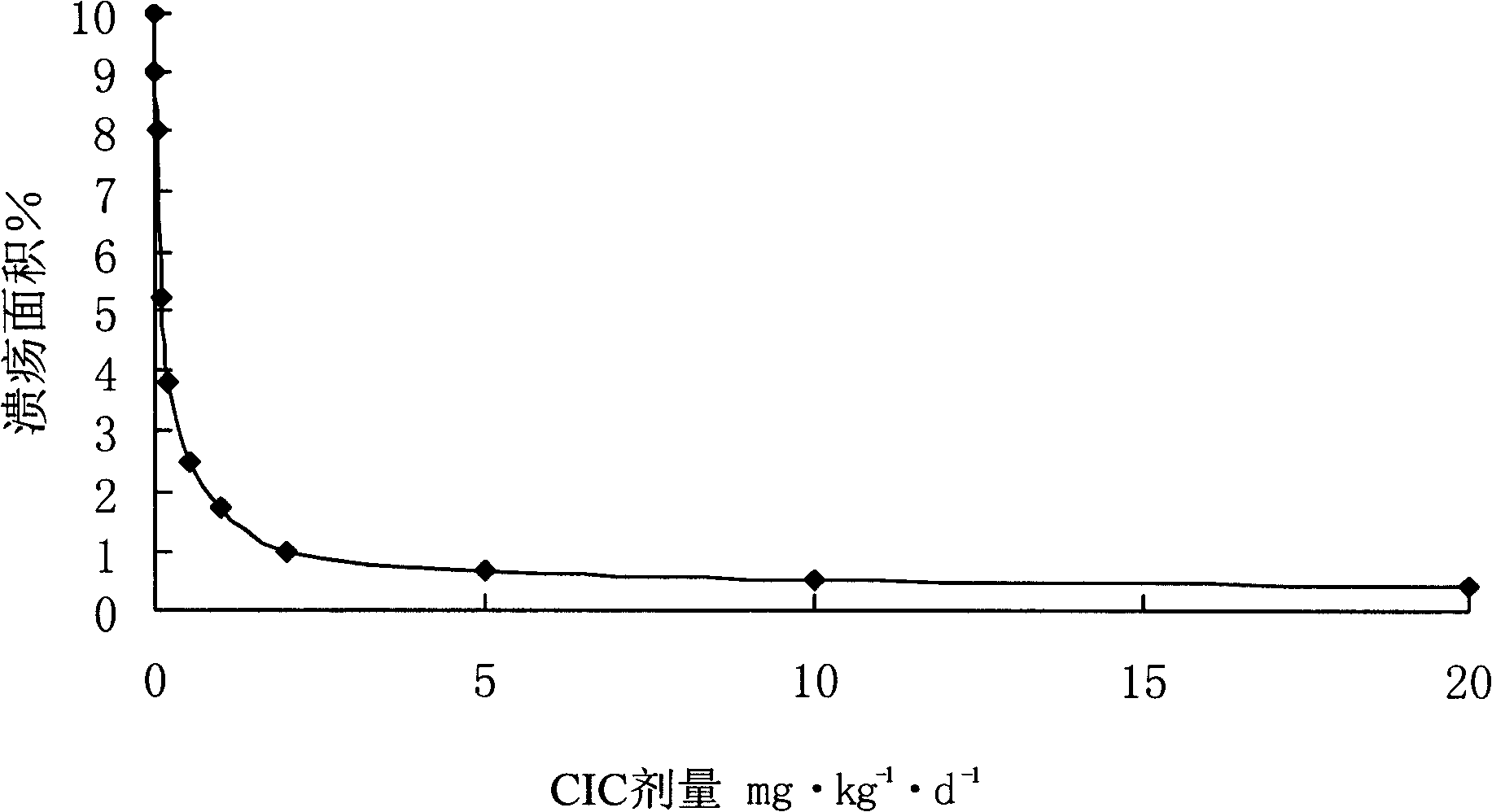
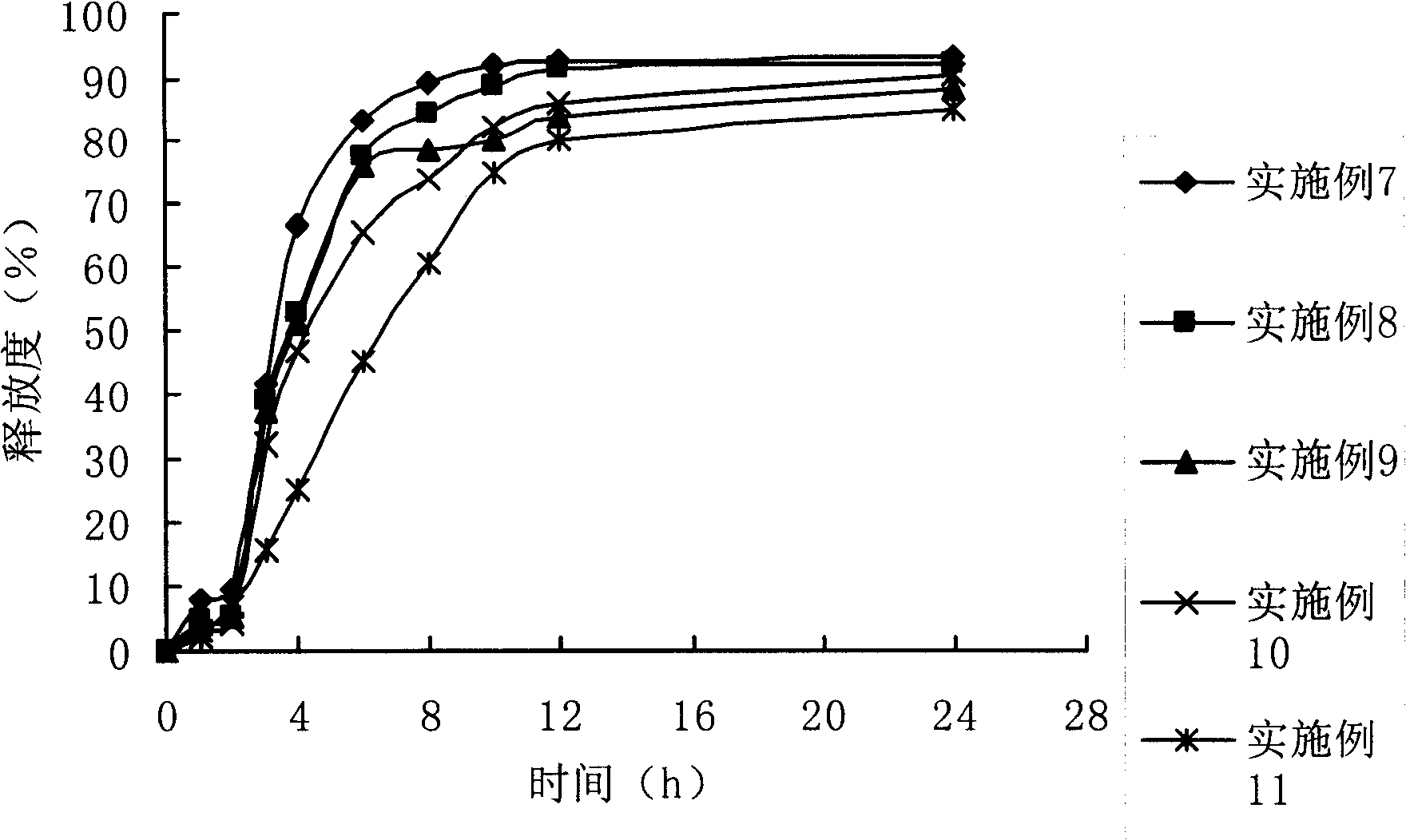
Medicament for treating inflammatory bowel disease
A technology for inflammatory bowel disease and medicine, which is applied in the field of colon-targeted pellets and preparation thereof, and can solve the problem that the active metabolite of ciclesonide that does not function as a system can be used, the bioavailability is small, the undisclosed avoidance or Alleviate problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Weigh 1 gram of ciclesonide, 9 grams of β-cyclodextrin, 6 grams of polyethylene glycol 6000, and 1 gram of talcum powder, and disperse the above materials in 45 grams of water under stirring; Slowly disperse in 10 grams FS30D (based on solid content) polymer suspension, passed through a 120-mesh sieve to prepare a medicinal water dispersion suspension (referred to as liquid); in addition, weigh 3 grams of talcum powder, 4.5 grams of triethyl citrate gram, add 20 grams of water and mix well to make a water-dispersed suspension, which is slowly added to the weighed 25 grams of A water-dispersed suspension was formed in FS30D, and the mixed solution was passed through a 120-mesh sieve to prepare a colon coating solution (referred to as solution). The colon-targeted pellets were prepared by spraying and solution on the sugar core pellets by fluidized bed coating method. The prepared micropills were assayed, and the results were: the peak time of the bulk drug (refer...
Embodiment 2
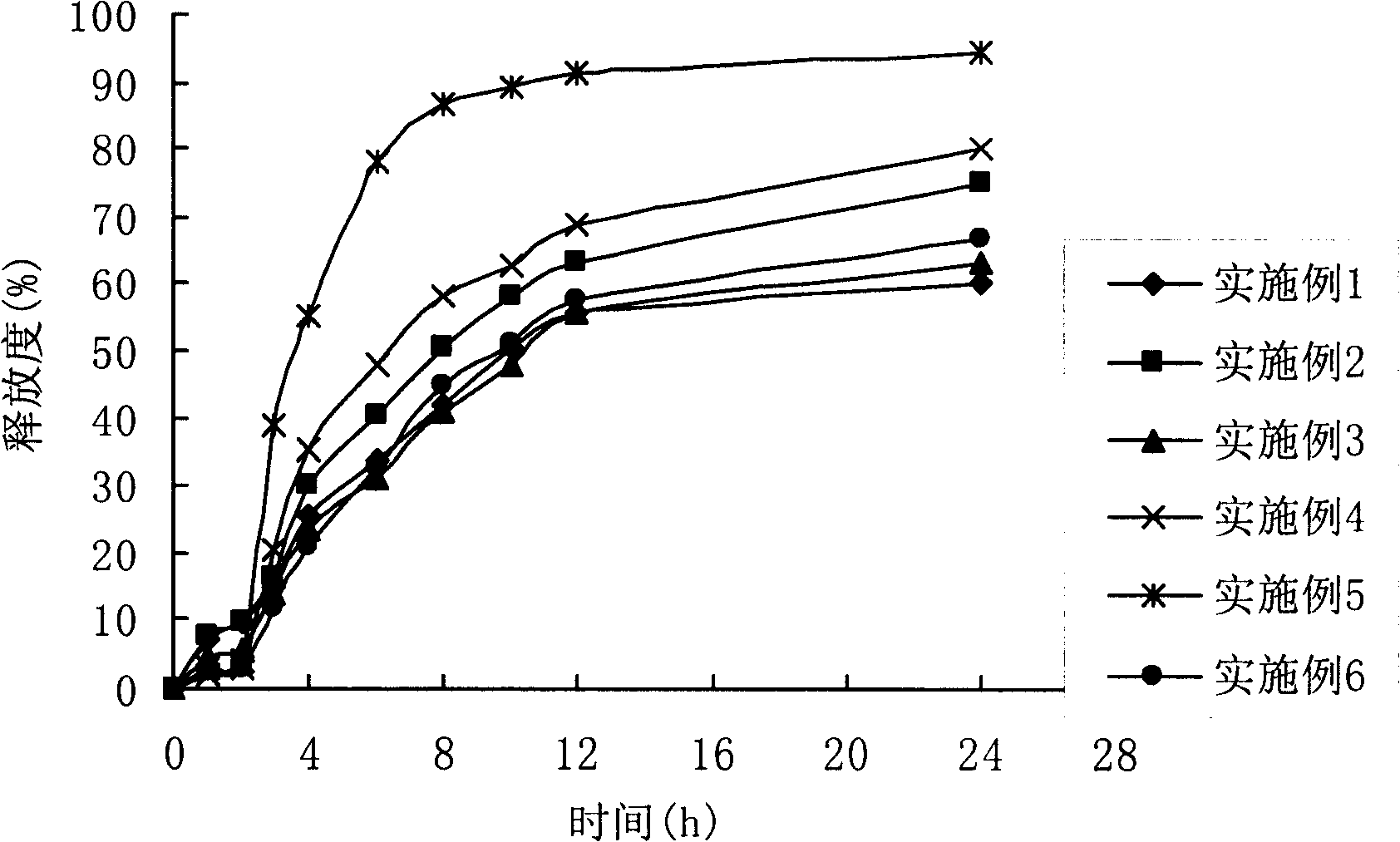
[0073] Weigh 40g ciclesonide, 120g beta-cyclodextrin, 105g polyethylene glycol 6000, 100g polyethylene glycol 4000, 20g talcum powder, and disperse the above materials in 600g water under stirring; Slowly disperse in 150g of FS30D (based on solid content) polymer suspension, pass through a 120-mesh sieve to prepare a medicinal water dispersion suspension (abbreviated as liquid); in addition, take 20g of talcum powder and 5g of triethyl citrate, Add 30g of water and mix well to make a water dispersion suspension, and slowly add the suspension to the weighed coating material under stirring 220g of FS30D (based on solid content) was used to form a water-dispersed suspension, and the mixture was passed through a 120-mesh sieve to prepare a colon coating solution (abbreviated as solution). The colon-targeted pellets were prepared by spraying and solution on the sugar core pellets by fluidized bed coating method. The measured release see figure 2 .
Embodiment 3
[0075] Weigh 35g ciclesonide, 70gβ-cyclodextrin, 20g polyethylene glycol 6000, 50g polyethylene glycol 4000, 20g talcum powder, and disperse the above materials in 350g water under stirring; Slowly disperse in 50g of FS30D (based on solid content) polymer suspension, passed through a 120 mesh sieve to prepare a medicinal water dispersion suspension (abbreviated as liquid); in addition, weigh 6g of talcum powder and 15g of triethyl citrate, Add 70g of water and mix well to make a water dispersion suspension, and slowly add the suspension to the weighed coating material under stirring FS30D170g forms a water-dispersed suspension, and passes the mixed solution through a 120-mesh sieve to prepare a colon coating solution (referred to as solution). Using the fluidized bed coating method, the solutions and were sprayed on the blank core to prepare colon-targeted pellets. For the results of the determination of the release rate, see figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com