Preparation method of 6-dehydronandrolone acetate
A technology of nandrolone acetate and acetic anhydride, applied in the directions of steroids, organic chemistry, etc., can solve the problems of low efficiency, high operation difficulty, low yield, etc., achieve less side reactions, avoid hydrolysis, and yield and the effect of improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
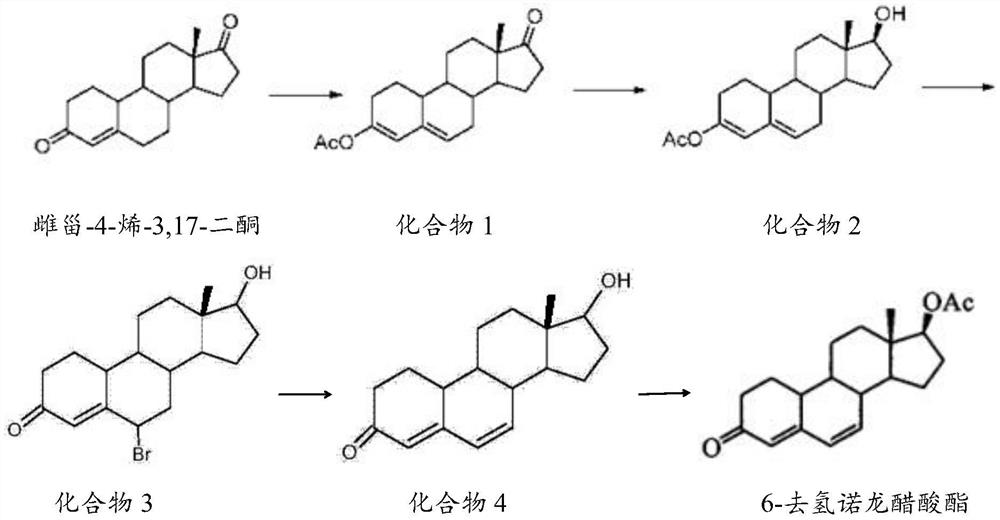
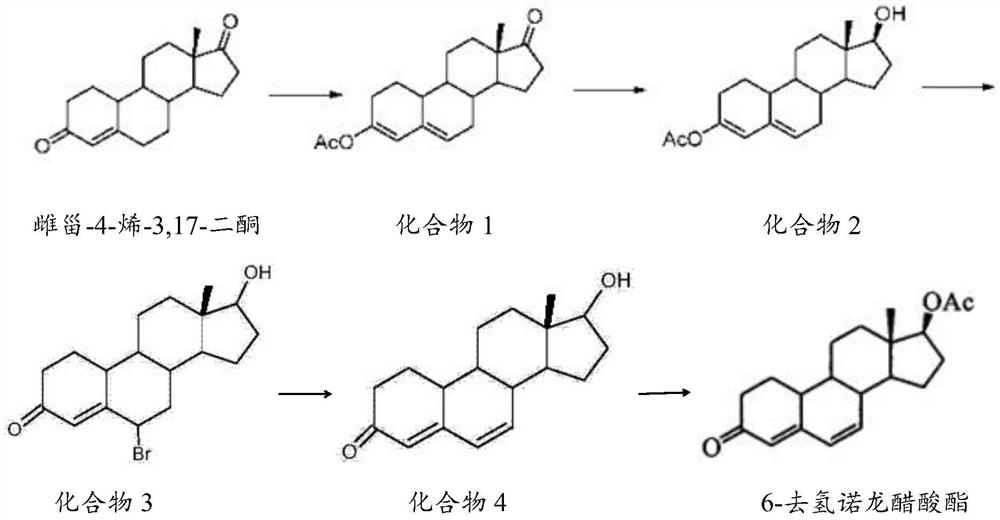
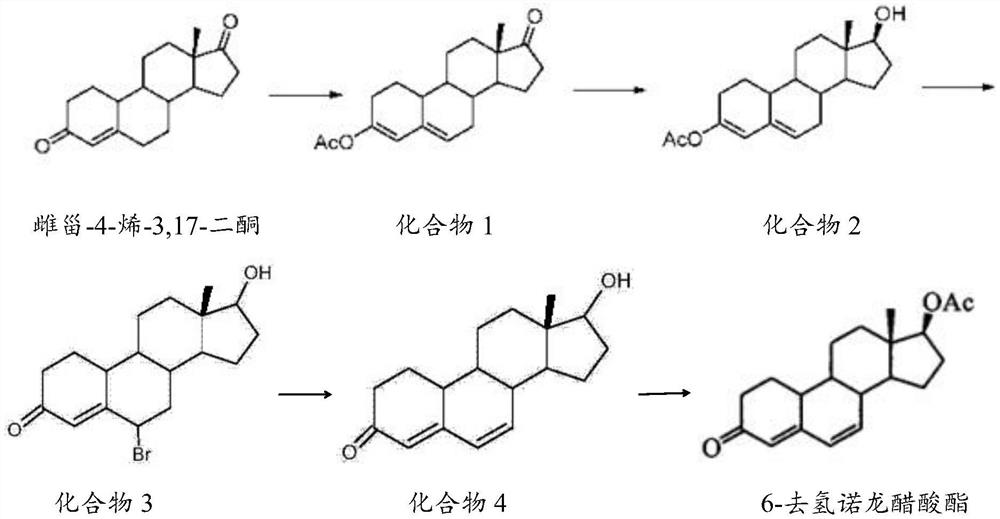
[0027] The preparation method of described 6-dehydronandrolone acetate comprises the steps:
[0028] 1) Catalytic reaction of estro-4-ene-3,17-dione, acetic anhydride and p-toluenesulfonic acid to obtain compound 1;
[0029] 2) reducing compound 1, borohydride and aluminum chloride to obtain compound 2;
[0030] 3) react compound 2, N-bromosuccinimide and DMF to obtain compound 3 solution;
[0031] 4) adding compound 3 solution and base to obtain compound 4;
[0032] 5) Catalyzed reaction of compound 4, acetic anhydride, triethylamine and dichloromethane to obtain 6-dehydronandrolone acetate.
[0033] The mass volume ratio of estro-4-ene-3,17-dione, acetic anhydride and p-toluenesulfonic acid in step 1) of the present invention is preferably 0.8-1.2g: 2-4mL: 0.05-0.07g, more preferably 1g: 3mL: 0.06g; the temperature of the catalytic reaction is preferably 18-25°C, more preferably 20-22°C; the time of the catalytic reaction is preferably 1.5-2.5h, more preferably 2h.
[00...
Embodiment 1
[0043]Dissolve 1g of estro-4-ene-3,17-dione in 3mL of acetic anhydride, add 0.06g of p-toluenesulfonic acid, react at 22°C for 0.4h, the reaction solution precipitates a white solid, continue the reaction for 1h, then add 60mL of saturated The sodium carbonate solution was stirred for 0.6 h, and when the solid was in the form of powder in the solution, it was directly filtered, and the solid was dried to obtain Compound 1 in the form of off-white powder. Control the temperature of the reaction system at 8°C, add 0.06g of lithium borohydride, 0.06g of sodium borohydride, 0.15g of aluminum trichloride, and 20mL of methanol, react at this temperature for 0.5h, raise the temperature to 40°C and continue the reaction for 1.5h, take a sample for TLC After the reaction of the raw materials is complete, the system is added to NH at 5°C 4 Cl solution, the organic phase was separated, dried and concentrated to obtain compound 2 as a white solid. Compound 2 was dissolved in 10 mL of DMF...
Embodiment 2
[0046] Dissolve 0.9g of estro-4-ene-3,17-dione in 2.5mL of acetic anhydride, add 0.05g of p-toluenesulfonic acid, react at 20°C for 0.4h, the reaction solution precipitates a white solid, continue the reaction for 1h, then add 60 mL of saturated sodium carbonate solution was stirred for 1 h, and when the solid was in the form of powder in the solution, it was directly filtered, and the solid was dried to obtain Compound 1 in the form of off-white powder. Control the temperature of the reaction system at 12°C, add 0.12g of lithium borohydride, 0.1g of aluminum trichloride, and 20mL of methanol, react at this temperature for 0.5h, raise the temperature to 30°C and continue the reaction for 1h, take samples for TLC until the raw materials are completely reacted, and the system Added to 5 °C NH 4 Cl solution, the organic phase was separated, dried and concentrated to obtain compound 2 as a white solid. Dissolve compound 2 in 12 mL of DMF (not completely dissolved), then add dropw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com