, polysorbate, sorbitol solution, sodium ethylenediaminetetraacetate, and germine were sequentially dissolved and stirred.
[0043] Subsequently, the active ingredient citral is added and mixed with rapid stirring until a homogeneous suspension is produced. The volume was increased to 50 liters with purified water and further mixed. Finally the suspension was evacuated to remove the generated air bubbles. At this point, the resulting suspension is ready to be dispensed into vials.
Embodiment 3
[0044] Example 3: Nasal Spray or Nasal Drops Containing Azelastine Hydrochloride (0.1%, Dissolved) and Citral (1%, Suspended)
[0045]
[0046] Pour 45kg of pure water into a suitable mixing container with a mixer, and rotate and mix microcrystalline cellulose RC591 at high speed. Then, the active ingredient azelastine hydrochloride, the excipient polysorbate, the sorbitol solution, sodium edetate, and germine were successively dissolved and stirred.
[0047] Subsequently, the active ingredient citral is added and mixed with rapid stirring until a homogeneous suspension is produced. The volume was increased to 50 liters with purified water and further mixed. Finally the suspension was evacuated to remove the generated air bubbles.
[0048] At this point, the resulting suspension is ready to be dispensed into vials.
[0049] According to the effect spectrum of some antihistamines and citral, it can be seen that the combined formulation of the two substances has a synergis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com