Preparation method of 1alpha-hydroxyl dehydroepiandrosterone
A technology of dehydroepiandrosterone and hydroxyl, applied in chemical instruments and methods, ketal steroids, steroids and other directions, can solve the problems of high technical requirements, poor selectivity, difficult separation, etc., and achieves broad industrial application prospects and improved The effect of location selectivity and security risk reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1 (transposition reaction, transposition reagent adopts potassium tert-butoxide and DBU)
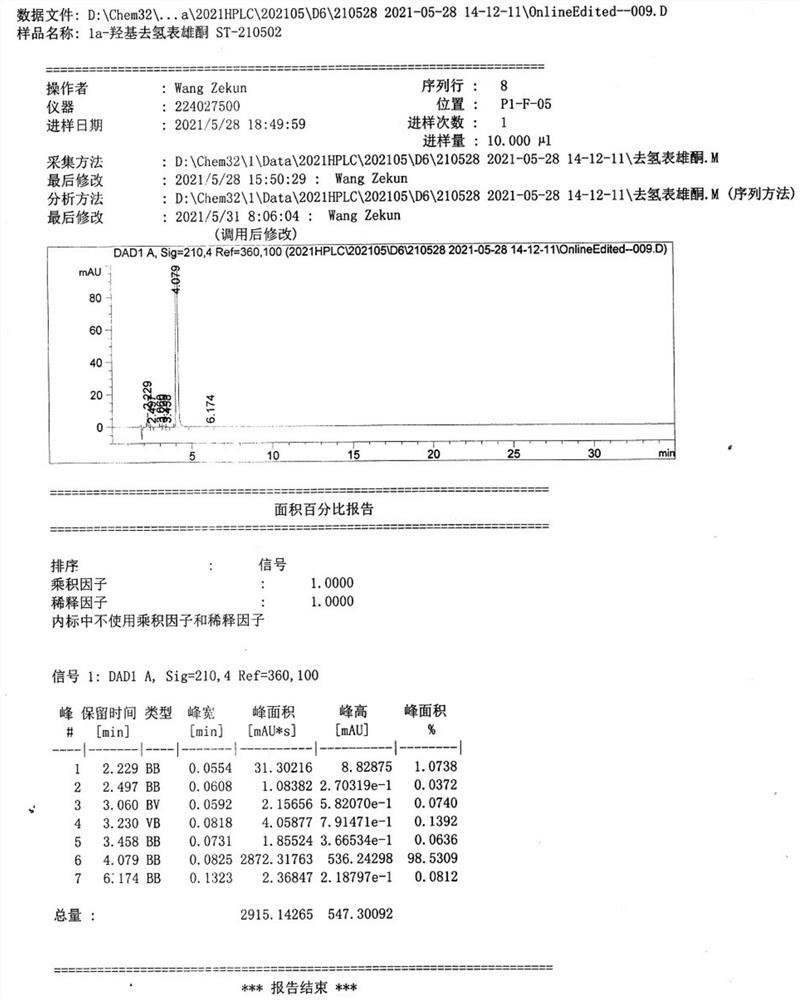
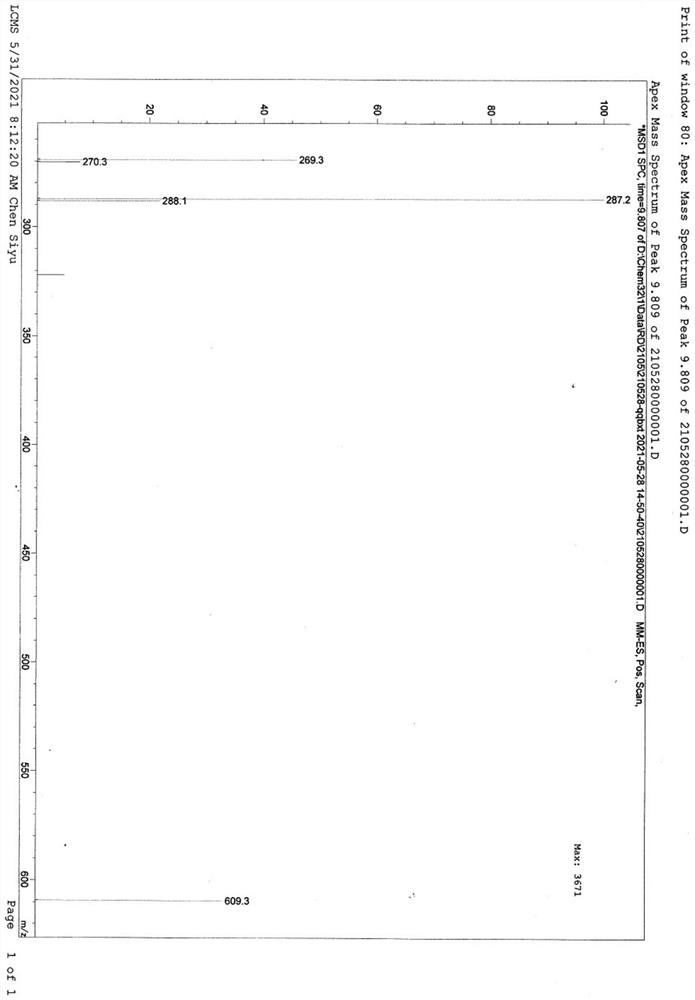
[0068] Transposition reaction: Add 110g of ketal product II and 330mL of dimethyl sulfoxide into the reactor, stir under nitrogen protection at a controlled temperature of 30-40°C, add 110g of potassium tert-butoxide and 55g of DBU, and keep the reaction for 6 hours. After the reaction is completed, Pour into acid water for water analysis, filter, wash the filter cake with drinking water until neutral, refine the filter cake with methanol to obtain 100.1 g of transposition product III, the weight yield is 91%, and the HPLC purity is 98.6%.
Embodiment 2
[0069] Embodiment 2 (transposition reaction, translocation reagent adopts potassium tert-butoxide and DBN)
[0070] Transposition reaction: Add 110g of ketal product II and 550mL of tert-butanol into the reaction kettle, stir at a temperature of 30-40°C under nitrogen protection, add 165g of potassium tert-butoxide and 110g of DBN, and keep the reaction for 3 hours. After the reaction is completed, pour Put into acid water for water analysis, filter, wash the filter cake with drinking water until neutral, refine the filter cake with methanol to obtain 94.6 g of transposition product III, the weight yield is 86%, and the HPLC purity is 96.6%.
[0071] Compared with the comparative example, when DBU or DBN is added to the transposition reagent, the yield and purity of the transposition product are improved, the post-treatment is simple, and the column separation operation with high environmental pollution and high cost is avoided.
Embodiment 3
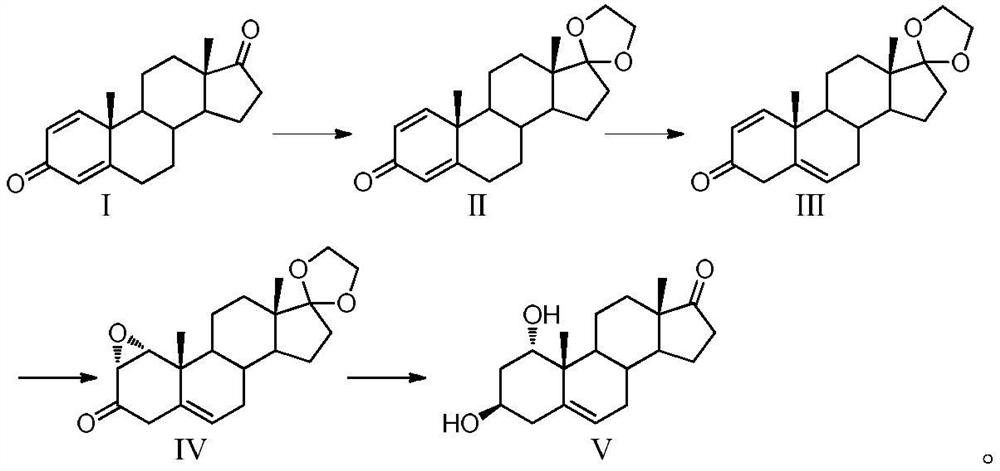
[0073] The first step, ketal reaction: put 500g of 1,4-androstenedione, 10g of p-toluenesulfonic acid and 1L of dichloromethane into the reaction kettle, pass N 2 , temperature control 15 ~ 20 ℃, stirring to dissolve. Slowly add 385 mL of triethyl orthoformate and 358.5 mL of ethylene glycol dropwise for 1 hour, and control the temperature at 15-25°C for 6-10 hours. After the heat preservation reaction is completed, add the prepared sodium bicarbonate solution into the reaction system, adjust the pH to 8-9, and stir for 20 minutes. Stand still, separate layers, collect the lower dichloromethane, and extract the aqueous layer twice with dichloromethane (500 mL). Combine the organic phases, control the vacuum degree above 0.08MPa and concentrate under reduced pressure to remove dichloromethane, add 2L of drinking water, stir at 25-35°C for 0.5-1 hour, filter with suction, wash the filter cake with drinking water until neutral, and filter the cake with cyclohexane Beat and dry ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com