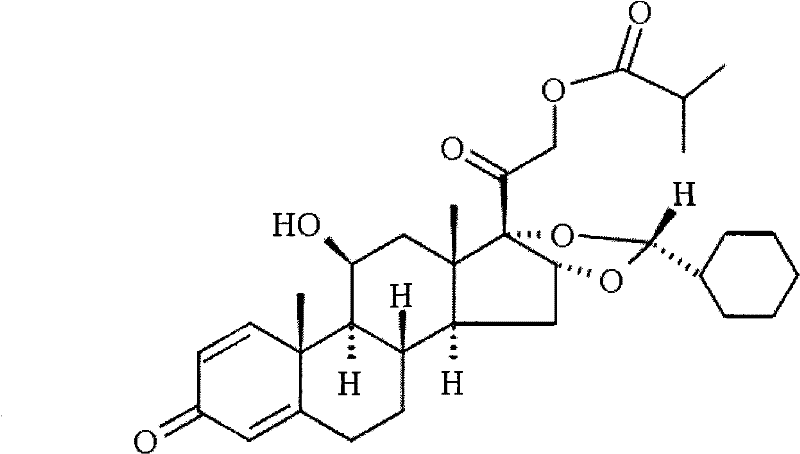
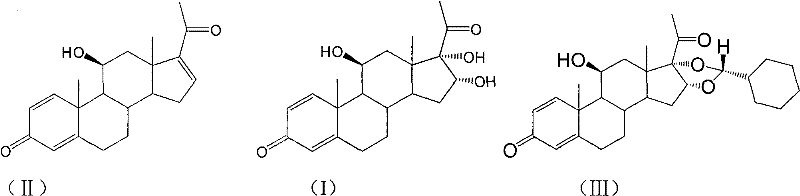
Novel 16,17-ketal intermediate for preparing ciclesonide
A technology of ciclesonide and cyclohexyl, which is applied to the application field in the preparation of ciclesonide and can solve problems such as difficulty and high content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Add 10mmol of the compound of formula (I), 15ml of nitromethane, and 13mmol of cyclohexylformaldehyde into a three-necked flask, stir, add 30mmol of perchloric acid dropwise at -5 to 0°C, drop it in 2 hours, then stir overnight at 15°C, and find A large amount of solids were precipitated, filtered, and the filter cake was dissolved with N, N-dimethylformamide (DMF). Under ice bath refrigeration, 10% NaHCO3 aqueous solution was added dropwise to neutralize, then extracted with ethyl acetate, organic The phase was dried over sodium sulfate and concentrated under reduced pressure, recrystallized with methanol, suction filtered, washed with water, dried to constant weight to obtain 8.6 mmol of the compound of formula (Ⅲ), 22R:22S=95:5.
[0068] Elemental analysis: C, 70.95; H, 8.24; O, 20.81
Embodiment 2
[0070] Add 50ml of dichloromethane, 15mmol of p-toluenesulfonic acid, and 15mmol of cyclohexylformaldehyde into a three-necked flask, stir, slowly add 10mmol of the compound of formula (I) at room temperature within 1h, stir for 5h, add 10% NaHCO3 aqueous solution to adjust the pH value to 7, The layers were separated, the aqueous layer was washed with 5ml of chloroform X 3 times, the chloroform was combined, concentrated by rotary distillation, recrystallized from methanol, filtered with suction, washed with water, dried to constant weight to obtain 8.4 mmol of the compound of formula (III), 22R:22S=92:8. ,
Embodiment 3
[0072] Add 10mmol of the compound of formula (I), 20ml of acetonitrile, 20ml of dichloromethane, and 18mmol of hydrofluoric acid into a three-neck flask, stir, and slowly add 20mmol of cyclohexylformaldehyde dropwise at 25°C, and after stirring for 4 hours, adjust the pH value with 10% NaHCO3 aqueous solution To 7, add 20ml of water, let stand to separate the layers, extract the water layer three times with dichloromethane, 10ml each time, combine the dichloromethane and concentrate by rotary distillation, recrystallize with methanol, suction filter, dry to constant weight, and obtain the formula ( III) Compound 8.1 mmol, 22R:22S=93:7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com