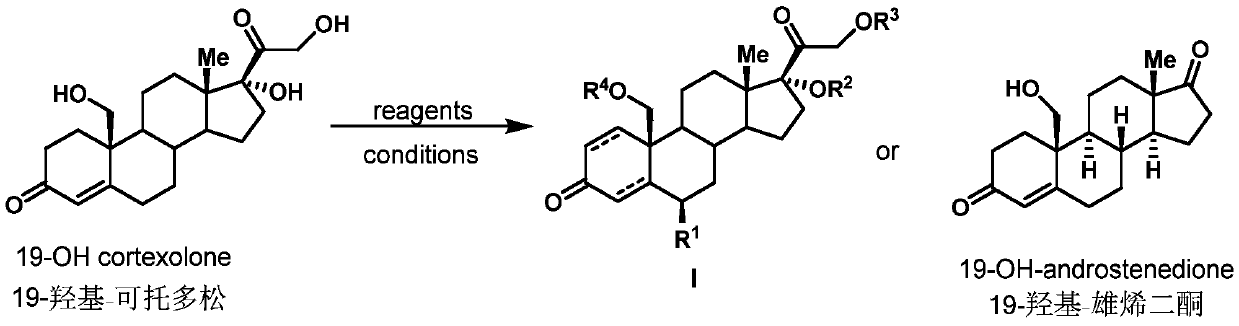
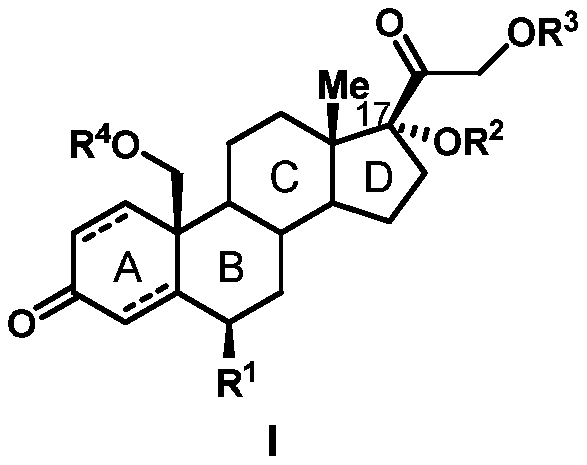
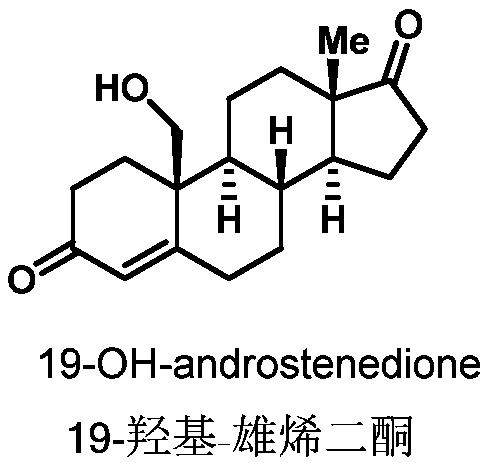
Preparation method of 19-hydroxylated cortodone derivatives and 19-hydroxyandrostenedione
A technology of hydroxyandrostenedione and cortodolone, applied in the direction of steroids, organic chemistry, etc., can solve the problems of harsh reaction conditions, limited use range, low yield, etc., to achieve optimized synthesis steps, cost reduction, The effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Preparation of 19-hydroxyl-cortodopine
[0033] (1) The strain Thanatephorus cucumeris NBRC 6298 was inoculated on PDA medium (potato 200g / L, glucose 20g / L and agar 15g / L) for activation culture in a constant temperature incubator at 30°C. After 6-10 days, inoculate the well-growth strains on the plate into a 250mL Erlenmeyer flask containing 100mL yeast liquid medium (glucose 25g / L, yeast extract 20g / L), and grow for about 2-3 days at 30°C and 200rpm , take 5mL of well-grown bacterial liquid and transfer it to a 250mL Erlenmeyer flask containing 100mL of fresh yeast liquid medium, continue to cultivate under the same conditions for 2-3 days, and put 25mg of cortodolone and ammonium ferrous sulfate into the In the bacterial culture medium (final iron ion concentration 1.5mmol / L), continue to cultivate and transform for about 3-4 days until the conversion rate reaches the highest, and obtain the compound 19-hydroxy-cortodone (conversion rate 45%).
[0034]...
Embodiment 2
[0037] Embodiment 2: the preparation of compound I-1
[0038]
[0039] 10 mg of 19-hydroxy-cortodone was dissolved in 1 mL of methanol, and then 10 μL of 3M aqueous sodium hydroxide solution and 20 μL of 30% hydrogen peroxide were added sequentially at 0°C. After stirring for 4 hours, the reaction solution was diluted with 5 mL of dichloromethane, and the organic phase was sequentially extracted with water and saturated brine. The combined organic phases were dried over anhydrous sodium sulfate, filtered under reduced pressure, and concentrated under reduced pressure. The obtained crude product was further separated and purified by silica gel column chromatography (eluent was dichloromethane:ethyl acetate=1:2, V / V ), yielding 6.6 mg of I-1 (58%). (c 0.46, CH 3 OH); HRMS(m / z): calcd for C 21 h 30 o 6 Na,[M+Na] + 401.1935; found, 401.1941; 1 H NMR (400MHz, Methanol-d 4 ( ddd,J=14.3,11.4,2.7Hz,1H),2.36–2.21(m,2H),2.20–2.10(m,1H),1.96–1.90(m,1H),1.86–1.73(m,3H), 1.68...
Embodiment 3
[0040] Embodiment 3: the preparation of compound 1-3
[0041]
[0042] 10 mg of 19-hydroxy-cortodone was dissolved in 1.5 mL of methanol, and 5.0 mg of 10% palladium on carbon was added under hydrogen atmosphere. The reaction was carried out at room temperature for 1 hour, and the reaction solution was filtered through celite to remove insoluble palladium carbon. The filtrate was concentrated under reduced pressure and dried to obtain the crude product I-2. I-2 obtained above was dissolved in 2 mL of dichloromethane, and then 10 mg of acetic anhydride and 3 mg of 4-dimethylaminopyridine (DMAP) were added. The reaction was stirred under argon at room temperature for 12 hours. The reaction solution was diluted with dichloromethane and extracted with saturated sodium bicarbonate and sodium chloride solutions successively. The obtained organic phase was dried over anhydrous sodium sulfate, filtered under reduced pressure, and concentrated, and the obtained crude product was fu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com