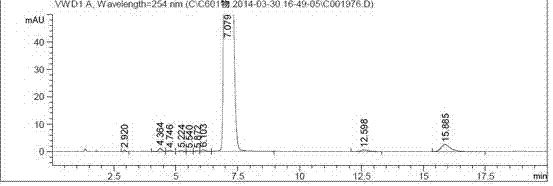
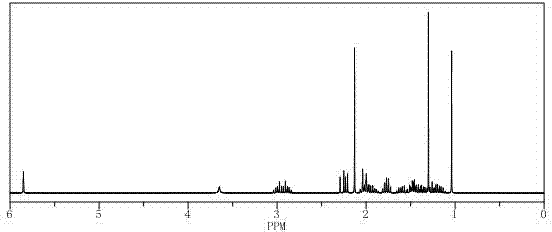
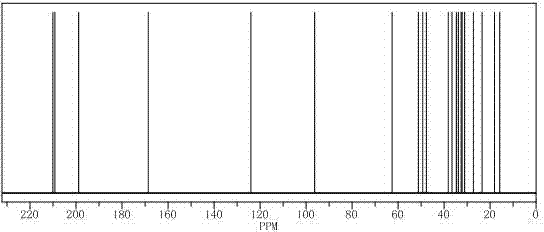
Preparation method of important intermediate of cortisone acetate
A technology of cortisone acetate and intermediates, which is applied in the field of preparation of cortisone acetate intermediates, can solve the problems of increasing the difficulty of wastewater treatment and the high price of TEMPO, and achieve good industrial application prospects, value and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A kind of preparation method of cortisone acetate intermediate, operates according to the following steps:
[0055] (1) Preparation of oxidizing reagent A
[0056] In the preparation tank, add 50Kg of water, then slowly add 3.6 Kg of concentrated hydrochloric acid, and stir for ten minutes. Then add 5Kg of potassium dichromate and stir to dissolve. Cool down to 10~15°C to obtain oxidizing reagent A—potassium dichromate solution. spare.
[0057] (2) Preparation of oxide 17α-hydroxypregn-4-ene-3,11,20-trione
[0058] In the reaction tank, add 200Kg tetrahydrofuran, 20Kg glacial acetic acid, 1Kg manganese chloride and 10 Kg raw material 11α, 17α-dihydroxypregna-4-ene-3,20-dione, stir to dissolve, and control the temperature at 0~ 5°C. Add the prepared potassium dichromate solution dropwise, control the temperature below 25°C, and complete the dropwise addition in about 2 hours. Stirring, thin-layer chromatography analysis, until the reaction of raw materials is compl...
Embodiment 2
[0060] A kind of preparation method of cortisone acetate intermediate, operates according to the following steps:
[0061] (1) Preparation of oxidizing reagent A
[0062] In the preparation tank, add 20Kg of water, then slowly add 5 Kg of concentrated nitric acid, and stir for ten minutes. Then add 4.8Kg manganese oxide, stir to dissolve. Cool down to 10~15°C to obtain oxidizing reagent A—manganese oxide solution. spare
[0063] (2) Preparation of oxide 17α-hydroxypregn-4-ene-3,11,20-trione
[0064] In the reaction tank, add 250Kg acetone, 10Kg glacial acetic acid, 1.2Kg manganese chloride and 10 Kg raw material 11α, 17α-dihydroxypregna-4-ene-3,20-dione, stir to dissolve, and control the temperature at 0 ~5°C. Add the prepared manganese oxide solution dropwise, control the temperature below 25°C, and complete the dropwise addition in about 1.5 hours. Stirring, thin-layer chromatography analysis, until the reaction of raw materials is complete. 5Kg aqueous solution of so...
Embodiment 3
[0066] A kind of preparation method of cortisone acetate intermediate, operates according to the following steps:
[0067] (1) Preparation of oxidizing reagent A
[0068] In the preparation tank, add 5Kg of water, then slowly add 5 Kg of concentrated sulfuric acid, and stir for ten minutes. Add 6Kg of chromium trioxide again, stir and dissolve. Cool down to 10~15°C to obtain oxidizing reagent A—chromium trioxide solution. spare
[0069] (2) Preparation of oxide 17α-hydroxypregn-4-ene-3,11,20-trione
[0070] In the reaction tank, add 250Kg dioxane, 5Kg glacial acetic acid, 1.5Kg manganese chloride and 10 Kg raw material 11α, 17α-dihydroxypregna-4-ene-3,20-dione, stir to dissolve, control The temperature is 10~15°C. Add the prepared chromium trioxide solution dropwise, control the temperature below 15°C, and complete the dropwise addition in about 0.5 hours. Stirring, thin-layer chromatography analysis, until the reaction of raw materials is complete. Add an aqueous solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com