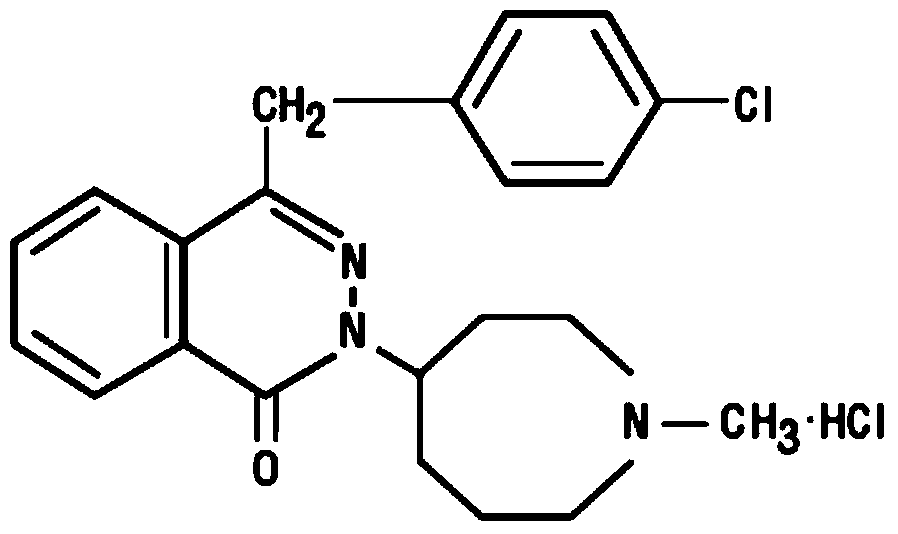
Pharmaceutical composition of azelastine hydrochloride
A technology of azelastine and composition, which is applied in the direction of drug combination, active ingredient of heterocyclic compound, antipyretic, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Embodiment 1: preparation pharmaceutical composition
[0141] formula:
[0142] Azelastine Hydrochloride
[0143] Preparation method: make azelastine or its medicinal salt and the transdermal absorption accelerator miscible with the polyhydric alcohol and PEG; then mix the obtained mixed solution with an appropriate amount of polyhydric alcohol fatty acid ester, and add polyhydric alcohol Fatty acid ester to the full amount, cooling, that is.
Embodiment 2
[0144] Embodiment 2: preparation pharmaceutical composition
[0145] formula:
[0146] Azelastine Hydrochloride
[0147] Preparation method: make azelastine or its medicinal salt and the transdermal absorption accelerator miscible with the polyhydric alcohol and PEG; then mix the obtained mixed solution with an appropriate amount of polyhydric alcohol fatty acid ester, and add polyhydric alcohol Fatty acid ester to the full amount, cooling, that is.
Embodiment 3
[0148] Embodiment 3: preparation pharmaceutical composition
[0149] formula:
[0150] Azelastine Hydrochloride
[0151] Glycerol Triacetate
[0152] Preparation method: make azelastine or its medicinal salt and the transdermal absorption accelerator miscible with the polyhydric alcohol and PEG; then mix the obtained mixed solution with an appropriate amount of polyhydric alcohol fatty acid ester, and add polyhydric alcohol Fatty acid ester to the full amount, cooling, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com